A Qualitative Needs Analysis of Skin Cancer Care from the Perspectives of Patients, Physicians, and Health Insurance Representatives—A Case Study from Eastern Saxony, Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Sampling
2.2.1. Focus Groups with Patients
2.2.2. Workshop with Physicians
2.2.3. Semi-Structured Interviews with Health Insurance Company Representatives
2.3. Survey Instruments
2.4. Data Collection
2.5. Data Evaluation
3. Results
3.1. Sample Description
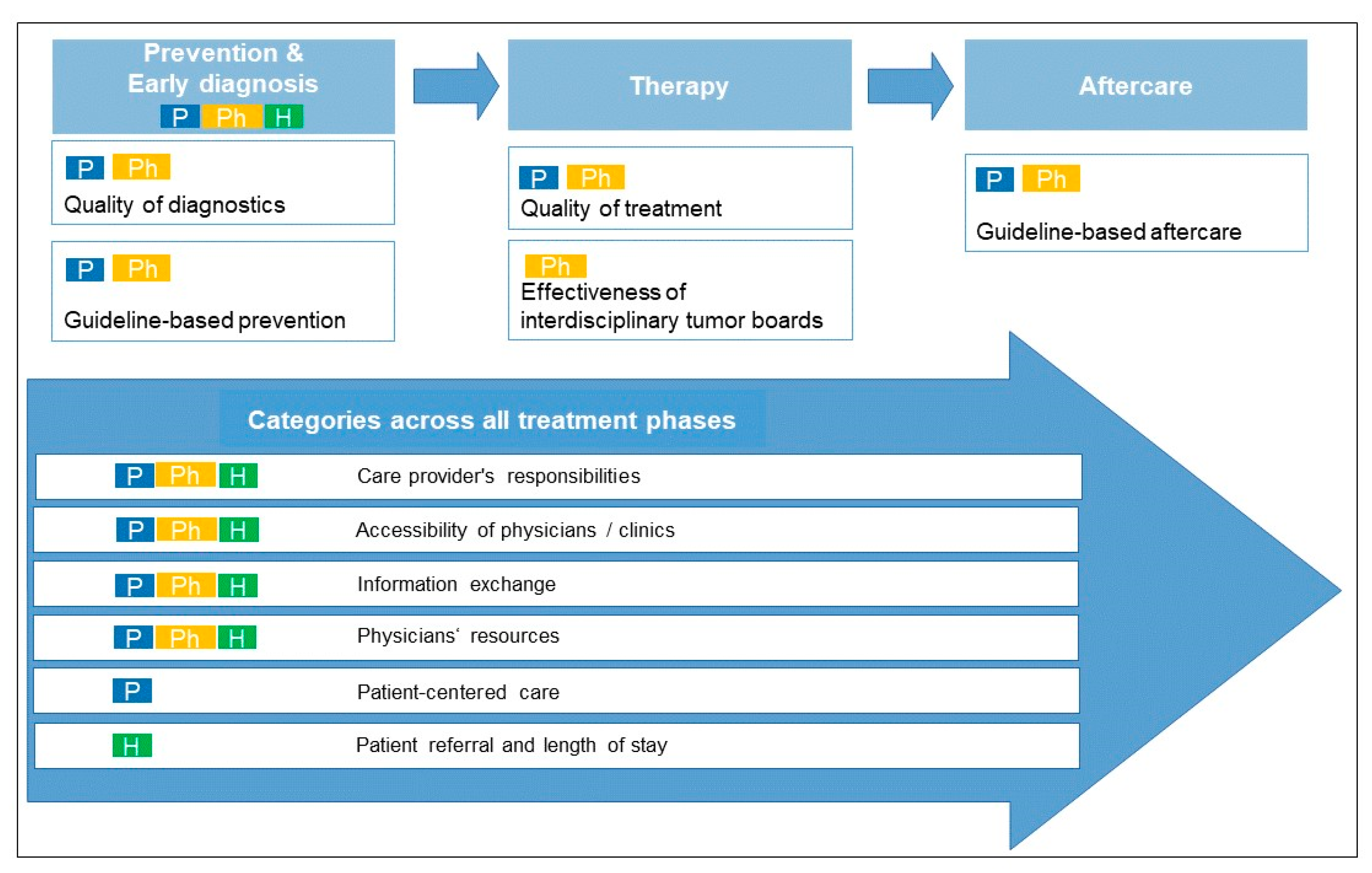
3.2. Categories: Optimization Potential in the Care of Patients with Skin Cancer in the Study Region
3.2.1. First Category: Prevention and Early Diagnosis of Skin Cancer
3.2.2. Second Category: Patient Referral and Length of Stay
3.2.3. Third Category: Accessibility of Physicians/Clinics
3.2.4. Fourth Category: Physician Resourcing
3.2.5. Fifth Category: Care Providers’ Responsibilities
3.2.6. Sixth Category: Quality of Diagnostics and Treatment
3.2.7. Seventh Category: Information Exchange
3.2.8. Eighth Category: Effectiveness of Interdisciplinary Tumor Boards
3.2.9. Nine Category: Guideline-Based Prevention and Aftercare
3.2.10. Tenth Category: Patient-Centered Care
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbe, C.; Keim, U.; Eigentler, T.K.; Amaral, T.; Katalinic, A.; Holleczek, B.; Martus, P.; Leiter, U. Time trends in incidence and mortality of cutaneous melanoma in Germany. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1272–1280. [Google Scholar] [CrossRef]
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 23 September 2021).
