P144 a Transforming Growth Factor Beta Inhibitor Peptide, Generates Antifibrogenic Effects in a Radiotherapy Induced Fibrosis Model
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
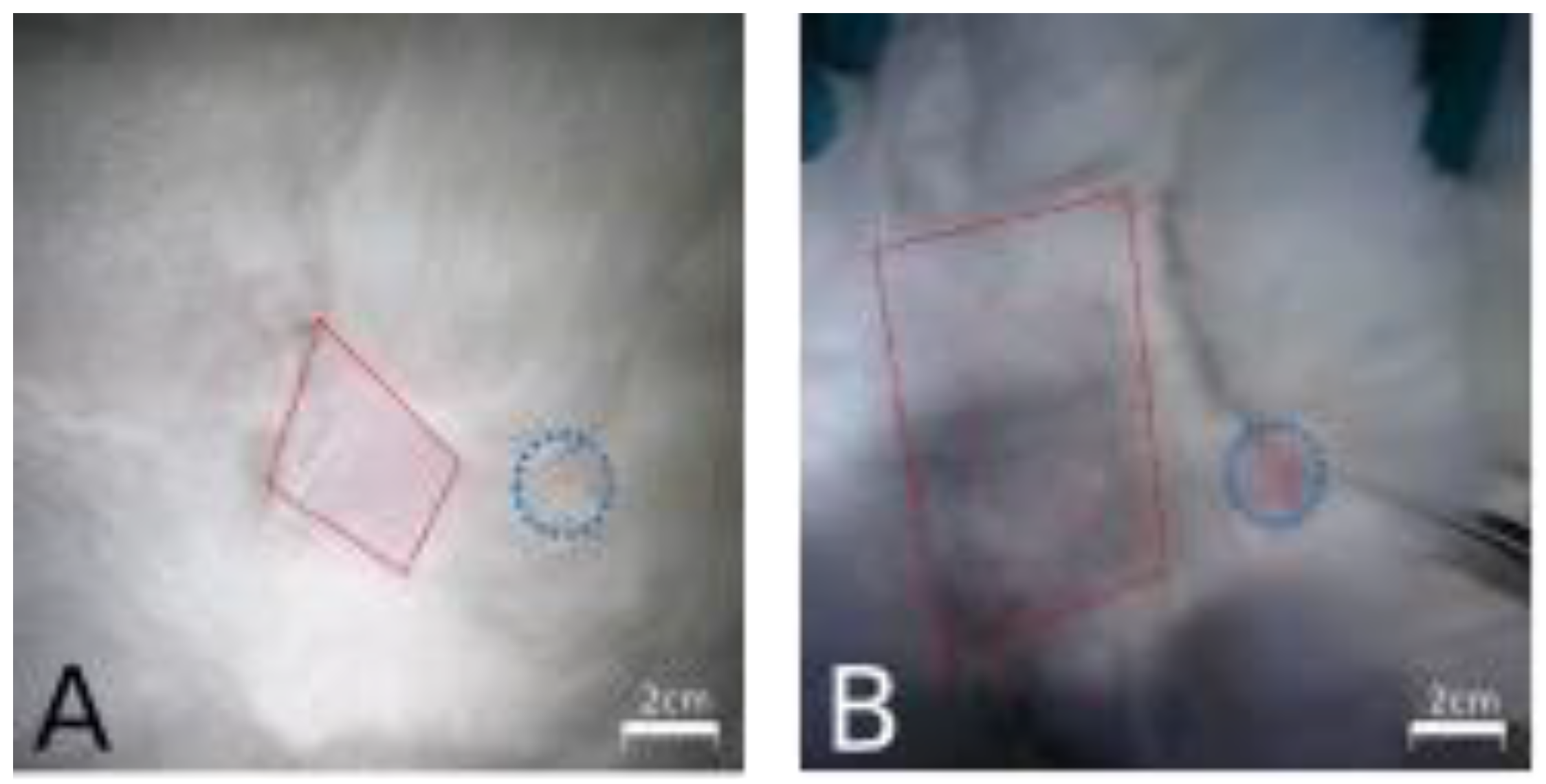
2.2. Surgical Technique
2.3. Brachytherapy
2.4. Drug Administration
2.5. Sacrifice and Histological Examinations
2.6. Histomorphometric and Immunohistochemical Analysis
2.7. Statistics
3. Results
3.1. Animal Model
3.2. Skin and Articular Range
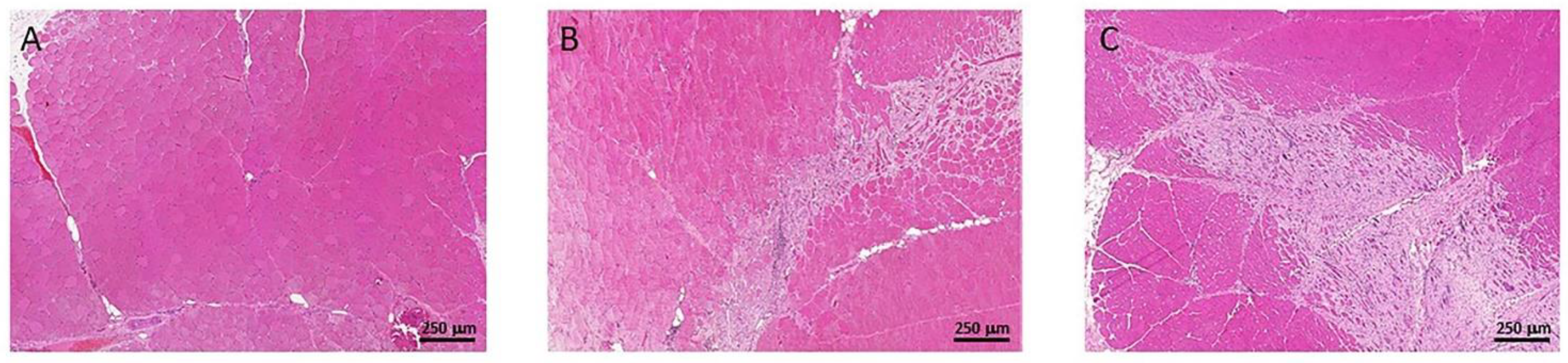
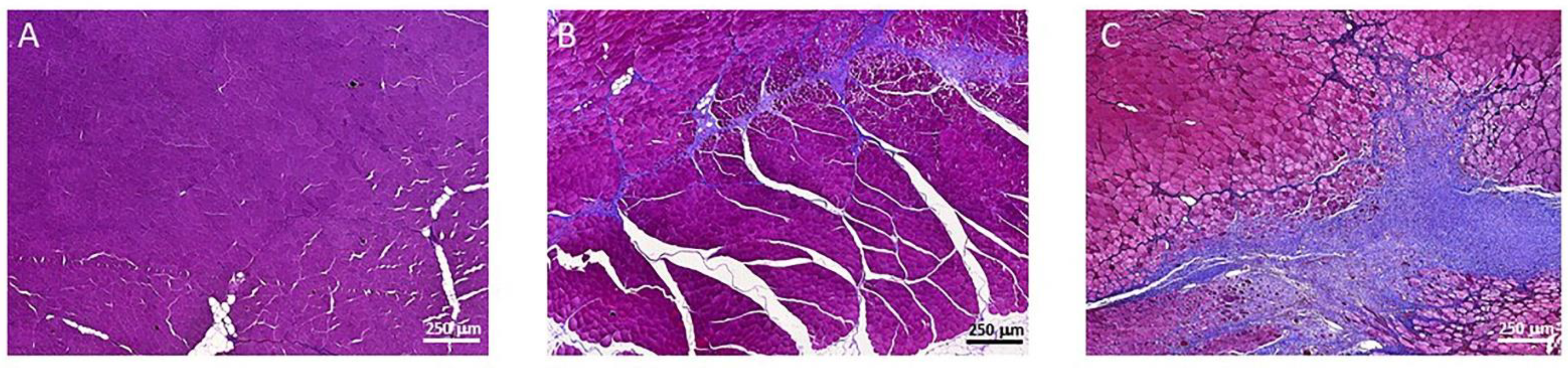
3.3. Muscle Fibrosis
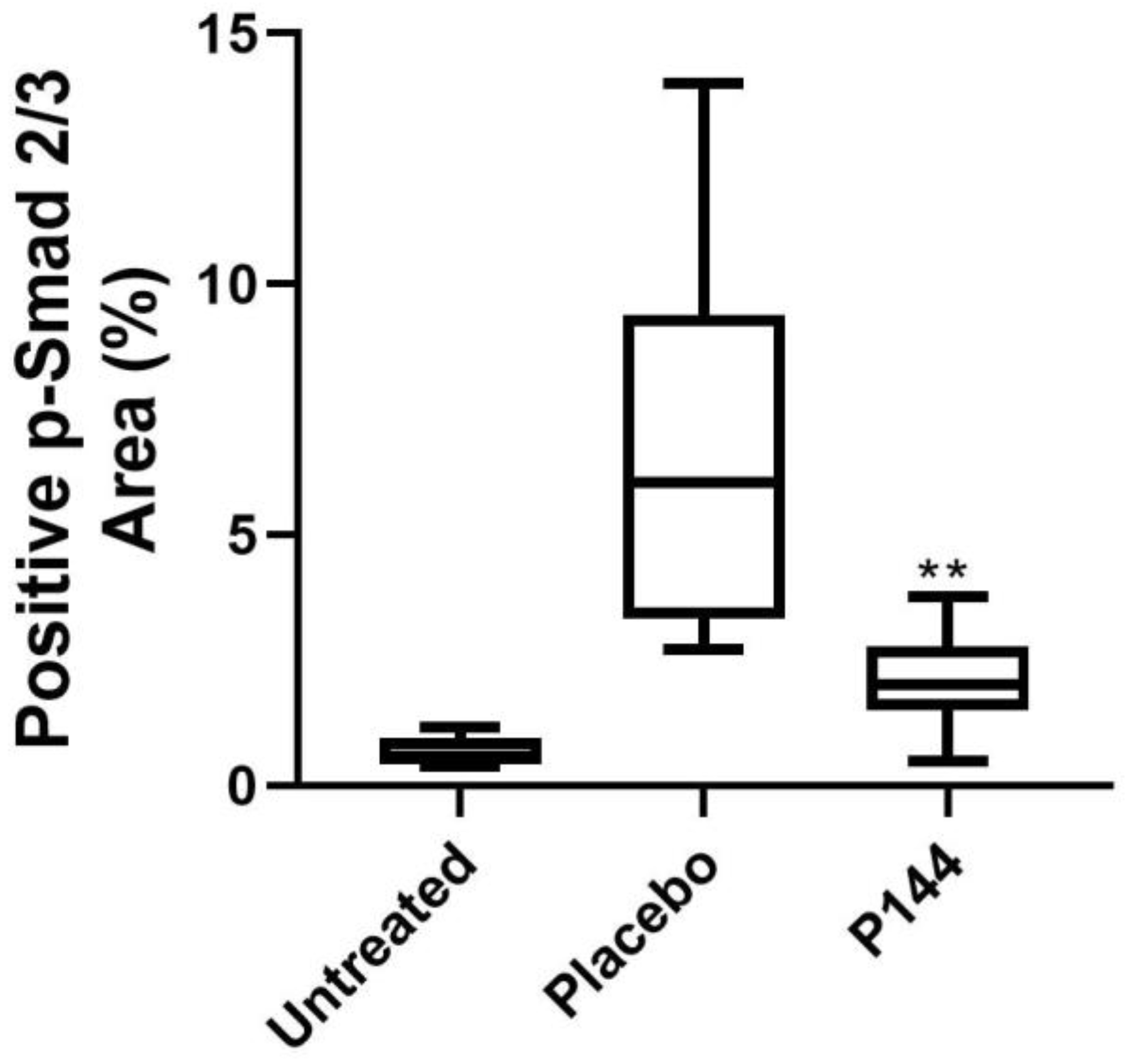
3.4. P-Smad2/3 Immunohistochemical Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezquerro, I.J.; Lasarte, J.J.; Dotor, J.; Castilla-Cortázar, I.; Bustos, M.; Peñuelas, I.; Blanco, G.; Rodríguez, C.; Lechuga, M.D.C.G.; Greenwel, P.; et al. A synthetic peptide from transforming growth factor β type III receptor inhibits liver fibrogenesis in rats with carbon tetrachloride liver injury. Cytokine 2003, 22, 12–20. [Google Scholar] [CrossRef]
- Beane, J.D.; Yang, J.C.; White, D.; Steinberg, S.M.; Rosenberg, S.A.; Rudloff, U. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-Year follow-up of a randomized prospective trial. Ann. Surg. Oncol. 2014, 21, 2484–2489. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Verma, V.; Takiar, R.; Vajapey, R.; Amarnath, S.; Murphy, E.; Mesko, N.W.; Lietman, S.; Joyce, M.; Anderson, P.; et al. Radiation Therapy in the Management of Soft Tissue Sarcoma. Am. J. Clin. Oncol. Cancer Clin. Trials 2016, 39, 630–635. [Google Scholar] [CrossRef]
- Habrand, J.L.; Le Pechoux, C. Radiation therapy in the management of adult soft tissue sarcomas. Ann. Oncol. 2004, 15, iv187–iv191. [Google Scholar] [CrossRef] [Green Version]
