Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article
Abstract
1. Introduction
2. Biologic Mechanism of Immune Checkpoint Inhibition
3. Immune-Related Adverse Events (irAEs)
4. Cutaneous Immune-Related Adverse Events (cirAEs)
| Cutaneous irAEs | Clinical Features | Histopathological Findings | Mainly Associated ICIs | Suggested Managements |
|---|---|---|---|---|
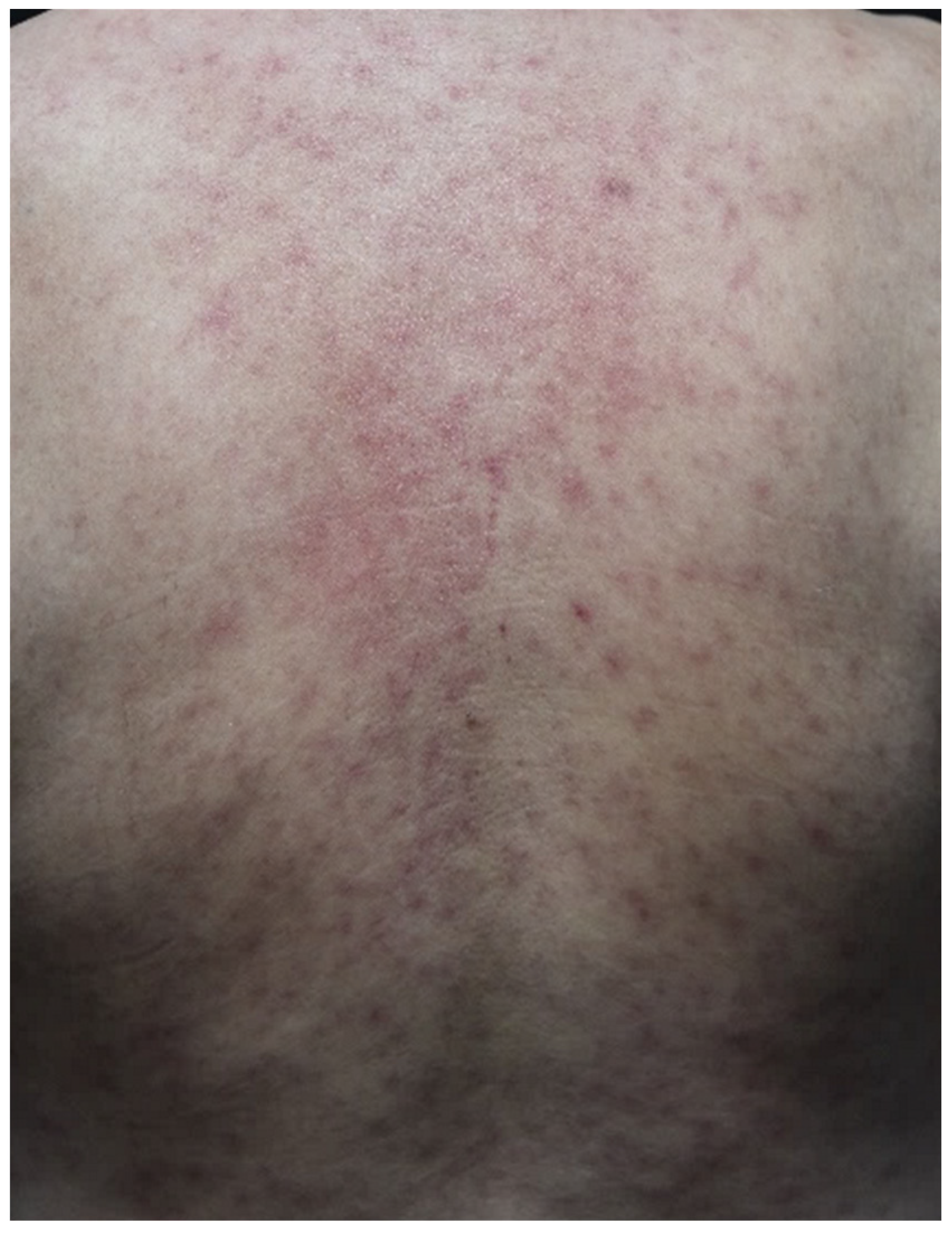
| Maculopapular eruption | Pruritic erythematous macules and papules coalescing into thin plaques, mostly on the trunk and extremities | Superficial, perivascular lymphocytes and eosinophils infiltrate into the upper dermis, mild epidermal spongiosis [33] | Anti-CTLA-4 > anti-PD-1/PD-L1 | Symptomatic management with emollients, topical steroids, and oral antihistamines; consider systemic corticosteroids and withholding ICIs in severe cases [33] |
| Pruritus | May be concomitant with maculopapular rash or develop on normal- appearing skin | - | Anti-CTLA-4 > anti-PD-1/PD-L1 | Topical emollients or oral antihistamines; consider topical/systemic corticosteroids or topical calcineurin inhibitors in severe cases; other therapies include aprepitant, doxepin, gabapentin, pregabalin, and naloxone [3,34,35] |
| Lichenoid dermatitis | Erythematous-to- violaceous scaly plaques with a localized or generalized distribution, mostly on the trunk and extremities; mucosal involvement is rarely reported | Hyperkeratosis, hypergranulosis, a sawtooth rete ridge pattern, lichenoid and interface lymphocytic infiltrates, basal vacuolar changes, parakeratosis, epidermal spongiosis and necrosis, and eosinophils may present [3,36,37,38] | Anti-PD-1/PD-L1 | High-potency topical steroids; consider systemic corticosteroids and withholding ICIs in severe cases; other therapies include oral acitretin and phototherapy [39,40,41] |
| Psoriasiform dermatitis | Sharply bordered, scaly, and erythematous plaques, mostly at extensor sites | Hyperkeratosis, hypogranulosis, acanthosis with elongated rete ridges, perivascular lymphocytic infiltration [42,43] | Anti-PD-1/PD-L1 | Topical corticosteroids, topical vitamin D analogs, or topical retinoids; phototherapy (NB-UVB) [44]; other therapies include acitretin, apremilast, and methotrexate [45]; biologic agents and systemic steroids should be carefully used (TNF-α inhibitors are contraindicated) |
| Vitiligo-like depigmentation (VLD) | Multiple depigmented flecked lesions coalescing into patches on photoexposed areas; the Koebner phenomenon (-) | Dermal lymphocytic infiltrates and a lack of melanocytes [46] | Anti-PD-1/ PD-L1 > anti-CTLA-4 | No effective treatment |
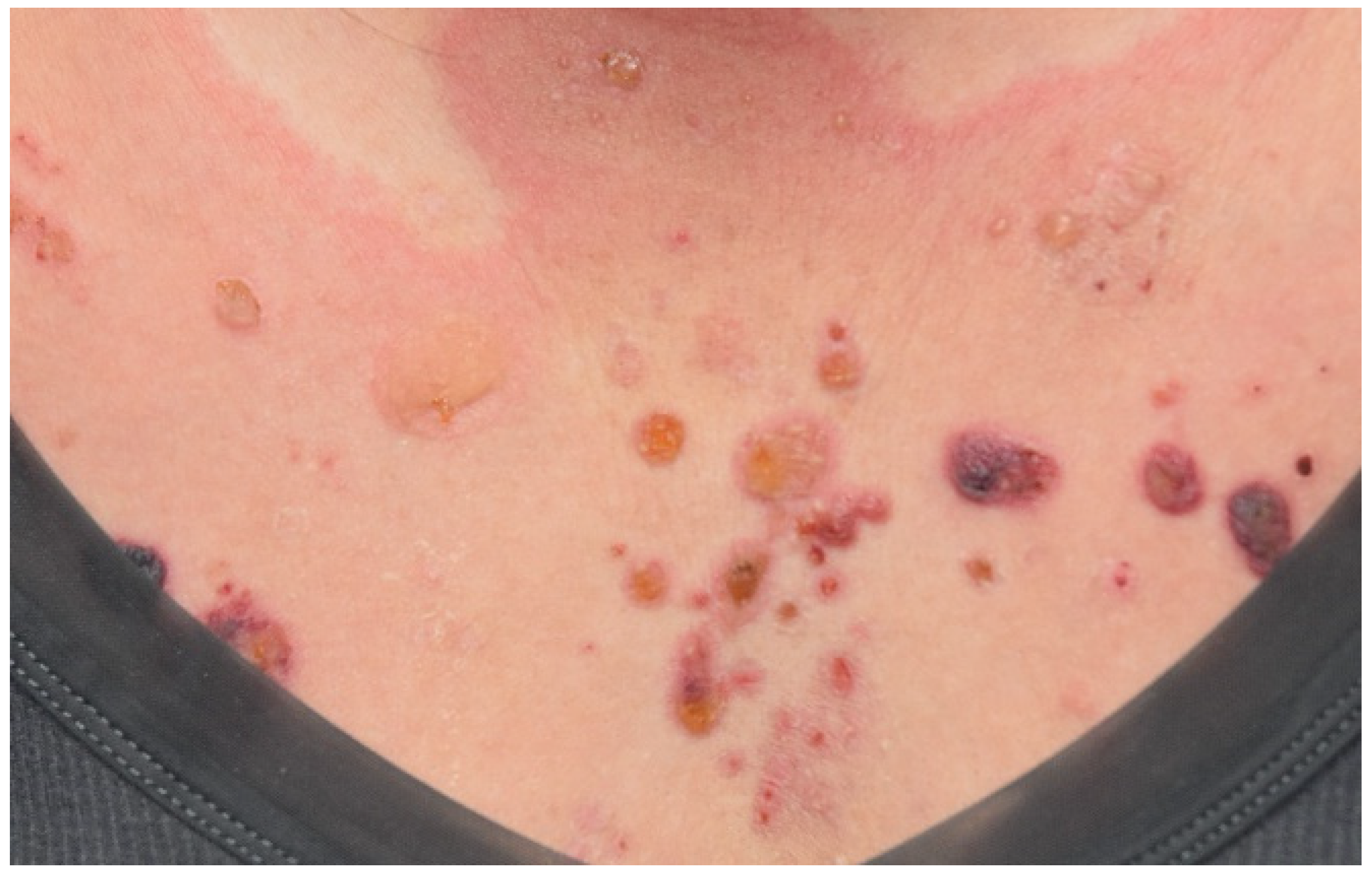