- Zentrum für Krebsregisterdaten. Krebs in Deutschland 2015/2016. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2019/krebs_in_deutschland_2019.pdf?__blob=publicationFile (accessed on 26 February 2021).
- Friedrich, S.; Kraywinkel, K. Faktenblatt: Epidemiologie des malignen Melanoms in Deutschland. Onkologe 2018, 24, 447–452. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Ugurel, S.; Röhmel, J.; Ascierto, P.A.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Long, G.V.; Lorigan, P.; McArthur, G.A.; et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies-update 2017. Eur. J. Cancer 2017, 83, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Bundesministerium für Gesundheit. Der nationale Krebsplan Stellt Sich vor. Available online: https://www.bundesgesundheitsministerium.de/themen/praevention/nationaler-krebsplan/der-nationale-krebsplan-stellt-sich-vor.html (accessed on 6 September 2021).
- Cancer Control Joint Action. European Guide on Quality Improvement in Comprehensive Cancer Control. Available online: https://cancercontrol.eu/archived/uploads/images/Guide/pdf/CanCon_Guide_FINAL_Web.pdf (accessed on 6 September 2021).
- Schoffer, O.; Schülein, S.; Arand, G.; Arnholdt, H.; Baaske, D.; Bargou, R.C.; Becker, N.; Beckmann, M.W.; Bodack, Y.; Böhme, B.; et al. Tumour stage distribution and survival of malignant melanoma in Germany 2002–2011. BMC Cancer 2016, 16, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmund, P.; Schmitt, J.; Roessler, M.; Meier, F.; Schoffer, O. Targeted and Checkpoint Inhibitor Therapy of Metastatic Malignant Melanoma in Germany, 2000–2016. Cancers (Basel) 2020, 12, 2354. [Google Scholar] [CrossRef] [PubMed]
- Bundesärztekammer. Prozessverbesserung in der Patientenversorgung Durch Kooperation und Koordination Zwischen den Gesundheitsberufen. Available online: http://www.bundesaerztekammer.de/fileadmin/user_upload/downloads/FachberufeProzessverbesserung.pdf (accessed on 10 December 2020).
- Braun, B.; Marstedt, G.; Sievers, C. Zur Bedeutung von Schnittstellen und Übergängen im Deutschen Gesundheitssystem; gesundheitsmonitor 3/2011 (Newsletter der Bertelsmann Stiftung und der BARMER GEK); Bertelsmann Stiftung: Gütersloh, Germany, 2011. [Google Scholar]
- Bond, T.R.; Estey, A.; Elwi, A. Cancer Strategic Clinical Network: Improving cancer care in Alberta. CMAJ 2019, 191, S13–S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melanoma Network of New Zealand. About MelNet. Available online: https://www.melnet.org.nz/index.php?p=about (accessed on 17 August 2021).
- Comprehensive Cancer Center Ostbayern. Regionales Netzwerk. Available online: https://www.ccco.de/regionales-netzwerk/ (accessed on 17 August 2021).