- Paulino, A.C. Late effects of radiotherapy for pediatric extremity sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 265–274. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Shen, A.H.; Lee, G.K.; Momeni, A.; Longaker, M.T.; Wan, D.C. Radiation-Induced Skin Fibrosis: Pathogenesis, Current Treatment Options, and Emerging Therapeutics. Ann. Plast. Surg. 2019, 83, S59–S64. [Google Scholar] [CrossRef]
- Martinez-Monge, R.; Cambeiro, M.; San-Julián, M.; Sierrasesúmaga, L. Use of brachytherapy in children with cancer: The search for an uncomplicated cure. Lancet Oncol. 2006, 7, 157–166. [Google Scholar] [CrossRef]
- Martínez-Monge, R.; San Julián, M.; Amillo, S.; Cambeiro, M.; Arbea, L.; Valero, J.; González-Cao, M.; Martín-Algarra, S. Perioperative high-dose-rate brachytherapy in soft tissue sarcomas of the extremity and superficial trunk in adults: Initial results of a pilot study. Brachytherapy 2005, 4, 264–270. [Google Scholar] [CrossRef]
- Dormand, E.L.; Banwell, P.E.; Goodacre, T.E.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.W. The skin: Its structure and response to ionizing radiation. Int. J. Radiat. Biol. 1990, 57, 751–773. [Google Scholar] [CrossRef] [PubMed]
- Abouarab, M.H.; Salem, I.L.; Degheidy, M.M.; Henn, D.; Hirche, C.; Eweida, A.; Uhl, M.; Kneser, U.; Kremer, T. Therapeutic options and postoperative wound complications after extremity soft tissue sarcoma resection and postoperative external beam radiotherapy. Int. Wound J. 2018, 15, 148–158. [Google Scholar] [CrossRef]
- Archambeau, J.O.; Pezner, R.; Wasserman, T. Pathophysiology of irradiated skin and breast. Int. J. Radiat. Oncol. 1995, 31, 1171–1185. [Google Scholar] [CrossRef]
- Denham, J.W.; Hauer-Jensen, M. The radiotherapeutic injury—A complex “wound”. Radiother. Oncol. 2002, 63, 129–145. [Google Scholar] [CrossRef]
