Evaluation of Open Surgical and Endovascular Treatment Options for Visceral Artery Erosions after Pancreatitis and Pancreatic Surgery
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Treatment Options for Erosion Bleeding
3.3. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansari, D.; Tingstedt, B.; Lindell, G.; Keussen, I.; Andersson, R. Hemorrhage after Major Pancreatic Resection: Incidence, Risk Factors, Management, and Outcome. Scand. J. Surg. SJS Off. Organ Finn. Surg. Soc. Scand. Surg. Soc. 2017, 106, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Stanley, J.C.; Frey, C.F.; Miller, T.A.; Lindenauer, S.M.; Child, C.G. Major arterial hemorrhage: A complication of pancreatic pseudocysts and chronic pancreatitis. Arch. Surg. 1960 1976, 111, 435–440. [Google Scholar] [CrossRef]
- Balthazar, E.J.; Fisher, L.A. Hemorrhagic complications of pancreatitis: Radiologic evaluation with emphasis on CT imaging. Pancreatology 2001, 1, 306–313. [Google Scholar] [CrossRef]
- Hassold, N.; Wolfschmidt, F.; Dierks, A.; Klein, I.; Bley, T.; Kickuth, R. Effectiveness and outcome of endovascular therapy for late-onset postpancreatectomy hemorrhage using covered stents and embolization. J. Vasc. Surg. 2016, 64, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Khuri, S.; Mansour, S.; Obeid, A.; Azzam, A.; Borzellino, G.; Kluger, Y. Postpancreatoduodenectomy Hemorrhage: Association between the Causes and the Severity of the Bleeding. Visc. Med. 2021, 37, 171–179. [Google Scholar] [CrossRef]
- Mendelson, R.M.; Anderson, J.; Marshall, M.; Ramsay, D. Vascular complications of pancreatitis. ANZ J. Surg. 2005, 75, 1073–1079. [Google Scholar] [CrossRef]
- Menger, M.D.; Plusczyk, T.; Vollmar, B. Microcirculatory derangements in acute pancreatitis. J. Hepato-Biliary-Pancreat. Surg. 2001, 8, 187–194. [Google Scholar] [CrossRef]
- Yoon, S.J.; Lee, O.; Jung, J.H.; Shin, S.H.; Heo, J.S.; Han, I.W. Does Preoperative Acute Pancreatitis Inevitably Delay Pancreatoduodenectomy in Patients with Periampullary Tumors? Cancers 2021, 13, 6289. [Google Scholar] [CrossRef] [PubMed]