| Bullous pemphigoid (BP) | Pruritic tense bullae overlying the urticarial plaques, mostly on the trunk and extremities | A subepidermal cleft with numerous eosinophils; DIF shows a linear deposition of C3 and IgG along the basement membrane zone | Anti-PD-1/PD-L1 | High-potency topical steroids or systemic corticosteroids depending on the extent of disease; other therapies include methotrexate, doxycycline, omalizumab, and rituximab [47,48,49,50,51] |
| SJS/TEN | Flaccid blister formation (Nikolsky’s sign +) and rapidly progressive and extensive epidermal necrosis and desquamation; mucosal involvement is common | Full-thickness epidermal necrolysis with extensive keratinocyte necrosis, subepidermal bullae, and dermal infiltrates with lymphocytes, eosinophils, and neutrophils | Anti-CTLA-4 > anti-PD-1/PD-L1 | Permanent cessation of ICIs, high-dose systemic corticosteroids and IVIG; intense supportive care (keeping a balance of electrolytes, fluid, and nutrition) and wound care; other therapies include TNF-α inhibitors, mycophenolate mofetil, cyclosporin, and plasmapheresis [52,53,54,55] |
4.1. Maculopapular Eruption (Eczema-like Dermatitis)
4.2. Pruritus
4.3. Lichenoid Dermatitis
4.4. Psoriasiform Dermatitis
4.5. Vitiligo-like Depigmentation (VLD)
4.6. Bullous Pemphigoid (BP)
4.7. Stevens–Johnson Syndrome (SJS)/Toxic Epidermal Necrolysis (TEN)
4.8. Other Less-Common cirAEs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Wahab, N.; Shah, M.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann. Intern. Med. 2018, 168, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Geisler, A.N.; Phillips, G.S.; Barrios, D.M.; Wu, J.; Leung, D.Y.M.; Moy, A.P.; Kern, J.A.; Lacouture, M.E. Immune checkpoint inhibitor-related dermatologic adverse events. J. Am. Acad. Dermatol. 2020, 83, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.T.; Johnson, D.B.; LeBoeuf, N.R.; Zwerner, J.P. Dewan AK.Cutaneous adverse events caused by immune checkpoint inhibitors. J. Am. Acad. Dermatol. 2021, 85, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Tentori, L.; Navarra, P. Ipilimumab: A novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol. Res. 2012, 65, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Ha, S.J.; Kim, H.R. Clinical Insights Into Novel Immune Checkpoint Inhibitors. Front. Pharmacol. 2021, 12, 681320. [Google Scholar] [CrossRef]
- Li, B.; Chan, H.L.; Chen, P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr. Med. Chem. 2019, 26, 3009–3025. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Dai, S.; Jia, R.; Zhang, X.; Fang, Q.; Huang, L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014, 290, 72–79. [Google Scholar] [CrossRef]
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Chen, T.W.-W.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 19 February 2022).