- Binatti, E.; Zoccatelli, G.; Zanoni, F.; Donà, G.; Mainente, F.; Chignola, R. Phagocytosis of Astaxanthin-Loaded Microparticles Modulates TGFβ Production and Intracellular ROS Levels in J774A.1 Macrophages. Mar. Drugs 2021, 19, 163. [Google Scholar] [CrossRef]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Burger, A.; Löffler, H.; Bamberg, M.; Rodemann, H.P. Molecular and cellular basis of radiation fibrosis. Int. J. Radiat. Biol. Phys. 1998, 73, 401–408. [Google Scholar] [CrossRef]
- Randall, K. Long-term expression of transforming growth factor TGF beta1 in mouse skin after localized beta-irradiation. Int. J. Radiat. Biol. 1996, 70, 351–360. [Google Scholar] [CrossRef]
- Randall, K.; Coggle, J.E. Expression of Transforming Growth Factor-β1 in Mouse Skin During the Acute Phase of Radiation Damage. Int. J. Radiat. Biol. 1995, 68, 301–309. [Google Scholar] [CrossRef]
- Martin, M.; Lefaix, J.L.; Delanian, S. TGF-β1 and radiation fibrosis: A master switch and a specific therapeutic target? Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 277–290. [Google Scholar] [CrossRef]
- Martin, M.; Lefaix, J.; Pinton, P.; Crechet, F.; Daburon, F. Temporal modulation of TGF-beta 1 and beta-actin gene expression in pig skin and muscular fibrosis after ionizing radiation—PubMed. Radiat. Res. 1993, 134, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Mosgau, S.; Wehrhan, F.; Grabenbauer, G.; Amann, K.; Radespiel-Tröger, M.; Neukam, F.W.; Rodel, F. Transforming growth factor beta1 and beta2 (TGFbeta2 / TGFbeta2) profile changes in previously irradiated free flap beds. Head Neck 2002, 24, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Mosgau, S.; Blaese, M.A.; Grabenbauer, G.; Wehrhan, F.; Kopp, J.; Amann, K.; Rodemann, H.P.; Rödel, F. Smad-3 and Smad-7 expression following anti-transforming growth factor beta 1 (TGFβ1)-treatment in irradiated rat tissue. Radiother. Oncol. 2004, 70, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Border, W.A.; Noble, N.A. Transforming Growth Factor β in Tissue Fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar] [CrossRef]
