Prognostic Relevance of Weight and Weight Loss during Multimodal Therapy for Oesophagogastric Tumours
Abstract
:1. Introduction
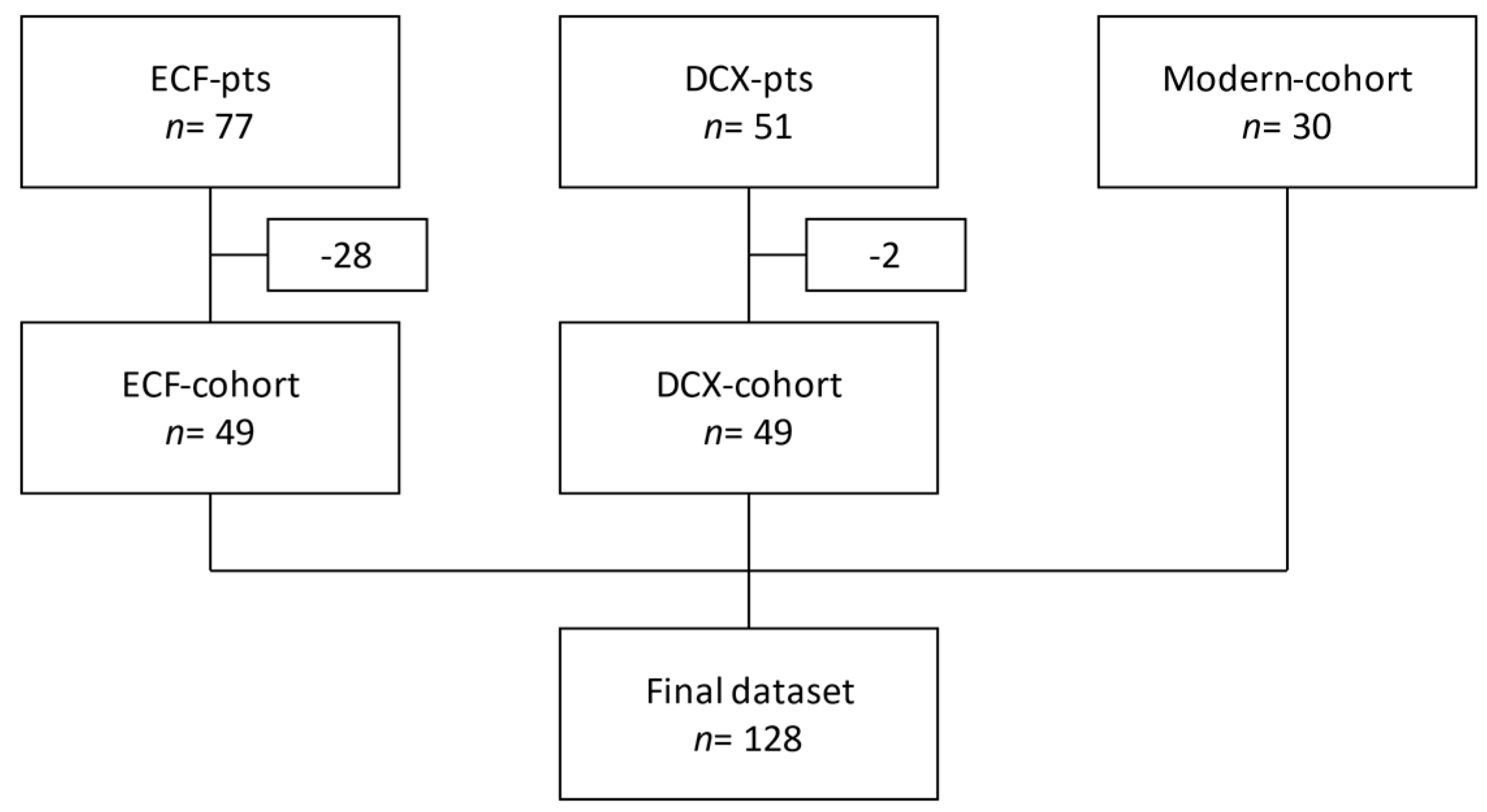
2. Materials and Methods
3. Results
3.1. Demographics
3.2. Chemotherapy
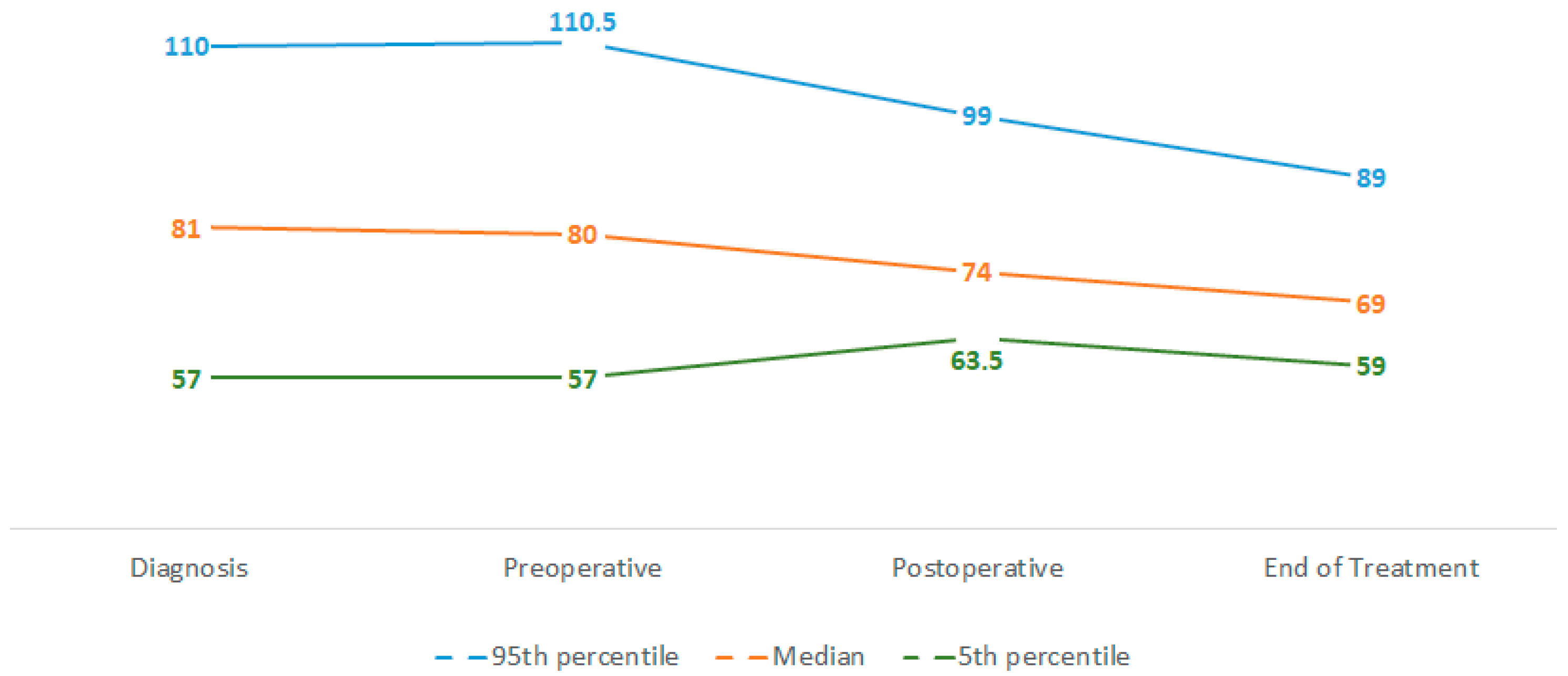
3.3. Weight and Weight Loss
3.4. Surgical Aspects
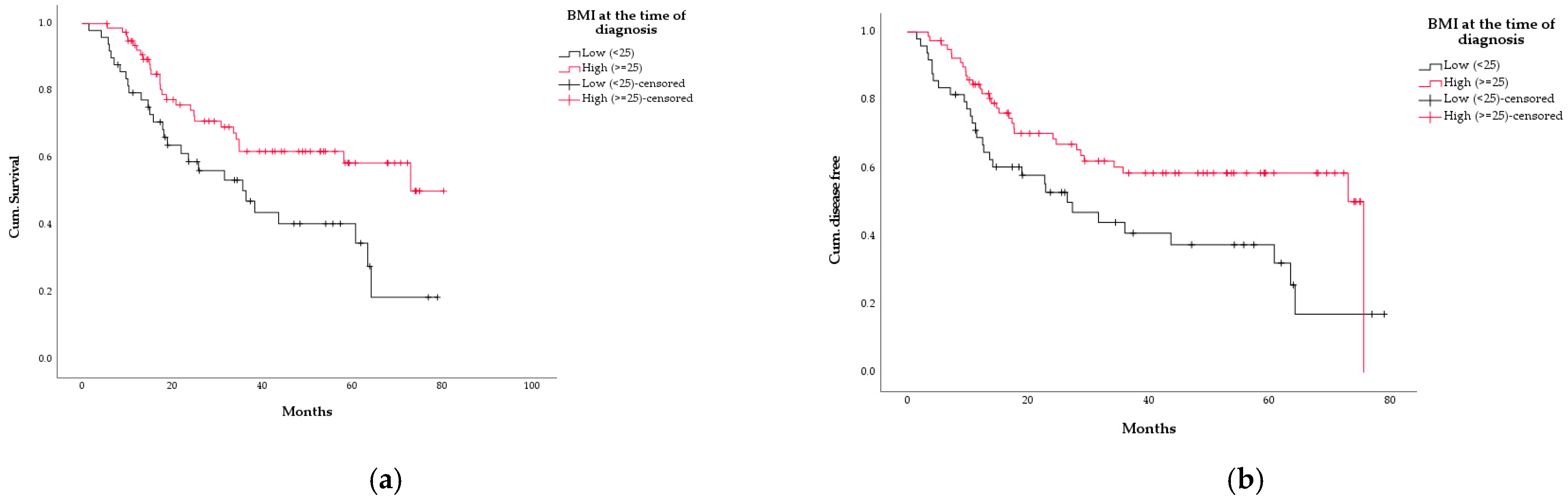
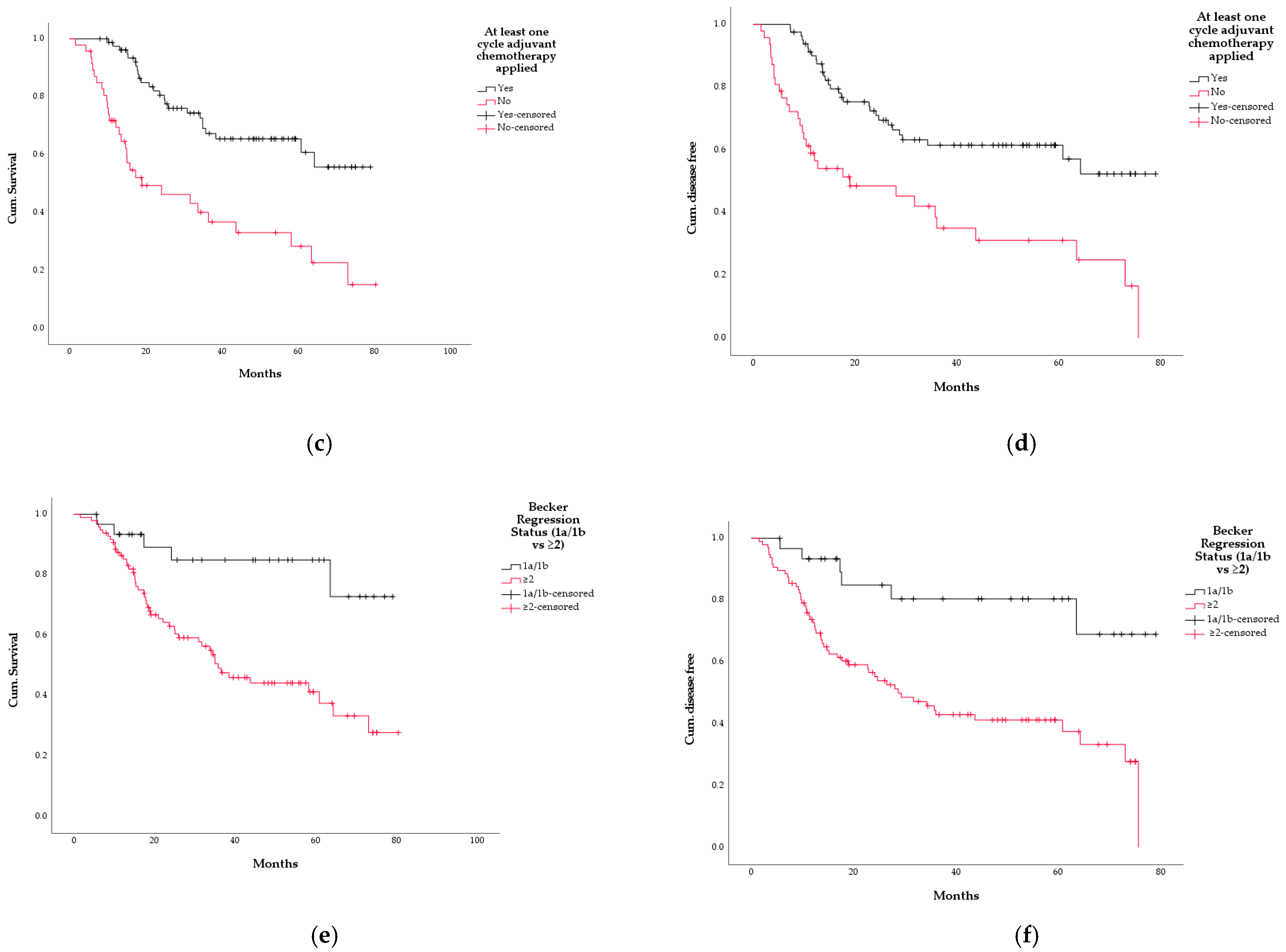
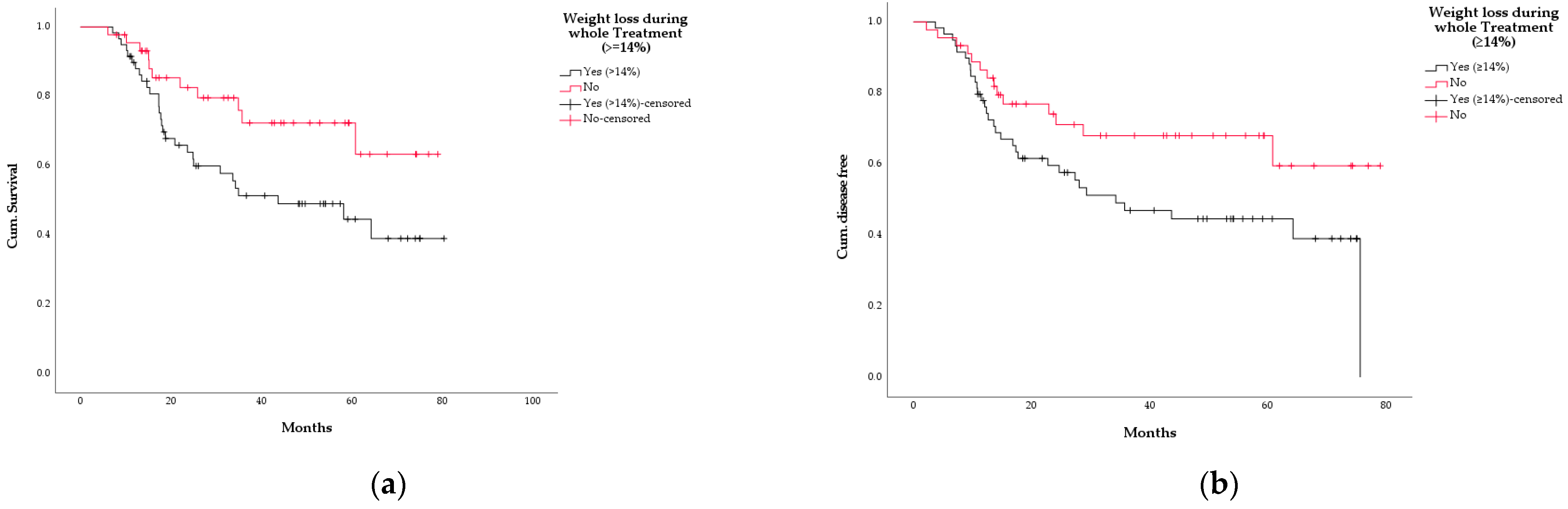
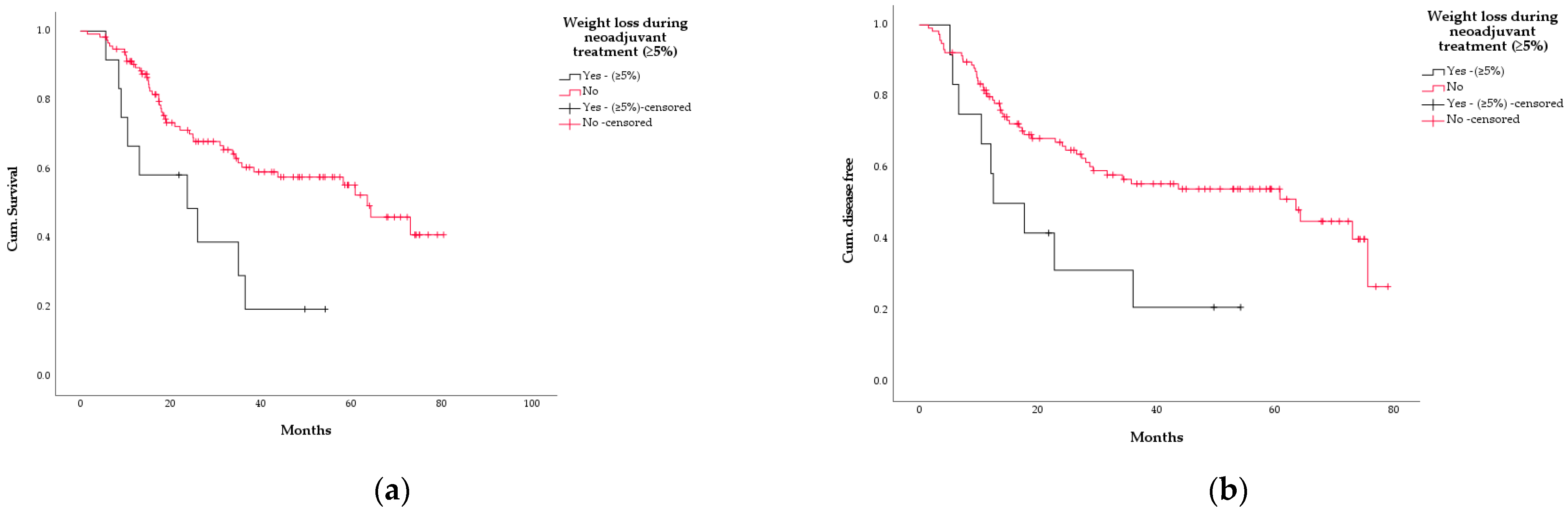
3.5. Survival
3.6. Association between WL and Other Variables
3.7. Cox Regression Analysis
4. Discussion
5. Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Abate, D. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [PubMed] [Green Version]
- Kim, J.; Bowlby, R. The Cancer Genome Atlas Research Network Analysis Working Group. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–175. [Google Scholar]
- Smyth, E.C.; Verheij, M. ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Mariette, C. ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v50–v57. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.; Al-Batran, S.-E. Deutsche Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten e.V. S3-Leitlinie Magenkarzinom—Diagnostik und Therapie der Adenokarzinome des Magens und ösophagogastralen Übergangs. Z. Gastroenterol. 2019, 57, 1517–1632. [Google Scholar]
- Porschen, R.; Fischbach, W. S3-Leitlinie—Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus. Z. Gastroenterol. 2019, 57, 336–418. [Google Scholar]
- Ryan, A.M.; Power, D.G. Cancer-Associated Malnutrition, Cachexia and Sarcopenia: The Skeleton in the Hospital Closet 40 Years Later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Dewys, W.D.; Begg, C. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Bozzetti, F.; SCRINIO Working Group. Screening the nutritional status in oncology: A preliminary report on 1000 outpatients. Support Care Cancer 2009, 17, 279–284. [Google Scholar] [CrossRef]
- Dalal, S. Lipid Metabolism in Cancer Cachexia. Ann. Palliat. Med. 2019, 8, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S. Cancer Cachexia: Understanding the Molecular Basis. Nat. Rev. Cancer 2014, 14, 754. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Senesse, P. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 2015, 33, 90–999. [Google Scholar] [CrossRef]
- Bichev, D.; Treese, C. High Impact of Histopathological Remission for Prognosis after Perioperative Chemotherapy with ECF and ECF-Like Regimens for Gastric and Gastroesophageal Adenocarcinoma. Oncology 2015, 89, 95–102. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Hofheinz, R.D. Perioperative Chemotherapy with Docetaxel, Cisplatin and Capecitabine (DCX) in Gastro-Oesophageal Adenocarcinoma: A Phase II Study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Ann. Oncol. 2012, 23, 2827–2834. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Chichester, UK, 2017. [Google Scholar]
- Becker, K.; Langer, R. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: A summary of 480 cases. Ann. Surg. 2011, 253, 934–939. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hofheinz, R.D. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar]
- WHO World Health Organization. Body Mass Index—BMI. Available online: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 31 January 2022).