- Kaiser, F.; Vehling-Kaiser, U.; Flieser-Hartl, M.; Weiglein, T. Das Onkologische und Palliativmedizinische Netzwerk Landshut: Lösungsansatz für die zukünftige ambulante und stationäre Versorgung von onkologischen und palliativmedizinischen Patienten in strukturschwachen ländlichen Gebieten. MMW Fortschr. Med. 2014, 156, 79–83. [Google Scholar] [CrossRef]
- Müller, H.L.; Blanke, J.-G.; Bonse, B.; Bosse, H.; Erkel, J.; Gitmans, R.; Kolb, R.; Krull, F.; Langlitz, J.; Liebner, T.; et al. Verbund PädOnko Weser-Ems--Regionale ambulante Versorgung pädiatrisch-onkologischer Patienten aus der Weser-Ems-Region im Rahmen einer Integrierten Versorgung. Klin. Padiatr. 2010, 222, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Landeshauptstadt Dresden. Bevölkerungsbestand. Available online: https://www.dresden.de/de/leben/stadtportrait/statistik/bevoelkerung-gebiet/Bevoelkerungsbestand.php (accessed on 17 December 2021).
- Klinische Krebsregister Sachsen. Jahresbericht der Klinischen Krebsregister in Sachsen 2010–2019. Available online: https://www.krebsregister-sachsen.de/fileadmin/user_upload/dokumente/auswertungen/Jahresbericht_KKR_Sachsen_2021.pdf (accessed on 17 December 2021).
- Demografieportal. Regionale Alterung. Available online: https://www.demografie-portal.de/DE/Fakten/aeltere-bevoelkerung-regional.html?nn=580048 (accessed on 10 December 2020).
- Uniklinikum Dresden. Hauttumorzentrum am Nationalen Centrum für Tumorerkrankungen Dresden (NCT/UCC). Available online: https://www.uniklinikum-dresden.de/de/das-klinikum/universitaetscentren/uhtc (accessed on 23 September 2021).
- Städtisches Klinikum Dresden. Hautkrebszentrum. Available online: https://www.klinikum-dresden.de/hz_khdf/#navigation (accessed on 23 September 2021).
- Greenbaum, T. The Handbook for Focus Group Research, 2nd ed.; SAGE Publications: Thousand Oaks, CA, USA; London, UK; New Delhi, India, 1998; pp. 1–16. [Google Scholar]
- Pelz, C.; Schmitt, A.; Meis, M. Knowledge Mapping als Methode zur Auswertung und Ergebnispräsentation von Fokusgruppen in der Markt- und Evaluationsforschung. Forum Qual. Soz./Forum Qual. Soc. Res. 2004, 5. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Critical Appraisal Skills Programme. CASP Qualitative Studies Checklist. 2018. Available online: https://casp-uk.net/casp-tools-checklists (accessed on 2 March 2022).
- Patton, M.Q. Qualitative Research & Evaluation Methods: Integrating Theory and Practice, 4th ed.; SAGE: Los Angeles, CA, USA; London, UK; New Delhi, India; Singapore; Washington, DC, USA, 2015; pp. 264–272. [Google Scholar]
- Misoch, S. Qualitative Interviews; De Gruyter: Berlin, Germany; München, Germany; Boston, MA, USA, 2015; pp. 189–198. [Google Scholar]
- Bundesinstitut Für Bau-, Stadt- und Raumforschung (BBSR). Laufende Stadtbeobachtung–Raumabgrenzungen. Available online: https://www.bbsr.bund.de/BBSR/DE/forschung/raumbeobachtung/Raumabgrenzungen/deutschland/gemeinden/StadtGemeindetyp/StadtGemeindetyp.html (accessed on 2 March 2022).
- Meier, U.; Diener, H.C. Kommission Integrierte Versorgung der Deutschen Gesellschaft für Neurologie (Ed.) Integrierte Versorgung in der Neurologie: Integrierte Versorgungskonzepte und Kooperative Versorgungsstrukturen; Thieme: Stuttgart, Germany; New York, NY, USA, 2007. [Google Scholar]
- Helfferich, C. Die Qualität Qualitativer Daten: Manual für Die Durchführung Qualitativer Interviews, 4th ed.; VS Verlag für Sozialwissenschaften: Wiesbaden, Germany, 2011; pp. 182–189. [Google Scholar]
- Mayring, P. Qualitative Inhaltsanalyse: Grundlagen und Techniken, 11th ed.; Beltz: Weinheim, Germany; Basel, Switzerland, 2010; pp. 48–109. [Google Scholar]
- Kuckartz, U. Qualitative Inhaltsanalyse: Methoden, Praxis, Computerunterstützung, 3rd ed.; Beltz Juventa: Weinheim, Germany; Basel, Switzerland, 2016; pp. 211–212. [Google Scholar]
- Lang, C.; Gottschall, M.; Sauer, M.; Köberlein-Neu, J.; Bergmann, A.; Voigt, K. “Da kann man sich ja totklingeln, geht ja keiner ran”–Schnittstellenprobleme zwischen stationärer, hausärztlicher und ambulant-fachspezialisierter Patientenversorgung aus Sicht Dresdner Hausärzte. Gesundheitswesen 2019, 81, 822–830. [Google Scholar] [CrossRef]
- Easley, J.; Miedema, B.; O’Brien, M.A.; Carroll, J.; Manca, D.; Webster, F.; Grunfeld, E. The role of family physicians in cancer care: Perspectives of primary and specialty care providers. Curr. Oncol. 2017, 24, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.; Harrison, J.D.; Young, J.M.; Butow, P.N.; Solomon, M.J.; Masya, L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv. Res. 2010, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Dossett, L.A.; Hudson, J.N.; Morris, A.M.; Lee, M.C.; Roetzheim, R.G.; Fetters, M.D.; Quinn, G.P. The primary care provider (PCP)-cancer specialist relationship: A systematic review and mixed-methods meta-synthesis. CA Cancer J. Clin. 2017, 67, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF). Diagnostik, Therapie und Nachsorge des Melanoms: Langversion 3.3, 2020, AWMF Registernummer: 032/024OL. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Melanom/Melanom_Version_3/LL_Melanom_Langversion_3.3.pdf (accessed on 2 November 2021).