- Ulrich, D.; Lichtenegger, F.; Eblenkamp, M.; Repper, D.; Pallua, N. Matrix metalloproteinases, tissue inhibitors of metalloproteinases, aminoterminal propeptide of procollagen type III, and hyaluronan in sera and tissue of patients with capsular contracture after augmentation with Trilucent breast implants. Plast. Reconstr. Surg. 2004, 114, 229–236. [Google Scholar] [CrossRef]
- Schultze-Mosgau, S.; Kopp, J.; Thorwarth, M.; Rödel, F.; Melnychenko, I.; Grabenbauer, G.G.; Amann, K.; Wehrhan, F. Plasminogen activator inhibitor-I-related regulation of procollagen I (α1 and α2) by antitransforming growth factor-β1 treatment during radiation-impaired wound healing. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 280–288. [Google Scholar] [CrossRef]
- Gallo-Oller, G.; Di Scala, M.; Aranda, F.; Dotor, J. Transforming growth factor beta (TGF-β) activity in immuno-oncology studies. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2020; Volume 636, pp. 129–172. ISBN 9780128206676. [Google Scholar]
- Santiago, B.; Gutierrez-Cañas, I.; Dotor, J.; Palao, G.; Lasarte, J.J.; Ruiz, J.; Prieto, J.; Borrás-Cuesta, F.; Pablos, J.L. Topical application of a peptide inhibitor of transforming growth factor-β1 ameliorates bleomycin-induced skin fibrosis. J. Investig. Dermatol. 2005, 125, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Dotor, J.; López-Vázquez, A.B.; Lasarte, J.J.; Sarobe, P.; García-Granero, M.; Riezu-Boj, J.I.; Martínez, A.; Feijoó, E.; López-Sagaseta, J.; Hermida, J.; et al. Identification of peptide inhibitors of transforming growth factor beta 1 using a phage-displayed peptide library. Cytokine 2007, 39, 106–115. [Google Scholar] [CrossRef]
- Llopiz, D.; Dotor, J.; Casares, N.; Bezunartea, J.; Díaz-Valdés, N.; Ruiz, M.; Aranda, F.; Berraondo, P.; Prieto, J.; Lasarte, J.J.; et al. Peptide inhibitors of transforming growth factor-β enhance the efficacy of antitumor immunotherapy. Int. J. Cancer 2009, 125, 2614–2623. [Google Scholar] [CrossRef]
