Two Distinct Clinical Patterns of Ibrutinib-to-Venetoclax Transition in Relapsed Chronic Lymphocytic Leukemia Patients
Abstract
:1. Introduction
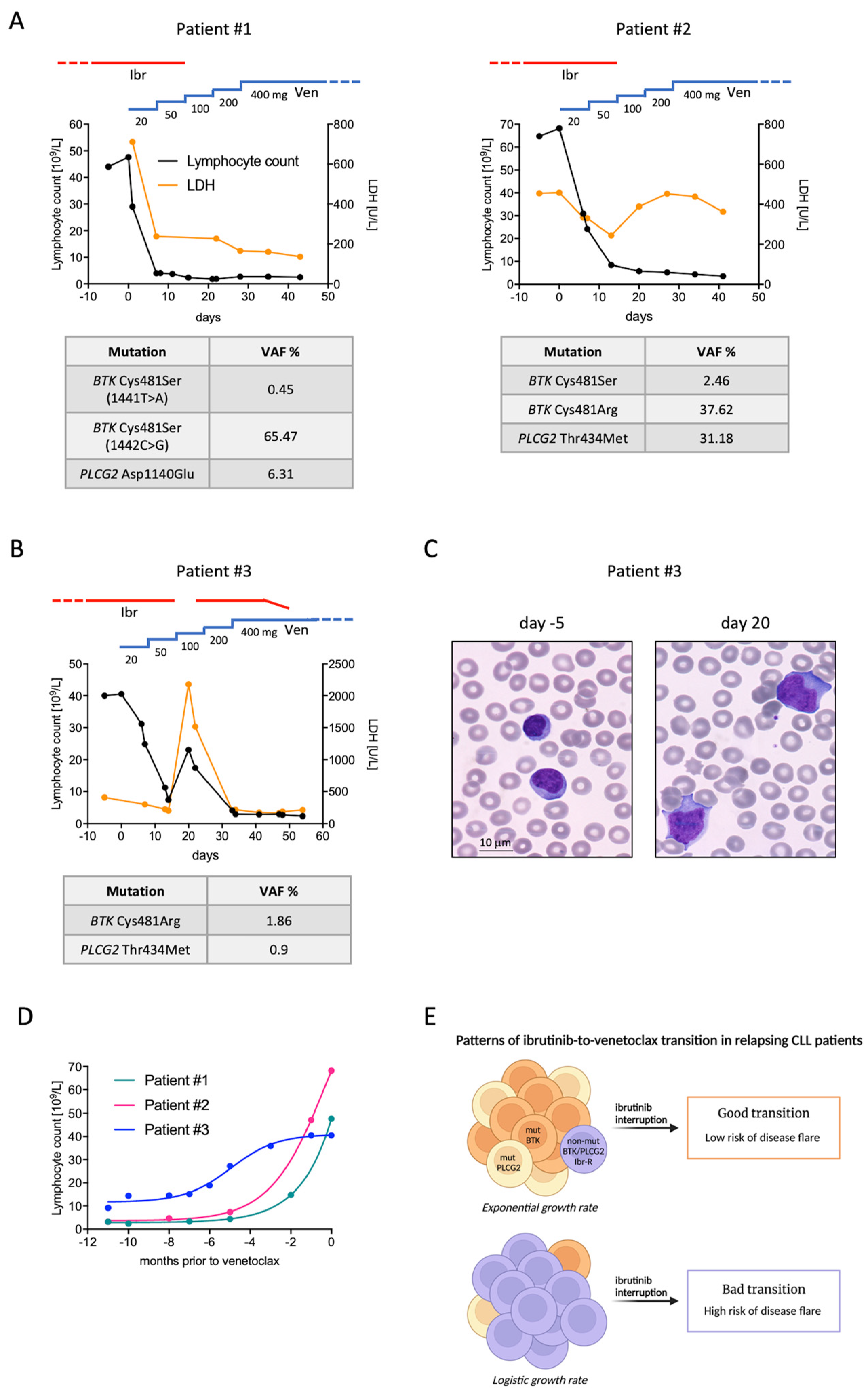
2. Pattern A: The Good Transition
3. Pattern B: The Bad Transition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, A.; Mato, A.R.; Rigolin, G.M.; Piciocchi, A.; Gentile, M.; Laurenti, L.; Allan, J.N.; Pagel, J.M.; Brander, D.M.; Hill, B.T.; et al. Efficacy of bendamustine and rituximab in unfit patients with previously untreated chronic lymphocytic leukemia. Indirect comparison with ibrutinib in a real-world setting. A GIMEMA-ERIC and US study. Cancer Med. 2020, 9, 8468–8479. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Ma, S.; Kipps, T.J.; Coutre, S.E.; Davids, M.S.; Eichhorst, B.; Hallek, M.; Byrd, J.C.; Humphrey, K.; Zhou, L.; et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood 2019, 134, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Mato, A.R.; Wierda, W.G.; Davids, M.S.; Choi, M.; Cheson, B.D.; Furman, R.R.; Lamanna, N.; Barr, P.M.; Zhou, L.; et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: An interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018, 19, 65–75. [Google Scholar] [CrossRef]
- Gribben, J.G. Practical management of tumour lysis syndrome in venetoclax-treated patients with chronic lymphocytic leukaemia. Br. J. Haematol. 2020, 188, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Kater, A.P.; Arslan, Ö.; Demikran, F.; Herishanu, Y.; Ferhanoglu, B.; Diaz, M.G.; Leber, B.; Montillo, M.; Panayiotidis, P.; Rossi, D.; et al. Efficacy of Venetoclax in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia: Primary Endpoint Analysis of the International Phase 3B Trial (Venice I). In Proceedings of the EHA Annual Congress 2020, Virtual, 11–21 June 2020. [Google Scholar]
- Kwok, M.; Wu, C.J. Clonal Evolution of High-Risk Chronic Lymphocytic Leukemia: A Contemporary Perspective. Front. Oncol. 2021, 11, 790004. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Landau, D.A.; Taylor-Weiner, A.; Bozic, I.; Zhang, H.; Sarosiek, K.; Wang, L.; Stewart, C.; Fan, J.; Hoellenriegel, J.; et al. Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nat. Commun. 2016, 7, 11589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landau, D.A.; Sun, C.; Rosebrock, D.; Herman, S.E.M.; Fein, J.; Sivina, M.; Underbayev, C.; Liu, D.; Hoellenriegel, J.; Ravichandran, S.; et al. The evolutionary landscape of chronic lymphocytic leukemia treated with ibrutinib targeted therapy. Nat. Commun. 2017, 8, 2185. [Google Scholar] [CrossRef] [PubMed]
- Agathangelidis, A.; Ljungstrom, V.; Scarfo, L. Highly similar genomic landscapes in monoclonal B-cell lymphocytosis and ultra-stable chronic lymphocytic leukemia with low frequency of driver mutations. Haematologica 2018, 103, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Maffei, R.; Fiorcari, S.; Atene, C.G.; Martinelli, S.; Scarfò, L.; Bonfiglio, S.; Maccaferri, M.; Ljungström, V.; Zucchini, P.; Forghieri, F.; et al. A single-tube multiplex method for monitoring mutations in cysteine 481 of Bruton Tyrosine Kinase (BTK) gene in chronic lymphocytic leukemia patients treated with ibrutinib. Leuk. Lymphoma 2021, 62, 2018–2021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrarini, I.; Gandini, F.; Zapparoli, E.; Rigo, A. Two Distinct Clinical Patterns of Ibrutinib-to-Venetoclax Transition in Relapsed Chronic Lymphocytic Leukemia Patients. Curr. Oncol. 2022, 29, 2792-2797. https://doi.org/10.3390/curroncol29040227
Ferrarini I, Gandini F, Zapparoli E, Rigo A. Two Distinct Clinical Patterns of Ibrutinib-to-Venetoclax Transition in Relapsed Chronic Lymphocytic Leukemia Patients. Current Oncology. 2022; 29(4):2792-2797. https://doi.org/10.3390/curroncol29040227
Chicago/Turabian StyleFerrarini, Isacco, Francesca Gandini, Ettore Zapparoli, and Antonella Rigo. 2022. "Two Distinct Clinical Patterns of Ibrutinib-to-Venetoclax Transition in Relapsed Chronic Lymphocytic Leukemia Patients" Current Oncology 29, no. 4: 2792-2797. https://doi.org/10.3390/curroncol29040227
APA StyleFerrarini, I., Gandini, F., Zapparoli, E., & Rigo, A. (2022). Two Distinct Clinical Patterns of Ibrutinib-to-Venetoclax Transition in Relapsed Chronic Lymphocytic Leukemia Patients. Current Oncology, 29(4), 2792-2797. https://doi.org/10.3390/curroncol29040227




