Linking Intermediate to Final “Real-World” Outcomes: Is Financial Toxicity a Reliable Predictor of Poorer Outcomes in Cancer?
Abstract
:1. Introduction
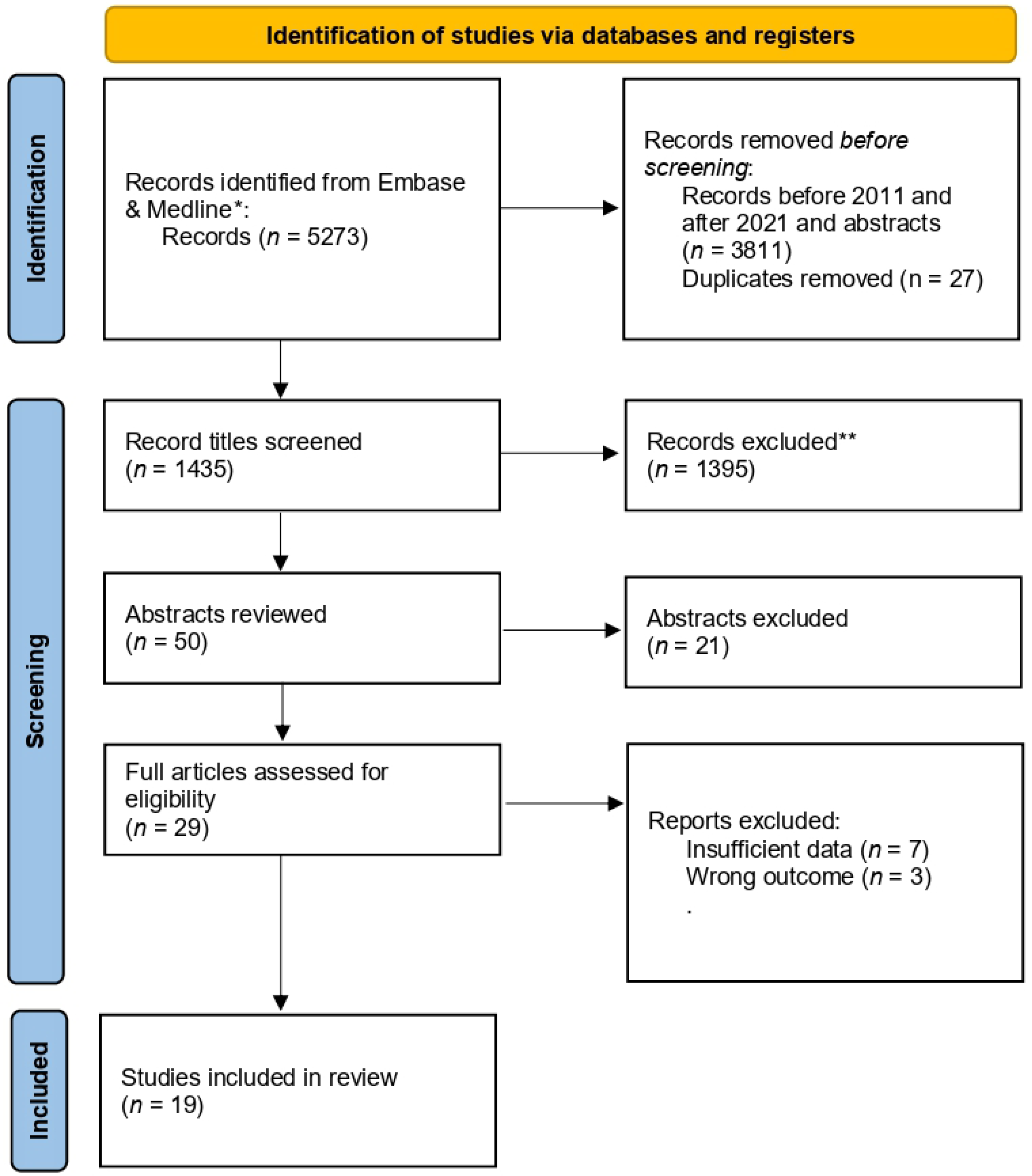
2. Search Methods
3. Current Evidence
3.1. Reduced Quality of Life
3.2. Reduced Overall Survival
3.3. Risks Based on Patient Characteristics
3.4. Implications
4. Future Research
5. Discussion
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Zafar, S.Y.; Abernethy, A.P. Financial Toxicity, Part I: A New Name for a Growing Problem. Oncology 2013, 27, 80–81. [Google Scholar] [PubMed]
- Durber, K.; Halkett, G.K.; McMullen, M.; Nowak, A.K. Measuring Financial Toxicity in Australian Cancer Patients—Validation of the COmprehensive Score for Financial Toxicity (FACT COST) Measuring Financial Toxicity in Australian Cancer Patients. Asia Pac. J. Clin. Oncol. 2021, 17, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.J.; Fitch, M.I.; Loree, J.M.; Carlson, L.E.; Turner, D.; Cheung, W.Y.; Gopaul, D.; Ellis, J.; Ringash, J.; Mathews, M. Patient and Family Financial Burden Associated with Cancer Treatment in Canada: A National Study. Support. Care Cancer 2021, 29, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.A.; Cole, A.P.; Lu, C.; Marchese, M.; Krimphove, M.J.; Friedlander, D.F.; Mossanen, M.; Kilbridge, K.L.; Kibel, A.S.; Trinh, Q.-D. The Impact of Underinsurance on Bladder Cancer Diagnosis, Survival, and Care Delivery for Individuals under the Age of 65 Years. Cancer 2020, 126, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Kaisaeng, N.; Harpe, S.E.; Carroll, N.V. Out-of-Pocket Costs and Oral Cancer Medication Discontinuation in the Elderly. J. Manag. Care Spec. Pharm. 2014, 20, 669–675. [Google Scholar] [CrossRef]
- Raborn, M.L.; Pelletier, E.M.; Smith, D.B.; Reyes, C.M. Patient Out-of-Pocket Payments for Oral Oncolytics: Results from a 2009 US Claims Data Analysis. J. Oncol. Pract. 2012, 8, 9s–15s. [Google Scholar] [CrossRef] [Green Version]
- Yezefski, T.; Schwemm, A.; Lentz, M.; Hone, K.; Shankaran, V. Patient Assistance Programs: A Valuable, yet Imperfect, Way to Ease the Financial Toxicity of Cancer Care. Semin. Hematol. 2018, 55, 185–188. [Google Scholar] [CrossRef]
- Hazell, S.Z.; Fu, W.; Hu, C.; Voong, K.R.; Lee, B.; Peterson, V.; Feliciano, J.L.; Nicholas, L.H.; McNutt, T.R.; Han, P.; et al. Financial Toxicity in Lung Cancer: An Assessment of Magnitude, Perception, and Impact on Quality of Life. Ann. Oncol. 2020, 31, 96–102. [Google Scholar] [CrossRef]