- Beltrami, G.; Rüdiger, H.A.; Mela, M.M.; Scoccianti, G.; Livi, L.; Franchi, A.; Campanacci, D.A.; Capanna, R. Limb salvage surgery in combination with brachytherapy and external beam radiation for high-grade soft tissue sarcomas. Eur. J. Surg. Oncol. 2008, 34, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Hermida, N.; López, B.; González, A.; Dotor, J.; Lasarte, J.J.; Sarobe, P.; Borrás-Cuesta, F.; Díez, J. A synthetic peptide from transforming growth factor-β1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovasc. Res. 2009, 81, 601–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäffer, M.; Weimer, W.; Wider, S.; Stülten, C.; Bongartz, M.; Budach, W.; Becker, H.D. Differential expression of inflammatory mediators in radiation-impaired wound healing. J. Surg. Res. 2002, 107, 93–100. [Google Scholar] [CrossRef]
- Anscher, M.S.; Thrasher, B.; Rabbani, Z.; Teicher, B.; Vujaskovic, Z. Antitransforming growth factor-β antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 876–881. [Google Scholar] [CrossRef]
- Collie, D.; Murchison, J.T.; Wright, S.H.; McLean, A.; Howard, L.; Del-Pozo, J.; Smith, S.; McLachlan, G.; Lawrence, J.; Kay, E.; et al. Nebulisation of synthetic lamellar lipids mitigates radiation-induced lung injury in a large animal model. Sci. Rep. 2018, 8, 13316. [Google Scholar] [CrossRef]
- Herskind, C.; Bamberg, M.; Rodemann, H.P. The role of cytokines in the development of normal-tissue reactions after radiotherapy. Strahlenther. Onkol. 1998, 174, 12–15. [Google Scholar]