- Makowiec, F.; Riediger, H.; Euringer, W.; Uhl, M.; Hopt, U.T.; Adam, U. Management of delayed visceral arterial bleeding after pancreatic head resection. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2005, 9, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Miura, F.; Asano, T.; Amano, H.; Yoshida, M.; Toyota, N.; Wada, K.; Kato, K.; Yamazaki, E.; Kadowaki, S.; Shibuya, M.; et al. Management of postoperative arterial hemorrhage after pancreato-biliary surgery according to the site of bleeding: Re-laparotomy or interventional radiology. J. Hepato-Biliary-Pancreat. Surg. 2009, 16, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Numoto, I.; Tsurusaki, M.; Oda, T.; Yagyu, Y.; Ishii, K.; Murakami, T. Transcatheter Arterial Embolization Treatment for Bleeding Visceral Artery Pseudoaneurysms in Patients with Pancreatitis or Following Pancreatic Surgery. Cancers 2020, 12, 2733. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.; Weerasinghe, S.; Lamond, N.; Younis, T.; Ramjeesingh, R. Palliative care consultation and aggressive care at end of life in unresectable pancreatic cancer. Curr. Oncol. Tor. Ont. 2019, 26, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhussan, A.; Bromma, K.; Bozdoğan, E.P.D.; Metcalfe, A.; Karasinska, J.; Beckham, W.; Alexander, A.S.; Renouf, D.J.; Schaeffer, D.F.; Chithrani, D.B. Investigation of Nano-Bio Interactions within a Pancreatic Tumor Microenvironment for the Advancement of Nanomedicine in Cancer Treatment. Curr. Oncol. Tor. Ont. 2021, 28, 1962–1979. [Google Scholar] [CrossRef]
- Lindner, K.; Binte, D.; Hoeppner, J.; Wellner, U.F.; Schulte, D.M.; Schmid, S.M.; Luley, K.; Buchmann, I.; Tharun, L.; Keck, T.; et al. Resection of Non-Functional Pancreatic Neuroendocrine Neoplasms—A Single-Center Retrospective Outcome Analysis. Curr. Oncol. Tor. Ont. 2021, 28, 3071–3080. [Google Scholar] [CrossRef] [PubMed]
- Addeo, P.; Cusumano, C.; Goichot, B.; Guerra, M.; Faitot, F.; Imperiale, A.; Bachellier, P. Simultaneous Resection of Pancreatic Neuroendocrine Tumors with Synchronous Liver Metastases: Safety and Oncological Efficacy. Cancers 2022, 14, 727. [Google Scholar] [CrossRef] [PubMed]