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, J.; Xu, B. Cutaneous adverse events associated with immune checkpoint blockade: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103376. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Park, H.; Malone, D.C.; Wang, C.-Y.; Wilson, D.L.; Yeh, Y.-M.; Boemmel-Wegmann, S.V.; Lo-Ciganic, W.-H. Immune Checkpoint Inhibitors and Immune-Related Adverse Events in Patients With Advanced Melanoma: A Systematic Review and Network Meta-analysis. JAMA Netw. Open 2020, 3, e201611. [Google Scholar] [CrossRef]
- Collins, L.K.; Chapman, M.S.; Carter, J.B.; Samie, F.H. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr. Probl. Cancer 2017, 41, 125–128. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Belum, V.R.; Benhuri, B.; Postow, M.A.; Hellmann, M.D.; Lesokhin, A.M.; Segal, N.H.; Motzer, R.J.; Wu, S.; Busam, K.J.; Wolchok, J.D.; et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur. J. Cancer 2016, 60, 12–25. [Google Scholar] [CrossRef]
- Shi, V.J.; Rodic, N.; Gettinger, S.; Leventhal, J.S.; Neckman, J.P.; Girardi, M.; Bosenberg, M.; Choi, J.N. Clinical and Histologic Features of Lichenoid Mucocutaneous Eruptions Due to Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Immunotherapy. JAMA Dermatol. 2016, 152, 1128–1136. [Google Scholar] [CrossRef]
- Coustal, C.; Du Thanh, A.; Roubille, F.; Assenat, E.; Maria, A.T.J. Rare cutaneous toxicity of immune checkpoint inhibitors: A case of durvalumab-induced dermatomyositis. Eur. J. Cancer 2021, 155, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, C.; Nelson, K.C.; Oliva, I.C.G.; Torres-Cabala, C.A.; Nagarajan, P.; Tetzlaff, M.T.; Ivan, D.; Hwu, W.-J.; Prieto, V.G.; Curry, J.L.; et al. Dermatologic toxicity from immune checkpoint blockade therapy with an interstitial granulomatous pattern. J. Cutan. Pathol. 2018, 45, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.; Panse, G.; Haldas, J.; Gettinger, S.N.; Leventhal, J.S. Pityriasis rubra pilaris-like erythroderma in the setting of pembrolizumab therapy responsive to acitretin. JAAD Case Rep. 2018, 4, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Nakano, K.; Udagawa, S.; Fukuda, N.; Nishizawa, A.; Ono, M.; Urasaki, T.; Tomomatsu, J.; Mochizuki, T.; Shiga, T.; et al. Chilblain lupus-like cutaneous reaction associated with systemic lupus erythematosus induced by immune checkpoint inhibitor. Rheumatology 2021, 61, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Pintova, S.; Sidhu, H.; Friedlander, P.A.; Holcombe, R.F. Sweet’s syndrome in a patient with metastatic melanoma after ipilimumab therapy. Melanoma Res. 2013, 23, 498–501. [Google Scholar] [CrossRef]
- Teulings, H.E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Min Lee, C.K.; Li, S.; Tran, D.C.; Zhu, G.A.; Kim, J.; Kwong, B.Y.; Chang, A.L.S. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: A retrospective case-control study. J. Am. Acad. Dermatol. 2018, 79, 1047–1052. [Google Scholar] [CrossRef]
- Jia, X.-H.; Geng, L.-Y.; Jiang, P.-P.; Xu, H.; Nan, K.-J.; Yao, Y.; Jiang, L.-L.; Sun, H.; Qin, T.-J.; Guo, H. The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. J. Exp. Clin. Cancer Res. 2020, 39, 284. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Berner, F.; Bomze, D.; Fässler, M.; Diem, S.; Cozzio, A.; Jörger, M.; Früh, M.; Driessen, C.; Lenz, T.L.; et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur. J. Cancer 2019, 107, 8–14. [Google Scholar] [CrossRef]
- Gault, A.; Anderson, A.E.; Plummer, R.; Stewart, C.; Pratt, A.G.; Rajan, N. Cutaneous immune-related adverse events in patients with melanoma treated with checkpoint inhibitors. Br. J. Dermatol. 2021, 185, 263–271. [Google Scholar] [CrossRef]
- Okiyama, N.; Tanaka, R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol. Int. 2022, 71, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Minkis, K.; Garden, B.C.; Wu, S.; Pulitzer, M.P.; Lacouture, M.E. The risk of rash associated with ipilimumab in patients with cancer: A systematic review of the literature and meta-analysis. J. Am. Acad. Dermatol. 2013, 69, e121–e128. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lacouture, M.E. Pruritus Associated with Targeted Anticancer Therapies and Their Management. Dermatol. Clin. 2018, 36, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, S.G.; Ständer, S.; Kang, H. PD-1 Blockade-Induced Pruritus Treated with a Mu-Opioid Receptor Antagonist. N. Engl. J. Med. 2018, 379, 1578–1579. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, M.T.; Nagarajan, P.; Chon, S.; Huen, A.; Diab, A.; Omar, P.; Aung, P.P.; Torres-Cabala, C.A.; Mays, S.R.; Prieto, V.G.; et al. Lichenoid Dermatologic Toxicity From Immune Checkpoint Blockade Therapy: A Detailed Examination of the Clinicopathologic Features. Am. J. Dermatopathol. 2017, 39, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Schaberg, K.B.; Novoa, R.A.; Wakelee, H.A.; Kim, J.; Cheung, C.; Srinivas, S.; Kwong, B.Y. Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. J. Cutan. Pathol. 2016, 43, 339–346. [Google Scholar] [CrossRef]