- Hanafy, N.A.N.; Fabregat, I.; Leporatti, S.; Kemary, M. El Encapsulating TGF-β1 inhibitory peptides P17 and P144 as a promising strategy to facilitate their dissolution and to improve their functionalization. Pharmaceutics 2020, 12, 421. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, J.; Kim, J.; Jeong, W.; Kim, J.S.; Jeong, B.K.; Shin, S.C.; Kim, J.H. Anthocyanins from black soybean seed coat prevent radiation-induced skin fibrosis by downregulating TGF-β and Smad3 expression. Arch. Dermatol. Res. 2018, 310, 401–412. [Google Scholar] [CrossRef]
- Qiu, S.S.; Dotor, J.; Hontanilla, B. Effect of P144®(Anti-TGF-β) in an “in Vivo” Human Hypertrophic Scar Model in Nude Mice. PLoS ONE 2015, 10, e0144489. [Google Scholar] [CrossRef]
- Zarranz-Ventura, J.; Fernández-Robredo, P.; Recalde, S.; Salinas-Alamán, A.; Borrás-Cuesta, F.; Dotor, J.; García-Layana, A. Transforming Growth Factor-Beta Inhibition Reduces Progression of Early Choroidal Neovascularization Lesions in Rats: P17 and P144 Peptides. PLoS ONE 2013, 8, e0065434. [Google Scholar] [CrossRef] [Green Version]
- Arribillaga, L.; Dotor, J.; Basagoiti, M.; Riezu-Boj, J.I.; Borrás-Cuesta, F.; Lasarte, J.J.; Sarobe, P.; Cornet, M.E.; Feijoó, E. Therapeutic effect of a peptide inhibitor of TGF-β on pulmonary fibrosis. Cytokine 2011, 53, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramann, R.; Dirocco, D.P.; Humphreys, B.D. Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J. Pathol. 2013, 231, 273–289. [Google Scholar] [CrossRef]
- Park, J.H.; Ryu, S.H.; Choi, E.K.; Ahn, S.D.; Park, E.; Choi, K.C.; Lee, S.W. SKI2162, an inhibitor of the TGF-ß type I receptor (ALK5), inhibits radiation-induced fibrosis in mice. Oncotarget 2015, 6, 4171–4179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flechsig, P.; Dadrich, M.; Bickelhaupt, S.; Jenne, J.; Hauser, K.; Timke, C.; Peschke, P.; Hahn, E.W.; Grone, H.J.; Yingling, J.; et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-β and BMP-associated proinflammatory and proangiogenic signals. Clin. Cancer Res. 2012, 18, 3616–3627. [Google Scholar] [CrossRef] [Green Version]