- Serrablo, A.; Serrablo, L.; Alikhanov, R.; Tejedor, L. Vascular Resection in Perihilar Cholangiocarcinoma. Cancers 2021, 13, 5278. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Blais, N.; Crowther, M.; Kavan, P.; Le Gal, G.; Moodley, O.; Shivakumar, S.; Suryanarayan, D.; Tagalakis, V.; Wu, C.; et al. Treatment Algorithm in Cancer-Associated Thrombosis: Updated Canadian Expert Consensus. Curr. Oncol. Tor. Ont. 2021, 28, 5434–5451. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Brugnatelli, S.; Maestri, R.; Boschi, F.; Filippi, B.; Perrone, L.; Barbieri, A.; Buonocore, D.; Dossena, M.; Verri, M. Peripheral Blood Lymphocyte Percentage May Predict Chemotolerance and Survival in Patients with Advanced Pancreatic Cancer. Association between Adaptive Immunity and Nutritional State. Curr. Oncol. Tor. Ont. 2021, 28, 3280–3296. [Google Scholar] [CrossRef]
- Enderes, J.; Teschke, J.; Manekeller, S.; Vilz, T.O.; Kalff, J.C.; Glowka, T.R. Chronic Liver Disease Increases Mortality Following Pancreatoduodenectomy. J. Clin. Med. 2021, 10, 2521. [Google Scholar] [CrossRef]
- Vorčák, M.; Sýkora, J.; Ďuríček, M.; Bánovčin, P.; Grendár, M.; Zeleňák, K. Endovascular Treatment of Gastrointestinal Hemorrhage. Medicina 2022, 58, 424. [Google Scholar] [CrossRef]
- Heiss, P.; Bachthaler, M.; Hamer, O.W.; Piso, P.; Herold, T.; Schlitt, H.J.; Feuerbach, S.; Zorger, N. Delayed visceral arterial hemorrhage following Whipple’s procedure: Minimally invasive treatment with covered stents. Ann. Surg. Oncol. 2008, 15, 824–832. [Google Scholar] [CrossRef]
- Puppala, S.; Patel, J.; McPherson, S.; Nicholson, A.; Kessel, D. Hemorrhagic complications after Whipple surgery: Imaging and radiologic intervention. AJR Am. J. Roentgenol. 2011, 196, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Yamaguchi, K.; Shimizu, S.; Morisaki, T.; Yokohata, K.; Chijiiwa, K.; Tanaka, M. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: The importance of early angiography. Arch. Surg. 1998, 133, 1099–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmar, B.; Menger, M.D. Microcirculatory dysfunction in acute pancreatitis. A new concept of pathogenesis involving vasomotion-associated arteriolar constriction and dilation. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP 2003, 3, 181–190. [Google Scholar] [CrossRef]