- Liu, X.; Qiu, H. Gastric cancer, nutritional status, and outcome. OncoTargets Ther. 2017, 10, 2107–2114. [Google Scholar] [CrossRef] [Green Version]
- Ock, C.Y.; Oh, D.Y. Weight Loss at the First Month of Palliative Chemotherapy Predicts Survival Outcomes in Patients with Advanced Gastric Cancer. Gastric Cancer 2016, 19, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Erickson, N.T. Early weight loss is an independent risk factor for shorter survival and increased side effects in patients with metastatic colorectal cancer undergoing first-line treatment within the randomized Phase III trial FIRE-3 (AIO KRK-0306). Int. J. Cancer. 2022, 150, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ma, Z. Clinicopathological factors affecting the effect of neoadjuvant chemotherapy in patients with gastric cancer. World J. Surg. Oncol. 2021, 19, 44. [Google Scholar] [CrossRef]
- Aoyama, T.; Sato, T. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int. J. Clin. Oncol. 2017, 22, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Yoshikawa, T. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann. Surg. Oncol. 2013, 20, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [PubMed]
- Koch, C.; Reitz, C. Sarcopenia as a prognostic factor for survival in patients with locally advanced gastroesophageal adenocarcinoma. PLoS ONE 2019, 14, e0223613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.J.; Xia, X.F. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer 2019, 9, 1004. [Google Scholar] [CrossRef]
- Miyamoto, R.; Inagawa, S. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef]
- Xishan, Z.; Ye, Z. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci. Rep. 2020, 10, 17373. [Google Scholar]
- Zhang, X.; Zhao, W. Combining the Fibrinogen-to-Pre-Albumin Ratio and Prognostic Nutritional Index (FPR-PNI) Predicts the Survival in Elderly Gastric Cancer Patients After Gastrectomy. Oncol. Targets Ther. 2020, 13, 8845–8859. [Google Scholar] [CrossRef]
| Variable n = 128 | Median | Range |
|---|---|---|
| Age | 64 | 29–83 |
| Weight | 81 kg | 45–118 kg |
| BMI | 25.7 kg/m2 | 16.5–39.4 kg/m2 |
| Frequency | Percentage | |
| Sex | ||
| Females | 20 | 15.6% |
| Males | 108 | 84.4% |
| Localisation | ||
| Oesophagus | 68 | 53.1% |
| Stomach (incl. AEG III) | 60 | 46.9% |
| Histology | ||
| Adenocarcinoma | 128 | 100% |
| Laurén Histology | ||
| Intestinal Type | 57 | 44.5% |
| Diffuse Type | 41 | 32% |
| Mixed Type | 10 | 7.8% |
| Not specified | 20 | 15.6% |
| WHO Histology | ||
| Papillary | 7 | 5.5% |
| Mucinous | 6 | 4.7% |
| Tubular | 26 | 20.3% |
| Signet Ring Cell | 38 | 29.7% |
| Undifferentiated | 10 | 7.8% |
| Not specified | 41 | 32% |
| Chemotherapy Performed | Frequency | Percentage |