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF). Diagnostik, Therapie und Nachsorge des Melanoms: Leitlinienreport, Version 3.2., 2019, AWMF Registernummer: 032/024OL. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Melanom/Melanom_Version_3/LL_Melanom_Leitlinienreport_3.2.pdf (accessed on 2 November 2021).
- Gemeinsamer Bundesausschuss. Bedarfsplanungs-Richtlinie. Available online: https://www.g-ba.de/richtlinien/4/ (accessed on 25 September 2021).
- Kassenärztliche Vereinigung Sachsen. Bedarfsplan 2020: Anlage 1.1.5.: Planungsblatt Hautärzte, 2020. Available online: https://www.kvsachsen.de/fileadmin/data/kvs/img/Aktuelles/Der_Weg_in_die_Praxis/200129_Bedarfsplan_2020_Stand_20200127_final_oeff.pdf (accessed on 12 October 2021).
- Hüther, M.; Südekum, J.; Voigtländer, M. (Eds.) Die Zukunft der Regionen in Deutschland: Zwischen Vielfalt und Gleichwertigkeit; IW Medien: Köln, Germany, 2019; pp. 251–564. [Google Scholar]
- Jünger, M.; Arnold, A.; Lutze, S. Teledermatologie zur notfallmedizinischen Patientenversorgung: Zweijahreserfahrungen mit teledermatologischer Notfallversorgung. Hautarzt 2019, 70, 324–328. [Google Scholar] [CrossRef]
- Mohr, P.; Tadmouri, A.; Suissa, J.; Alivon, M.; Meyer, N. 1140P A digital companion for patients with BRAF-mutant advanced melanoma treated with targeted therapies: TAVIE skin app. Ann. Oncol. 2020, 31, S763. [Google Scholar] [CrossRef]
- Kruse, C.S.; Karem, P.; Shifflett, K.; Vegi, L.; Ravi, K.; Brooks, M. Evaluating barriers to adopting telemedicine worldwide: A systematic review. J. Telemed. Telecare 2018, 24, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Melanoma Network of New Zealand. MelNet Strategic Plan. Available online: https://www.melnet.org.nz/uploads/Strategic-Plan.pdf (accessed on 15 August 2021).