- Hwang, S.J.; Carlos, G.; Wakade, D.; Byth, K.; Kong, B.Y.; Chou, S.; Carlino, M.S.; Kefford, R.; Fernandez-Penas, P. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J. Am. Acad. Dermatol. 2016, 74, 455–461.e1. [Google Scholar] [CrossRef]
- Muntyanu, A.; Netchiporouk, E.; Gerstein, W.; Gniadecki, R.; Litvinov, I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J. Cutan. Med. Surg. 2021, 25, 59–76. [Google Scholar] [CrossRef]
- Donaldson, M.; Owen, J.L.; Chae, Y.K.; Choi, J.N. Management of Persistent Pruritus and Lichenoid Reaction Secondary to Nivolumab With Narrowband Ultraviolet B Phototherapy. Front. Oncol. 2018, 8, 405. [Google Scholar] [CrossRef]
- Lindner, A.K.; Schachtner, G.; Tulchiner, G.; Staudacher, N.; Steinkohl, F.; Nguyen, V.A.; Horninger, W.; Pichler, R. Immune-related lichenoid mucocutaneous erosions during anti-PD-1 immunotherapy in metastatic renal cell carcinoma—A case report. Urol. Case Rep. 2019, 23, 1–2. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Miura, T.; Mori, T.; Ishikawa, M.; Yamamoto, T. Occurrence of Psoriasiform Eruption During Nivolumab Therapy for Primary Oral Mucosal Melanoma. JAMA Dermatol. 2015, 151, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Matsumoto, S.; Takeda, Y.; Sugiyama, H. Systemic Psoriasiform Dermatitis Appeared after the Administration of Pembrolizumab. Intern. Med. 2020, 59, 871–872. [Google Scholar] [CrossRef] [PubMed]
- Ma, V.T.; Katzman, C.S.; Palmbos, P.L.; Patel, R.M.; Gudjonsson, J.E.; Alva, A.S. NB-UVB phototherapy in the treatment of anti-PD-1 inhibitor induced psoriasis: A case report. Curr. Probl. Cancer Case Rep. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Nikolaou, V.; Sibaud, V.; Fattore, D.; Sollena, P.; Ortiz-Brugués, A.; Giacchero, D.; Romano, M.C.; Riganti, J.; Lallas, K.; Peris, K.; et al. Immune checkpoint-mediated psoriasis: A multicenter European study of 115 patients from the European Network for Cutaneous Adverse Event to Oncologic Drugs (ENCADO) group. J. Am. Acad. Dermatol. 2021, 84, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Boussemart, L.; Mateus, C.; Routier, E.; Boutros, C.; Cazenave, H.; Viollet, R.; Thomas, M.; Roy, S.; Benannoune, N. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated with Pembrolizumab. JAMA Dermatol. 2016, 152, 45–51. [Google Scholar] [CrossRef]
- Siegel, J.; Totonchy, M.; Damsky, W.; Berk-Krauss, J.; Castiglione, F., Jr.; Sznol, M.; Petrylak, D.P.; Fischbach, N.; Goldberg, S.B.; Decker, R.H.; et al. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: A retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J. Am. Acad. Dermatol. 2018, 79, 1081–1088. [Google Scholar] [CrossRef]
- Haug, V.; Behle, V.; Benoit, S.; Kneitz, H.; Schilling, B.; Goebeler, M.; Gesierich, A. Pembrolizumab-associated mucous membrane pemphigoid in a patient with Merkel cell carcinoma. Br. J. Dermatol. 2018, 179, 993–994. [Google Scholar] [CrossRef]
- Lopez, A.T.; Geskin, L. A Case of Nivolumab-Induced Bullous Pemphigoid: Review of Dermatologic Toxicity Associated with Programmed Cell Death Protein-1/Programmed Death Ligand-1 Inhibitors and Recommendations for Diagnosis and Management. Oncologist 2018, 23, 1119–1126. [Google Scholar] [CrossRef]
- Barrios, D.M.; Phillips, G.S.; Geisler, A.N.; Trelles, S.R.; Markova, A.; Noor, S.J.; Quigley, E.A.; Haliasos, H.C.; Moy, A.P.; Schram, A.M.; et al. IgE blockade with omalizumab reduces pruritus related to immune checkpoint inhibitors and anti-HER2 therapies. Ann. Oncol. 2021, 32, 736–745. [Google Scholar] [CrossRef]
- Sowerby, L.; Dewan, A.K.; Granter, S.; Gandhi, L.; LeBoeuf, N.R. Rituximab Treatment of Nivolumab-Induced Bullous Pemphigoid. JAMA Dermatol. 2017, 153, 603–605. [Google Scholar] [CrossRef]
- Keerty, D.; Koverzhenko, V.; Belinc, D.; LaPorta, K.; Haynes, E. Immune-Mediated Toxic Epidermal Necrolysis. Cureus 2020, 12, e9587. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; He, C.; Zhang, L.; Liu, X.; Li, Y.; Wang, H.; Guo, X.; Zhou, J.; Duan, L.; Wang, M.; et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac. Cancer 2020, 11, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Vivar, K.L.; Deschaine, M.; Messina, J.; Divine, J.M.; Rabionet, A.; Patel, N.; Harrington, M.A.; Seminario-Vidal, L. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J. Cutan. Pathol. 2017, 44, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Khan, H.N. Nivolumab-Induced Toxic Epidermal Necrolysis: Rare but Fatal Complication of Immune Checkpoint Inhibitor Therapy. Cureus 2021, 13, e15017. [Google Scholar] [CrossRef]