- Gallo-Oller, G.; Vollmann-Zwerenz, A.; Meléndez, B.; Rey, J.A.; Hau, P.; Dotor, J.; Castresana, J.S. P144, a Transforming Growth Factor beta inhibitor peptide, generates antitumoral effects and modifies SMAD7 and SKI levels in human glioblastoma cell lines. Cancer Lett. 2016, 381, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Zubeldia, I.; Dotor, J.; Redrado, M.; Bleau, A.M.; Manrique, I.; de Aberasturi, A.L.; Villalba, M.; Calvo, A. Co-migration of colon cancer cells and CAFs induced by TGFβ1 enhances liver metastasis. Cell Tissue Res. 2015, 359, 829–839. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.Q.; Zhou, X.Q.; Liu, Q.B.; Chen, L.; Cai, F. NEAT1 induces osteosarcoma development by modulating the miR-339-5p/TGF-β1 pathway. J. Cell. Physiol. 2019, 234, 5097–5105. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Morande, S.; Dotor, J.; San-Julian, M. P144 a Transforming Growth Factor Beta Inhibitor Peptide, Generates Antifibrogenic Effects in a Radiotherapy Induced Fibrosis Model. Curr. Oncol. 2022, 29, 2650-2661. https://doi.org/10.3390/curroncol29040217
Cruz-Morande S, Dotor J, San-Julian M. P144 a Transforming Growth Factor Beta Inhibitor Peptide, Generates Antifibrogenic Effects in a Radiotherapy Induced Fibrosis Model. Current Oncology. 2022; 29(4):2650-2661. https://doi.org/10.3390/curroncol29040217
Chicago/Turabian StyleCruz-Morande, Sebastián, Javier Dotor, and Mikel San-Julian. 2022. "P144 a Transforming Growth Factor Beta Inhibitor Peptide, Generates Antifibrogenic Effects in a Radiotherapy Induced Fibrosis Model" Current Oncology 29, no. 4: 2650-2661. https://doi.org/10.3390/curroncol29040217
APA StyleCruz-Morande, S., Dotor, J., & San-Julian, M. (2022). P144 a Transforming Growth Factor Beta Inhibitor Peptide, Generates Antifibrogenic Effects in a Radiotherapy Induced Fibrosis Model. Current Oncology, 29(4), 2650-2661. https://doi.org/10.3390/curroncol29040217