- Porter, M.E. What is value in health care? N. Engl. J. Med. 2010, 363, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Herbst, F.A.; Heckel, M.; Stiel, S.; Ostgathe, C. Kompetent vernetzt–optimal versorgt!: Förderliche Faktoren der Zusammenarbeit in hospizlich-palliativen Versorgungsnetzwerken in Bayern. Bundesgesundheitsblatt Gesundh. Gesundh. 2017, 60, 37–44. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number | |
|---|---|---|
| Age | Years: M (SD) 1, range | 67 (±12.3), 45–83 |
| Not specified | 1 | |
| Gender | Female | 12 |
| Male | 7 | |
| Not specified | 1 | |
| Stage of disease (according to AJCC 2017) | Stage I | 7 |
| Stage II | 5 | |
| Stage III | 4 | |
| Stage IV | 4 | |
| Size of the place of residence | Large city (≥100,000 inhabitants) | 9 |
| Small-to-medium sized city (≥5000 inhabitants) | 7 | |
| Rural community (<5000 inhabitants) | 3 | |
| Not specified | 1 |
| Characteristics | Number | |
|---|---|---|
| Age | Years: M (SD) 1, range | 49 (±8.6), 38–62 |
| Not specified | 1 | |
| Gender | Female | 6 |
| Male | 2 | |
| Specialist discipline | Dermatologists in outpatient practices | 4 |
| Oncologists in outpatient practices | 2 | |
| Clinicians from the field of dermatooncology | 2 | |
| Places of practice | Large city (≥100,000 inhabitants) | 2 |
| Small-to-medium sized city (≥5000 inhabitants) | 5 | |
| Not specified | 1 |
| Category | Stakeholder Group | Sample Quotes |
|---|---|---|
| Prevention and early diagnosis of skin cancer | Patients | “They always have the pictures that look ghastly there and then say, yeah, well if I had something like that I’d go to the physician. You’d have to really show first, like I had this little spot now, that that could be a skin cancer.” |
| Physicians | “And skin tumor […], it sounds so harmless. It’s just not colon cancer. Yes, that’s where some of the other specialties are much further ahead of us in enlightenment.” | |
| Health insurance company representatives | “What I’ve already said, prevention I think needs to be improved generally in mainstream care. For example […] that the age limit must be set lower. […] Payers need to educate patients more. Conversely, of course, the physicians’ outpatient practices, which must also better educate patients. Not only if [a patient] happens to arrive there, but you can inform patients via email, regular mail, saying: attention, it’s time now, you can stop by and do skin cancer screening.” | |
| Patient referral and length of stay | Health insurance company representatives | “The problem I see is mainly in the collaboration in outpatient practice between the GPs and the dermatologists, because the problem is that patients who visit the GP are referred too late. Or in the context of […] the budget problem, too long in the physician’s outpatient practice of the general practitioner, because the general practitioner says, that’s my patient, I don’t want to give him away.” |
| Accessibility of physicians/clinics | Patients | “[…] so I’m going to be 84 soon now—for me, the drive to [name of a city] is very exhausting. […] I was satisfied, but I can’t do it by myself anymore with trams, buses and everything.” |
| Physicians | “But she [a clinic employee] is not always available, so if you have someone [a patient] where you now say that you would like to place them in the clinic quickly. And that’s always the difficult part, to reach the [employee].” | |
| Health insurance company representatives | “There’s been a lot of problems with visiting the outpatient practice. First of all to reach the practice at all, no matter how, telephone or online appointments, homepage, that is all such a problem. Secondly, when do you get an appointment both with the general practitioner, especially with the dermatologist. […] Because if I have to wait a long time to even reach someone, then get another appointment and wait three months for it, then in case of doubt I don’t even go.” | |
| Physician resourcing | Patients | “So in [place name], there’s only one dermatologist left. I’m already looking for a new dermatologist in my new neighborhood. That’s practically impossible. My dermatologist, she’s retiring in a few years too. At that point, I don’t even know where I’m going to go then, and you’re talking about a network here.” |
| Physicians | “And yes, unfortunately, of course, one thing you hear a lot is you can’t get appointments with the local practitioners.” | |
| Health insurance company representatives | “So there is insufficient coverage here in Saxony, it has to be said. But the problem is getting physicians there. So they’re just not there. […] Infrastructure is the be-all and end-all. And the infrastructure in these village areas is unfortunately just not what it should be.” | |
| Care providers’ responsibilities | Patients | “[…] after the other clinic […] no longer guaranteed me this outpatient examination—That was then the [physician] who referred me to the [name of a clinic] and who said: ‘You’re in good hands there.’ But how I get there and all, of course they don’t care.” |
| Physicians | “However, I have to say, I’m always a big fan of the GP-centered care principle, […] we get ten euro for the patient per quarter, the GP gets up to 85. He’s allowed to do a little bit for it. Because that is very often the case that they say […] I’ll send it to you right away, you do it and you also know what you have to do for the lab, I have no idea. We’re lucky sometimes […] that we […] divide up […].” | |