- Bottlaender, L.; Amini-Adle, M.; Maucort-Boulch, D.; Robinson, P.; Thomas, L.; Dalle, S. Cutaneous adverse events: A predictor of tumour response under anti-PD-1 therapy for metastatic melanoma, a cohort analysis of 189 patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2096–2105. [Google Scholar] [CrossRef]
- Curry, J.L.; Tetzlaff, M.T.; Nagarajan, P.; Drucker, C.; Diab, A.; Hymes, S.R.; Duvic, M.; Hwu, W.-J.; Wargo, J.A.; Cabala, C.A.T.C.; et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J. Cutan. Pathol. 2017, 44, 158–176. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Apalla, Z.; Papageorgiou, C.; Lallas, A.; Delli, F.; Fotiadou, C.; Kemanetzi, C.; Lazaridou, E. Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Literature Review. Dermatol. Pract. Concept. 2021, 11, e2021155. [Google Scholar] [CrossRef]
- Sibaud, V.; Meyer, N.; Lamant, L.; Vigarios, E.; Mazieres, J.; Delord, J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 2016, 28, 254–263. [Google Scholar] [CrossRef]
- Ito, J.; Fujimoto, D.; Nakamura, A.; Nagano, T.; Uehara, K.; Imai, Y.; Tomii, K. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer 2017, 109, 58–61. [Google Scholar] [CrossRef]
- Joseph, R.W.; Cappel, M.; Goedjen, B.; Gordon, M.; Kirsch, B.; Gilstrap, C.; Bagaria, S.; Jambusaria-Pahlajani, A. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol. Res. 2015, 3, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Fässler, M.; Rammlmair, A.; Feldmeyer, L.; Suter, V.G.A.; Gloor, A.D.; Horn, M.; Deml, K.; Beltraminelli, H.; Borradori, L. Mucous membrane pemphigoid and lichenoid reactions after immune checkpoint inhibitors: Common pathomechanisms. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e112–e115. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Masuzawa, M.; Amoh, Y. Oral lichenoid reaction showing multiple ulcers associated with anti-programmed death cell receptor-1 treatment: A report of two cases and published work review. J. Dermatol. 2018, 45, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Eid, C.; Belum, V.R.; Combemale, P.; Barres, B.; Lamant, L.; Mourey, L.; Gomez-Roca, C.; Estilo, C.L.; Motzer, R.; et al. Oral lichenoid reactions associated with anti-PD-1/PD-L1 therapies: Clinicopathological findings. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, e464–e469. [Google Scholar] [CrossRef]
- Guggina, L.M.; Yanes, D.A.; Choi, J.N. Inverse lichenoid drug eruption associated with nivolumab. JAAD Case Rep. 2016, 3, 7–9. [Google Scholar] [CrossRef][Green Version]
- Strickley, J.D.; Vence, L.M.; Burton, S.K.; Callen, J.P. Nivolumab-induced lichen planus pemphigoides. Cutis 2019, 103, 224–226. [Google Scholar]
- Chang, H.-C.; Chang, Y.-S.; Lee, H.-L.; Lin, M.-H. Bullous lichen planus-like reactions in a patient with renal cancer after receiving anti-programmed cell death-1 therapy. Dermatol. Sin. 2020, 38, 55–58. [Google Scholar]
- Bonigen, J.; Raynaud-Donzel, C.; Hureaux, J.; Kramkimel, N.; Blom, A.; Jeudy, G.; Breton, A.-L.; Hubiche, T.; Bedane, C.; Legoupil, D.; et al. Anti-PD1-induced psoriasis: A study of 21 patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e254–e257. [Google Scholar] [CrossRef]
- De Bock, M.; Hulstaert, E.; Kruse, V.; Brochez, L. Psoriasis Vulgaris Exacerbation during Treatment with a PD-1 Checkpoint Inhibitor: Case Report and Literature Review. Case Rep. Dermatol. 2018, 10, 190–197. [Google Scholar] [CrossRef]
- Seervai, R.N.H.; Heberton, M.; Cho, W.C.; Gill, P.; Murphy, M.B.; Aung, P.P.; Nagarajan, P.; Torres-Cabala, C.A.; Patel, A.B.; Ruiz-Bañobre, J.; et al. Severe de novo pustular psoriasiform immune-related adverse event associated with nivolumab treatment for metastatic esophageal adenocarcinoma. J. Cutan. Pathol. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Imai, Y.; Ayithan, N.; Wu, X.; Yuan, Y.; Wang, L.; Hwang, S.T. Cutting Edge: PD-1 Regulates Imiquimod-Induced Psoriasiform Dermatitis through Inhibition of IL-17A Expression by Innate γδ-Low T Cells. J. Immunol. 2015, 195, 421–425. [Google Scholar] [CrossRef]
- Yu, S.; Wu, X.; Shi, Z.; Huynh, M.; Jena, P.K.; Sheng, L.; Zhou, Y.; Han, D.; Wan, Y.-J.Y.; Hwang, S.T. Diet-induced obesity exacerbates imiquimod-mediated psoriasiform dermatitis in anti-PD-1 antibody-treated mice: Implications for patients being treated with checkpoint inhibitors for cancer. J. Dermatol. Sci. 2020, 97, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Pantelyushin, S.; Haak, S.; Ingold, B.; Kulig, P.; Heppner, F.L.; Navarini, A.A.; Becher, B. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Investig. 2012, 122, 2252–2256. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, I.W.; Leventhal, J.S. Cutaneous Toxicities of Immune Checkpoint Inhibitors: The Role of the Dermatologist. Yale J. Biol. Med. 2020, 93, 123–132. [Google Scholar] [PubMed]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Ségui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef]