- Chiang, K.-C.; Chen, T.-H.; Hsu, J.-T. Management of chronic pancreatitis complicated with a bleeding pseudoaneurysm. World J. Gastroenterol. 2014, 20, 16132–16137. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Król, R.; Mrowiec, S. Vascular Resection in Pancreatectomy—Is It Safe and Useful for Patients with Advanced Pancreatic Cancer? Cancers 2022, 14, 1193. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, T.; Schulz, H.-U.; Tautenhahn, J.; Halloul, Z.; Effenberger, O.; Lippert, H.; Bürger, T. Entzündliche Arrosionsblutungen aus ViszeralarterienInterventionelles und gefässchirurgisches Management nach vorwiegend pankreaschirurgischen Eingriffen. Der Chir. Z. Fur Alle Geb. Der Oper. Medizen 2004, 75, 1021–1028. [Google Scholar]
- Yekebas, E.F.; Wolfram, L.; Cataldegirmen, G.; Habermann, C.R.; Bogoevski, D.; Koenig, A.M.; Kaifi, J.; Schurr, P.G.; Bubenheim, M.; Nolte-Ernsting, C.; et al. Postpancreatectomy hemorrhage: Diagnosis and treatment: An analysis in 1669 consecutive pancreatic resections. Ann. Surg. 2007, 246, 269–280. [Google Scholar] [CrossRef]
- Roulin, D.; Cerantola, Y.; Demartines, N.; Schäfer, M. Systematic review of delayed postoperative hemorrhage after pancreatic resection. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2011, 15, 1055–1062. [Google Scholar] [CrossRef]
- Radojkovic, M.D.; Radisavljevic, M.; Radojkovic, D.; Tasic, S.; Nestorovic, M.; Stevanovic, G. Pankreatitli hastalarda gastrointestinal kanama için embolizasyon: İki olgu raporu ve literatür taraması. Ulus. Travma Ve Acil Cerrahi Derg. Turk. Trauma Emerg. Surg. TJTES 2021, 27, 590–594. [Google Scholar]
- Zeyara, A.; Tingstedt, B.; Andersson, B. Late postpancreatectomy hemorrhage from the gastroduodenal artery stump into an insufficient hepaticojejunostomy: A case report. J. Med. Case Rep. 2021, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Zaydfudim, V.; Truty, M.; Reid-Lombardo, K.M.; Kendrick, M.; Que, F.; Nagorney, D.; Andrews, J.; Farnell, M. Management of a delayed post-pancreatoduodenectomy haemorrhage using endovascular techniques. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2015, 17, 902–908. [Google Scholar] [CrossRef] [Green Version]
- Biondetti, P.; Fumarola, E.M.; Ierardi, A.M.; Carrafiello, G. Bleeding complications after pancreatic surgery: Interventional radiology management. Gland. Surg. 2019, 8, 150–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernon, M.M.; Krige, J.E.J.; Jonas, E.; Kloppers, J.C.; Burmeister, S.; Naidoo, N.G.; Beningfield, S.J. Severe post-pancreatoduodenectomy haemorrhage: An analytical review based on 118 consecutive pancreatoduodenectomy patients in a South African Academic Hospital. S. Afr. J. Surg. Suid-Afrik. Tydskr. Vir Chir. 2016, 54, 23–28. [Google Scholar]