|---|---|---|
| Neoadjuvant | 128 | 100% |
| Adjuvant | 81 | 63.3% |
| Nr. of cycles (neoadjuvant) | ||
| ≤2 | 11 | 8.6% |
| 3–4 | 114 | 89.1% |
| ≥5 | 3 | 2.3% |
| No. of cycles (adjuvant) | ||
| none | 47 | 36.7% |
| ≤2 | 19 | 14.8% |
| ≥3 | 62 | 48.4% |
| Regimens 1 | Frequency | Percentage |
|---|---|---|
| Neoadjuvant (n = 128) | ||
| DCX | 49 | 38.3% |
| ECF | 45 | 35.2% |
| FLOT | 21 | 16.4% |
| FLOT-like | 8 | 6.2% |
| ECF-like | 5 | 3.9% |
| Adjuvant (n = 81) | ||
| DCX | 36 | 44,4% |
| ECF | 20 | 24.7% |
| FLOT | 11 | 13.6% |
| FLOT-like | 9 | 11.1% |
| ECF-like | 4 | 4.9% |
| Others | 1 | 1.2% |
| Median WL 1 | Range 2 | |
|---|---|---|
| Neoadjuvant Phase | 0% | WL 23%–WG 16% |
| Adjuvant Phase | 4% | WL 24%–WG 8% |
| Whole Treatment | 14% | WL 33%–WG 7% |
| Variable | Sig. | exp(B) | 95% CI |
|---|---|---|---|
| Becker regression 1a/1b | 0.008 | 0.068 | 0.009–0.503 |
| Adjuvant chemotherapy performed | 0.001 | 0.310 | 0.159–0.601 |
| BMI < 25 kg/m2 | 0.162 | 1.595 | 0.829–3.071 |
| WL ≥ 5% neoadjuvant | 0.075 | 2.224 | 0.923–5.359 |
| WL ≥ 14% whole treatment | 0.140 | 1.744 | 0.833–3.653 |
| Variable | Sig. | exp(B) | 95% CI |
|---|---|---|---|
| Becker regression 1a/1b | 0.003 | 0.114 | 0.027–0.478 |
| Adjuvant chemotherapy performed | 0.006 | 0.415 | 0.222–0.776 |
| BMI < 25 kg/m2 | 0.162 | 1.542 | 0.841–2.830 |
| WL ≥ 5% neoadjuvant | 0.176 | 1.810 | 0.766–4.273 |
| WL ≥ 14% whole treatment | 0.260 | 1.471 | 0.752–2.881 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, A.; Bichev, D.; Högner, A.; Bartels, P.; Ballhausen, A.; Treese, C.; Biebl, M.; Thuss-Patience, P. Prognostic Relevance of Weight and Weight Loss during Multimodal Therapy for Oesophagogastric Tumours. Curr. Oncol. 2022, 29, 2706-2719. https://doi.org/10.3390/curroncol29040221
Lorusso A, Bichev D, Högner A, Bartels P, Ballhausen A, Treese C, Biebl M, Thuss-Patience P. Prognostic Relevance of Weight and Weight Loss during Multimodal Therapy for Oesophagogastric Tumours. Current Oncology. 2022; 29(4):2706-2719. https://doi.org/10.3390/curroncol29040221
Chicago/Turabian StyleLorusso, Alessandro, Dmitry Bichev, Anica Högner, Prisca Bartels, Alexej Ballhausen, Christoph Treese, Matthias Biebl, and Peter Thuss-Patience. 2022. "Prognostic Relevance of Weight and Weight Loss during Multimodal Therapy for Oesophagogastric Tumours" Current Oncology 29, no. 4: 2706-2719. https://doi.org/10.3390/curroncol29040221
APA StyleLorusso, A., Bichev, D., Högner, A., Bartels, P., Ballhausen, A., Treese, C., Biebl, M., & Thuss-Patience, P. (2022). Prognostic Relevance of Weight and Weight Loss during Multimodal Therapy for Oesophagogastric Tumours. Current Oncology, 29(4), 2706-2719. https://doi.org/10.3390/curroncol29040221