- Savage, L.J.; Wittmann, M.; McGonagle, D.; Helliwell, P.S. Ustekinumab in the Treatment of Psoriasis and Psoriatic Arthritis. Rheumatol. Ther. 2015, 2, 1–16. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Yan, B.Y.; Chan, T.C.; Krueger, J.G. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J. Immunol. 2018, 201, 1605–1613. [Google Scholar] [CrossRef]
- Yun, S.J.; Oh, I.-J.; Park, C.K.; Kim, Y.-C.; Kim, H.B.; Kim, H.-K.; Hong, A.R.; Kim, I.-Y.; Ahn, S.-J.; Na, K.-J.; et al. Vitiligo-like depigmentation after pembrolizumab treatment in patients with non-small cell lung cancer: A case report. Transl. Lung Cancer Res. 2020, 9, 1585–1590. [Google Scholar] [CrossRef]
- Yin, E.S.; Totonchy, M.B.; Leventhal, J.S. Nivolumab-associated vitiligo-like depigmentation in a patient with acute myeloid leukemia: A novel finding. JAAD Case Rep. 2017, 3, 90–92. [Google Scholar] [CrossRef]
- Liu, R.C.; Consuegra, G.; Chou, S.; Peñas, P.F. Vitiligo-like depigmentation in oncology patients treated with immunotherapies for nonmelanoma metastatic cancers. Clin. Exp. Dermatol. 2019, 44, 643–646. [Google Scholar] [CrossRef]
- Guida, M.; Strippoli, S.; Maule, M.; Quaglino, P.; Ramondetta, A.; Sileni, V.C.; Cappellini, G.A.; Queirolo, P.; Ridolfi, L.; Del Vecchio, M.; et al. Immune checkpoint inhibitor associated vitiligo and its impact on survival in patients with metastatic melanoma: An Italian Melanoma Intergroup study. ESMO Open 2021, 6, 100064. [Google Scholar] [CrossRef]
- Larsabal, M.; Marti, A.; Jacquemin, C.; Rambert, J.; Thiolat, D.; Dousset, L.; Taieb, A.; Dutriaux, C.; Prey, S.; Boniface, K.; et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J. Am. Acad. Dermatol. 2017, 76, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Lommerts, J.E.; Bekkenk, M.W.; Luiten, R.M. Vitiligo induced by immune checkpoint inhibitors in melanoma patients: An expert opinion. Expert Opin. Drug Saf. 2021, 20, 883–888. [Google Scholar] [CrossRef] [PubMed]
- De Golian, E.; Kwong, B.Y.; Swetter, S.M.; Pugliese, S.B. Cutaneous Complications of Targeted Melanoma Therapy. Curr. Treat. Options Oncol. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Suppa, M.; Bruderer, P.; Sirtaine, N.; Aspeslagh, S.; Kerger, J. A Late Dermatologic Presentation of Bullous Pemphigoid Induced by Anti-PD-1 Therapy and Associated with Unexplained Neurological Disorder. Case Rep. Oncol. 2021, 14, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Schindler, K.; Querfeld, C.; Busam, K.; Cunningham, J.; Page, D.B.; Postow, M.A.; Weinstein, A.; Lucas, A.S.; Ciccolini, K.T.; et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol. Res. 2016, 4, 383–389. [Google Scholar] [CrossRef]
- Carlos, G.; Anforth, R.; Chou, S.; Clements, A.; Fernandez-Peñas, P. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Res. 2015, 25, 265–268. [Google Scholar] [CrossRef]
- Ba, A.T.L.; Khanna, T.; Antonov, N.; Audrey-Bayan, C.; Geskin, L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int. J. Dermatol. 2018, 57, 664–669. [Google Scholar]
- Chou, P.-S.; Chou, T.-C.; Chang, C.-H.; Yu, S.; Lee, C.-H. Chronic eczematous dermatitis in patients with neurodegenerative diseases may be an early marker of bullous pemphigoid. Med. Hypotheses 2017, 103, 86–89. [Google Scholar] [CrossRef]
- Hammers, C.M.; Stanley, J.R. Mechanisms of Disease: Pemphigus and Bullous Pemphigoid. Annu. Rev. Pathol. 2016, 11, 175–197. [Google Scholar] [CrossRef]
- Sharma, P.; Barnes, M.; Nabeel, S.; LiPera, W. Pembrolizumab-Induced Bullous Pemphigoid Treated With Rituximab. JCO Oncol. Pract. 2020, 16, 764–766. [Google Scholar] [CrossRef]
- Wesolow, J.T.; Jalali, S.; Clark, L.D. A Rare Case of Bullous Pemphigoid Secondary to Checkpoint Inhibitor Immunotherapy: A Tense Situation. Cureus 2021, 13, e16169. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, A.V.; Rzepka, P.V.; Shearer, S.M.; Scrape, S.R.; Olencki, T.E.; Kaffenberger, B.H. Novel use of combination therapeutic plasma exchange and rituximab in the treatment of nivolumab-induced bullous pemphigoid. Int. J. Dermatol. 2018, 57, 1372–1374. [Google Scholar] [CrossRef]
- Bs, N.J.M.; Ravi, V.; Cheng, K.; Bach, D.Q.; Worswick, S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: A systematic review. Int. J. Dermatol. 2020, 59, e183–e188. [Google Scholar]
- Chirasuthat, P.; Chayavichitsilp, P. Atezolizumab-Induced Stevens-Johnson Syndrome in a Patient with Non-Small Cell Lung Carcinoma. Case Rep. Dermatol. 2018, 10, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Antonazzo, I.C.; La Placa, M.; Ardizzoni, A.; Poluzzi, E.; De Ponti, F. Serious Cutaneous Toxicities with Immune Checkpoint Inhibitors in the U.S. Food and Drug Administration Adverse Event Reporting System. Oncologist 2019, 24, e1228–e1231. [Google Scholar] [CrossRef]