- Perrone, F.; Jommi, C.; Di Maio, M.; Gimigliano, A.; Gridelli, C.; Pignata, S.; Ciardiello, F.; Nuzzo, F.; de Matteis, A.; Del Mastro, L.; et al. The Association of Financial Difficulties with Clinical Outcomes in Cancer Patients: Secondary Analysis of 16 Academic Prospective Clinical Trials Conducted in Italy. Ann. Oncol. 2016, 27, 2224–2229. [Google Scholar] [CrossRef]
- Delgado-Guay, M.; Ferrer, J.; Rieber, A.G.; Rhondali, W.; Tayjasanant, S.; Ochoa, J.; Cantu, H.; Chisholm, G.; Williams, J.; Frisbee-Hume, S.; et al. Financial Distress and Its Associations with Physical and Emotional Symptoms and Quality of Life Among Advanced Cancer Patients. Oncologist 2015, 20, 1092–1098. [Google Scholar] [CrossRef] [Green Version]
- Fenn, K.M.; Evans, S.B.; McCorkle, R.; DiGiovanna, M.P.; Pusztai, L.; Sanft, T.; Hofstatter, E.W.; Killelea, B.K.; Knobf, M.T.; Lannin, D.R.; et al. Impact of Financial Burden of Cancer on Survivors’ Quality of Life. J. Oncol. Pract. 2014, 10, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Lathan, C.S.; Cronin, A.; Tucker-Seeley, R.; Zafar, S.Y.; Ayanian, J.Z.; Schrag, D. Association of Financial Strain with Symptom Burden and Quality of Life for Patients with Lung or Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1732–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, S.Y.; McNeil, R.B.; Thomas, C.M.; Lathan, C.S.; Ayanian, J.Z.; Provenzale, D. Population-Based Assessment of Cancer Survivors’ Financial Burden and Quality of Life: A Prospective Cohort Study. J. Oncol. Pract. 2015, 11, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.L.; Butler, E.N.; Stevens, J.; Lathan, C.S.; Noone, A.-M.; Ward, K.C.; Harlan, L.C. Receipt of Chemotherapy among Medicare Patients with Cancer by Type of Supplemental Insurance. J. Clin. Oncol. 2015, 33, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, S.D.; Bansal, A.; Fedorenko, C.R.; Blough, D.K.; Overstreet, K.A.; Shankaran, V.; Newcomb, P. Financial Insolvency as a Risk Factor for Early Mortality among Patients with Cancer. J. Clin. Oncol. 2016, 34, 980–986. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.J.; Iovoli, A.J.; Attwood, K.; Wooten, K.E.; Arshad, H.; Gupta, V.; McSpadden, R.P.; Kuriakose, M.A.; Markiewicz, M.R.; Chan, J.M.; et al. Association of Significant Financial Burden with Survival for Head and Neck Cancer Patients Treated with Radiation Therapy. Oral Oncol. 2021, 115, 105196. [Google Scholar] [CrossRef]