| Health insurance company representatives | “[…] the vanity between participating care providers must disappear. It’s not that what I say is right, it’s […] treatment pathways. They pretend, the GP can’t say I’m right and you’re not right or the dermatologist says you’re not right. But that this collaboration track can be improved there.” | |
| Quality of diagnostics and treatment | Patients | “I had a different process now in that respect. I had a GP, she was always looking at the files or the computer, but basically never examined me. And that was too much for me at some point. And then I talked to my wife and she put me with another physician. And he asked during the first examination, what kind of spot do you have back here. And that’s when I said I wouldn’t look at myself in the mirror in the back. And that’s when he immediately referred me to the dermatologist. […] That is, if I hadn’t changed GPs and that’s always difficult too, getting a new one.” |
| Physicians | “[…] whether we dermatologists have done ourselves such a favor by including general practitioners in the skin check, because […] I also get diagnoses on referral slips. […] So with diagnoses you’ve never heard before.” | |
| Information exchange | Patients | “And to top it all off, yes, I went back to my dermatologist in [place name] several weeks later. Then I thought, well, now my physician will have all the findings here and will tell me something about it again. There was nothing there. She didn’t even know that.” |
| Physicians | “[…] A patient who has been cared for there for years and I don’t really know what the status is. […]“ | |
| Health insurance company representatives | “[…] The classic is, of course, discharge management after hospital treatment. In practice, this is a problem that we hear time and time again. Data is submitted late or not at all and then the physician is running after the physician’s letters […].” | |
| Effectiveness of interdisciplinary tumor boards | Physicians | “Simply because so much is changing so quickly in our field that many other disciplines sometimes still [have] views from X years ago […]” “But also this presenting in the tumor board, so unfortunately I’ve seen some tumor boards where it’s really presenting where the dermatologist says I have a patient. Melanoma at that and that stage. I’d like to do that and that right now. And then everyone looks at the wall, yeah well next patient […].” |
| Guideline-based prevention and aftercare | Patients | “What bothered me afterwards, though, was that there was essentially no aftercare. Further metastases then occurred, so the woman had to have surgery in [month name and year]. And only then was therapy initiated. Why? The question comes to me. Why?” |
| Physicians | “I think even with guideline-based aftercare, there are already some issues like that with outpatient-based colleagues. For example, when I look at squamous cell carcinoma, which is not melanoma, but theoretically one would have to do an ultrasound every quarter. For example, that doesn’t really work out either because they don’t really have the facilities.” “And we have the ones where the patients credibly assure us, well I didn’t have to take my clothes off there for a skin check. […] the physician says, he always makes the cross in the computer, that he has done it and if something is wrong, I should come to you in such and such a way. […] the general practitioner, at least in our regions, is not a really reliable entity.” | |
| Patient-centered care | Patients | “The diagnosis really shocks you, doesn’t it? […] No one can tell you what will happen next. And then came all the formalities and going here and going there and stuff. So that was soon worse.” “[…] it’s hard to manage everything. And all the files you get […] and all the appointments. It’s a burden to me. And I haven’t even thought about psychosocial treatment. I didn’t ask for it. No one came to help me there, either.” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathiebe, J.; Reinhardt, L.; Bergmann, M.; Lindauer, M.; Herrmann, A.; Strasser, C.; Meier, F.; Schmitt, J. A Qualitative Needs Analysis of Skin Cancer Care from the Perspectives of Patients, Physicians, and Health Insurance Representatives—A Case Study from Eastern Saxony, Germany. Curr. Oncol. 2022, 29, 2583-2598. https://doi.org/10.3390/curroncol29040212
Mathiebe J, Reinhardt L, Bergmann M, Lindauer M, Herrmann A, Strasser C, Meier F, Schmitt J. A Qualitative Needs Analysis of Skin Cancer Care from the Perspectives of Patients, Physicians, and Health Insurance Representatives—A Case Study from Eastern Saxony, Germany. Current Oncology. 2022; 29(4):2583-2598. https://doi.org/10.3390/curroncol29040212
Chicago/Turabian StyleMathiebe, Josephine, Lydia Reinhardt, Maike Bergmann, Marina Lindauer, Alina Herrmann, Cristin Strasser, Friedegund Meier, and Jochen Schmitt. 2022. "A Qualitative Needs Analysis of Skin Cancer Care from the Perspectives of Patients, Physicians, and Health Insurance Representatives—A Case Study from Eastern Saxony, Germany" Current Oncology 29, no. 4: 2583-2598. https://doi.org/10.3390/curroncol29040212
APA StyleMathiebe, J., Reinhardt, L., Bergmann, M., Lindauer, M., Herrmann, A., Strasser, C., Meier, F., & Schmitt, J. (2022). A Qualitative Needs Analysis of Skin Cancer Care from the Perspectives of Patients, Physicians, and Health Insurance Representatives—A Case Study from Eastern Saxony, Germany. Current Oncology, 29(4), 2583-2598. https://doi.org/10.3390/curroncol29040212