- Chen, C.-B.; Wu, M.-Y.; Ng, C.Y.; Lu, C.-W.; Wu, J.; Kao, P.-H.; Yang, C.-K.; Peng, M.-T.; Huang, C.-Y.; Chang, W.-C.; et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag. Res. 2018, 10, 1259–1273. [Google Scholar] [CrossRef]
- Salati, M.; Pifferi, M.; Baldessari, C.; Bertolini, F.; Tomasello, C.; Cascinu, S.; Barbieri, F. Stevens-Johnson syndrome during nivolumab treatment of NSCLC. Ann. Oncol. 2018, 29, 283–284. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Zarbo, A.; Belum, V.; Sibaud, V.; Oudard, S.; Postow, M.; Hsieh, J.; Motzer, R.; Busam, K.; Lacouture, M. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br. J. Dermatol. 2017, 176, 1649–1652. [Google Scholar] [CrossRef]
- Assi, H.; Wilson, K.S. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: Two illustrative cases. Curr. Oncol. 2013, 20, e165–e169. [Google Scholar] [CrossRef]
- Antoury, L.; Maloney, N.J.; Bach, D.Q.; Goh, C.; Cheng, K. Alopecia areata as an immune-related adverse event of immune checkpoint inhibitors: A review. Dermatol. Ther. 2020, 33, e14171. [Google Scholar] [CrossRef] [PubMed]
- Dasanu, C.A.; Lippman, S.M.; Plaxe, S.C. Persistently curly hair phenotype with the use of nivolumab for squamous cell lung cancer. J. Oncol. Pharm. Pract. 2017, 23, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.; Carvajal, R.D.; Postow, M.A.; Wolchok, J.D.; Krueger, J.G. Definite regression of cutaneous melanoma metastases upon addition of topical contact sensitizer diphencyprone to immune checkpoint inhibitor treatment. Exp. Dermatol. 2016, 25, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Furudate, S.; Kakizaki, A.; Kambayashi, Y.; Haga, T.; Hashimoto, A.; Aiba, S. Contact immunotherapy enhances the therapeutic effects of nivolumab in treating in-transit melanoma: Two cases reports. J. Dermatol. 2016, 43, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Gkiozos, I.; Kopitopoulou, A.; Kalkanis, A.; Vamvakaris, I.N.; Judson, M.A.; Syrigos, K.N. Sarcoidosis-Like Reactions Induced by Checkpoint Inhibitors. J. Thorac. Oncol. 2018, 13, 1076–1082. [Google Scholar] [CrossRef]
- Tetzlaff, M.T.; Nelson, K.; Diab, A.; Staerkel, G.A.; Nagarajan, P.; Torres-Cabala, C.A.; Chasen, B.A.; Wargo, J.A.; Prieto, V.G.; Amaria, R.N.; et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: A marker of therapy response in a subset of melanoma patients. J. ImmunoTherapy Cancer 2018, 6, 14. [Google Scholar] [CrossRef]
- Laroche, A.; Chinchilla, E.A.; Bourgeault, E.; Doré, M.-A. Erythema Nodosum as the Initial Presentation of Nivolumab-Induced Sarcoidosis-Like Reaction. J. Cutan. Med. Surg. 2018, 22, 627–629. [Google Scholar] [CrossRef]
- Pach, J.; Moody, K.; Ring, N.; Panse, G.; Zhang, M.; Deverapalli, S.; Leventhal, J. Erythema nodosum-like panniculitis associated with immune checkpoint inhibitor therapy: Two cases reporting a rare cutaneous adverse event. JAAD Case Rep. 2021, 13, 118–120. [Google Scholar] [CrossRef]
- Tetzlaff, M.T.; Jazaeri, A.A.; Torres-Cabala, C.A.; Korivi, B.R.; Landon, G.A.; Nagarajan, P.; Choksi, A.; Chen, L.; Uemura, M.; Aung, P.; et al. Erythema nodosum-like panniculitis mimicking disease recurrence: A novel toxicity from immune checkpoint blockade therapy-Report of 2 patients. J. Cutan. Pathol. 2017, 44, 1080–1086. [Google Scholar] [CrossRef]
- Seban, R.-D.; Vermersch, C.; Champion, L.; Bonsang, B.; Roger, A.; Ghidaglia, J. Immune-Related Erythema Nodosum Mimicking in Transit Melanoma Metastasis on [18F]-FDG PET/CT. Diagnostics 2021, 11, 747. [Google Scholar] [CrossRef]
- Ravi, V.; Maloney, N.J.; Worswick, S. Neutrophilic dermatoses as adverse effects of checkpoint inhibitors: A review. Dermatol. Ther. 2019, 32, e13074. [Google Scholar] [CrossRef] [PubMed]
- Gormley, R.; Wanat, K.; Elenitsas, R.; Giles, J.; McGettigan, S.; Schuchter, L.; Takeshita, J. Ipilimumab-associated Sweet syndrome in a melanoma patient. J. Am. Acad. Dermatol. 2014, 71, e211–e213. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, H.A.; Akkus, E.; Heper, A.O.; Akay, B.N.; Urun, Y.; Utkan, G. Sweet’s syndrome under ipilimumab therapy and a brief comparison of the cases in literature. J. Oncol. Pharm. Pract. 2020, 26, 1762–1764. [Google Scholar] [CrossRef] [PubMed]
- Welborn, M.E.; Kubicki, S.L.; Patel, A.B. Pyoderma Gangrenosum Following Initiation of Immune Checkpoint Inhibitor Therapy. J. Immunother. Precis. Oncol. 2020, 1, 82–84. [Google Scholar] [CrossRef]
- Tsibris, H.; Lian, C.; Ho, A. Pembrolizumab-associated pyoderma gangrenosum in a patient with metastatic squamous cell carcinoma. Dermatol. Online J. 2021, 27, 27. [Google Scholar] [CrossRef]
- Rudolph, B.M.; Staib, F.; Von Stebut, E.; Hainz, M.; Grabbe, S.; Loquai, C. Neutrophilic disease of the skin and intestines after ipilimumab treatment for malignant melanoma-simultaneous occurrence of pyoderma gangrenosum and colitis. Eur. J. Dermatol. 2014, 24, 268–269. [Google Scholar] [CrossRef]