- Klein, J.; Bodner, W.; Garg, M.; Kalnicki, S.; Ohri, N. Pretreatment Financial Toxicity Predicts Progression-Free Survival Following Concurrent Chemoradiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. Future Oncol. 2019, 15, 1697–1705. [Google Scholar] [CrossRef]
- Ward, E.; Jemal, A.; Cokkinides, V.; Singh, G.K.; Cardinez, C.; Ghafoor, A.; Thun, M. Cancer Disparities by Race/Ethnicity and Socioeconomic Status. CA Cancer J. Clin. 2004, 54, 78–93. [Google Scholar] [CrossRef]
- Kent, E.E.; Forsythe, L.P.; Yabroff, K.R.; Weaver, K.E.; de Moor, J.S.; Rodriguez, J.L.; Rowland, J.H. Are Survivors Who Report Cancer-Related Financial Problems More Likely to Forgo or Delay Medical Care? Cancer 2013, 119, 3710–3717. [Google Scholar] [CrossRef]
- Abbott, D.E.; Voils, C.L.; Fisher, D.A.; Greenberg, C.C.; Safdar, N. Socioeconomic Disparities, Financial Toxicity, and Opportunities for Enhanced System Efficiencies for Patients with Cancer. J. Surg. Oncol. 2017, 115, 250–256. [Google Scholar] [CrossRef]
- Lau-Min, K.; Prakash, P.; Jo, E.; Thrift, A.P.; Hilsenbeck, S.; Musher, B.L. Outcomes among Minority Patients with Metastatic Colorectal Cancer in a Safety-Net Health Care System. Clin. Colorectal Cancer 2020, 19, e49–e57. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, C.J. Linking Intermediate to Final “Real-World” Outcomes: Is Financial Toxicity a Reliable Predictor of Poorer Outcomes in Cancer? Curr. Oncol. 2022, 29, 2483-2489. https://doi.org/10.3390/curroncol29040202
Longo CJ. Linking Intermediate to Final “Real-World” Outcomes: Is Financial Toxicity a Reliable Predictor of Poorer Outcomes in Cancer? Current Oncology. 2022; 29(4):2483-2489. https://doi.org/10.3390/curroncol29040202
Chicago/Turabian StyleLongo, Christopher J. 2022. "Linking Intermediate to Final “Real-World” Outcomes: Is Financial Toxicity a Reliable Predictor of Poorer Outcomes in Cancer?" Current Oncology 29, no. 4: 2483-2489. https://doi.org/10.3390/curroncol29040202
APA StyleLongo, C. J. (2022). Linking Intermediate to Final “Real-World” Outcomes: Is Financial Toxicity a Reliable Predictor of Poorer Outcomes in Cancer? Current Oncology, 29(4), 2483-2489. https://doi.org/10.3390/curroncol29040202