- Thomas, R.; Patel, H.; Scott, J. Dermatomyositis Flare With Immune Checkpoint Inhibitor Therapy for Melanoma. Cureus 2021, 13, e14387. [Google Scholar] [CrossRef]
- Shibata, C.; Kato, J.; Toda, N.; Imai, M.; Fukumura, Y.; Arai, J.; Kurokawa, K.; Kondo, M.; Takagi, K.; Kojima, K.; et al. Paraneoplastic dermatomyositis appearing after nivolumab therapy for gastric cancer: A case report. J. Med. Case Rep. 2019, 13, 168. [Google Scholar] [CrossRef]
- Estenaga, A.; Rodriguez-Garijo, N.; Tomás-Velázquez, A.; Antoñanzas-Pérez, J.; Alvarez-Gigli, M.L.; García-Tobar, L.; Espaa-Alonso, A.; Salido-Vallejo, R. Immunotherapy-intensified paraneoplastic dermatomyositis. Indian J. Dermatol. Venereol. Leprology. 2021, 88, 93–96. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Buser, T.; Willi, N.; Rothschild, S.I.; Wicki, A.; Schiller, P.; Cathomas, G.; Zippelius, A.; Mertz, K.D. Grover’s-like drug eruption in a patient with metastatic melanoma under ipilimumab therapy. J. Immunother. Cancer 2016, 4, 47. [Google Scholar] [CrossRef][Green Version]
- Uemura, M.; Fa’ak, F.; Haymaker, C.; McQuail, N.; Sirmans, E.; Hudgens, C.W.; Barbara, L.; Bernatchez, C.; Curry, J.L.; Hwu, P.; et al. A case report of Grover’s disease from immunotherapy-a skin toxicity induced by inhibition of CTLA-4 but not PD-1. J. Immun. Ther. Cancer 2016, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Thuraisingam, T.; Chergui, M.; Nguyen, K. Nivolumab-associated DRESS syndrome: A case report. JAAD Case Rep. 2019, 5, 216–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ai, L.; Gao, J.; Zhao, S.; Li, Q.; Cui, Y.-H.; Liu, Q.; Wu, D.; Wang, Y.; Jin, X.; Ji, Y.; et al. Nivolumab-associated DRESS in a genetic susceptible individual. J. Immunother. Cancer 2021, 9, e002879. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Uchi, H.; Haratake, N.; Takamori, S.; Toyozawa, R.; Miura, N.; Yamaguchi, M.; Seto, T.; Takenoyama, M. Acute Generalized Exanthematous Pustulosis Caused by the Combination of Pembrolizumab Plus Chemotherapy in a Patient With Squamous-Cell Carcinoma. Clin. Lung Cancer 2020, 21, e54–e56. [Google Scholar] [CrossRef]
- Page, B.; Borradori, L.; Beltraminelli, H.; Yawalkar, N.; Hunger, R. Acute generalized exanthematous pustulosis associated with ipilimumab and nivolumab. J. Eur. Acad. Dermatol. Venereol. 2017, 32, e256–e257. [Google Scholar] [CrossRef]
- Chang, H.-C.; Ko, P.-H.; Chang, Y.-S.; Chou, P.-C. Acute generalized exanthematous pustulosis induced by atezolizumab in a patient with lung adenocarcinoma. Lung Cancer 2021, 159, 175–176. [Google Scholar] [CrossRef]
- Hwang, S.J.; Carlos, G.; Wakade, D.; Sharma, R.; Fernandez-Penas, P. Ipilimumab-induced acute generalized exanthematous pustulosis in a patient with metastatic melanoma. Melanoma Res. 2016, 26, 417–420. [Google Scholar] [CrossRef]

| ICIs | Target | Indications |
|---|---|---|
| Ipilimumab | CTLA-4 | CRC, HCC, melanoma, mesothelioma, NSCLC, RCC |
| Nivolumab | PD-1 | CRC, esophageal SCC, HCC, HL, HNSCC, melanoma, mesothelioma, NSCLC, RCC, urothelial carcinoma |
| Pembrolizumab | PD-1 | breast cancer, cervical cancer, CRC, CSCC, endometrial carcinoma, esophageal carcinoma, gastric carcinoma, HCC, HL, HNSCC, melanoma, mesothelioma, MCC, NSCLC, large B-cell lymphoma, RCC, SCLC, urothelial carcinoma |
| Cemiplimab | PD-1 | BCC, CSCC, NSCLC |
| Atezolizumab | PD-L1 | breast cancer, HCC, melanoma, NSCLC, SCLC, urothelial carcinoma |
| Durvalumab | PD-L1 | NSCLC, SCLC, urothelial carcinoma |
| Avelumab | PD-L1 | MCC, RCC, urothelial carcinoma |
| Less-Common cirAEs | Description | Suggested Managements |
|---|---|---|
| Alopecia areata/ universalis [101,102,103] |
|
|
| Sarcoidosis/ sarcoidosis-like reactions [107,108,109] |
| Dependent on the extent of Involvement.
|
| Erythema nodosum (EN) [109,110,111,112] |
|
|
| Sweet syndrome [26,113,114,115] |
|
|
| Pyoderma gangrenosum (PG) [113,116,117,118] |
|
|
| Dermatomyositis (DM) [22,119,120,121] |
|
|
| Grover’s disease (GD) [122,123] |
|
|
| Drug reaction with eosinophilia and systemic symptoms (DRESS) [124,125] |
|
|
| Acute generalized exanthematous pustulosis (AGEP) [126,127,128,129] |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Yu, H.-S.; Yu, S. Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article. Curr. Oncol. 2022, 29, 2871-2886. https://doi.org/10.3390/curroncol29040234
Chen C-H, Yu H-S, Yu S. Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article. Current Oncology. 2022; 29(4):2871-2886. https://doi.org/10.3390/curroncol29040234
Chicago/Turabian StyleChen, Chieh-Hsun, Hsin-Su Yu, and Sebastian Yu. 2022. "Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article" Current Oncology 29, no. 4: 2871-2886. https://doi.org/10.3390/curroncol29040234
APA StyleChen, C.-H., Yu, H.-S., & Yu, S. (2022). Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article. Current Oncology, 29(4), 2871-2886. https://doi.org/10.3390/curroncol29040234