- Duarte Garcés, A.A.; Andrianello, S.; Marchegiani, G.; Piccolo, R.; Secchettin, E.; Paiella, S.; Malleo, G.; Salvia, R.; Bassi, C. Reappraisal of post-pancreatectomy hemorrhage (PPH) classifications: Do we need to redefine grades A and B? HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2018, 20, 702–707. [Google Scholar] [CrossRef] [Green Version]
- Wellner, U.F.; Kulemann, B.; Lapshyn, H.; Hoeppner, J.; Sick, O.; Makowiec, F.; Bausch, D.; Hopt, U.T.; Keck, T. Postpancreatectomy hemorrhage—incidence, treatment, and risk factors in over 1,000 pancreatic resections. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2014, 18, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH): An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Müssle, B.; Wierick, A.; Distler, M.; Weitz, J.; Welsch, T. Falciform ligament wrap for prevention of gastroduodenal artery bleed after pancreatoduodenectomy. J. Surg. Res. 2017, 207, 215–222. [Google Scholar] [CrossRef]
- Müssle, B.; Zühlke, L.; Wierick, A.; Sturm, D.; Grählert, X.; Distler, M.; Rahbari, N.N.; Weitz, J.; Welsch, T. Pancreatoduodenectomy with or without prophylactic falciform ligament wrap around the gastroduodenal artery stump for prevention of pancreatectomy hemorrhage. Trials 2018, 19, 222. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, A.; Luo, T.; Li, J.; Liu, D.; Cao, F.; Li, J.; Li, F. Strategy and management of severe hemorrhage complicating pancreatitis and post-pancreatectomy. Diagn. Interv. Radiol. Ank. Turk. 2019, 25, 81–89. [Google Scholar] [CrossRef]

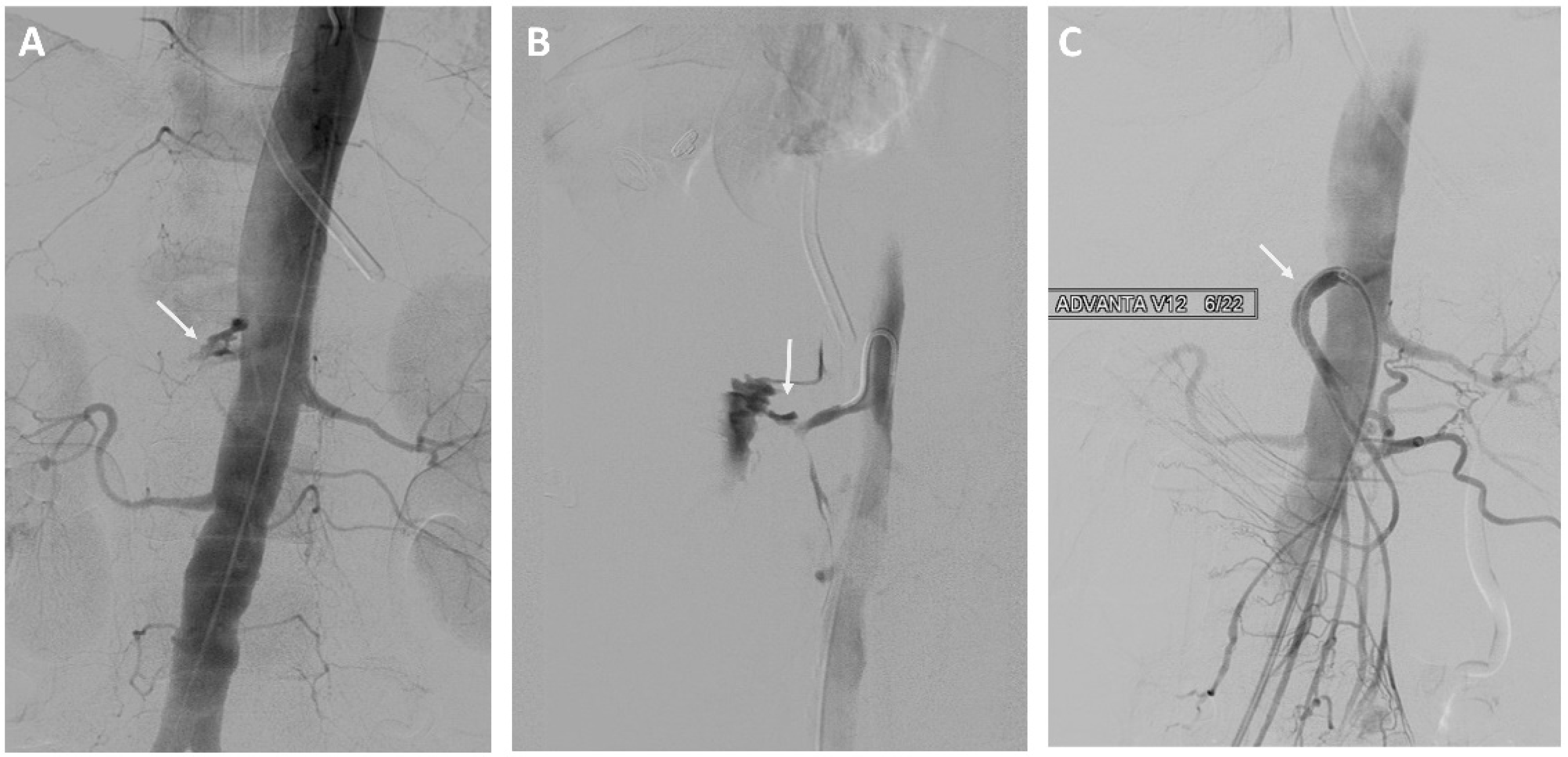

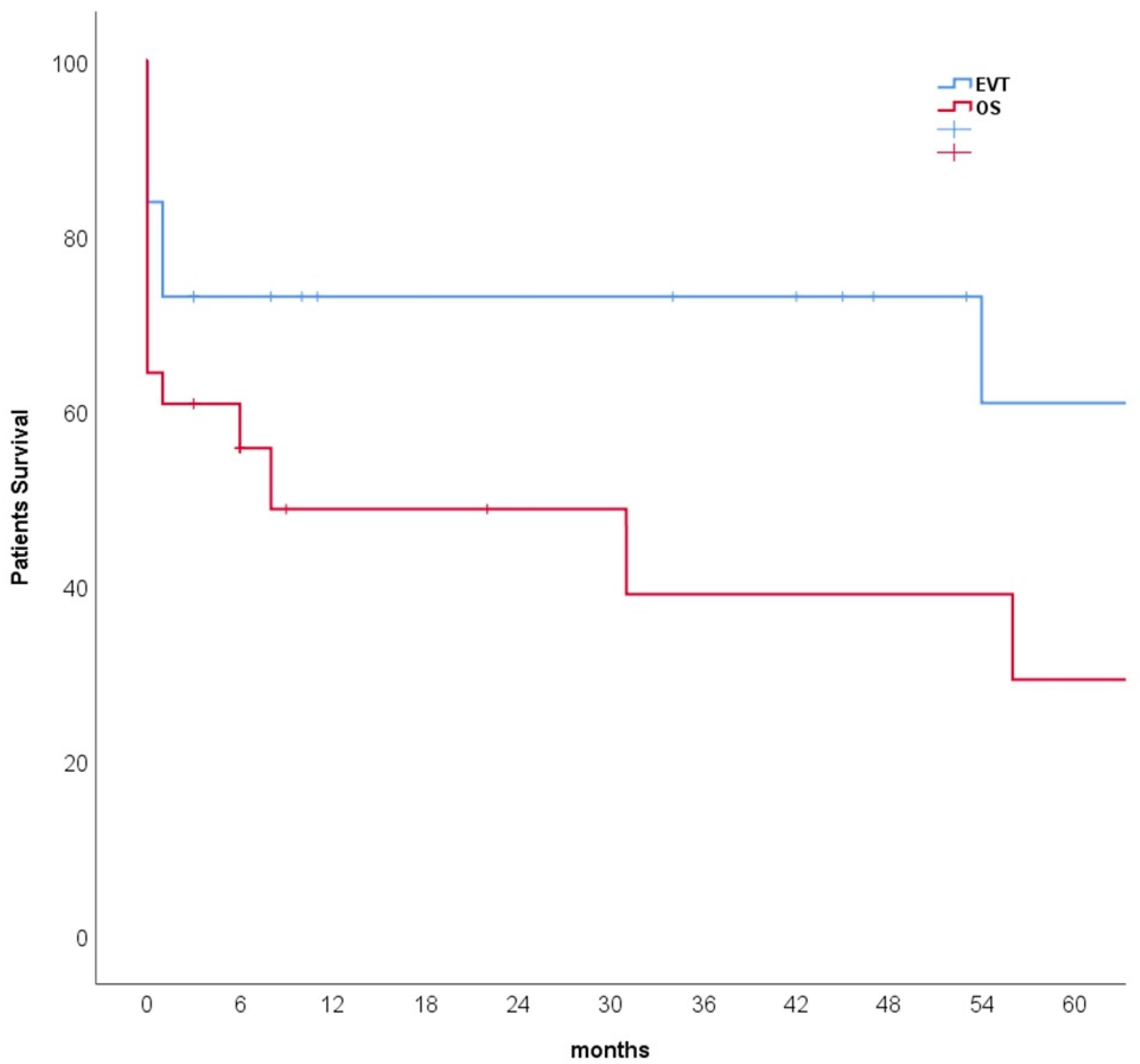
| Characteristic | Total n (%) | EVT n (%) | OS n (%) | p-Value |
|---|---|---|---|---|
| Patients | 65 | 37 (57) | 28 (43) | |
| Age | 62 ± 14 | 63 ± 13 | 62 ± 15 | 0.678 |
| Male | 46 (71) | 25 (67) | 21 (75) | 0.514 |
| Risk factors | ||||
| Coronary artery disease | 5 (8) | 4 (11) | 1 (4) | 0.380 |
| Diabetes mellitus | 16 (25) | 9 (24) | 7 (25) | 0.950 |
| Hypertension | 26 (40) | 16 (43) | 10 (38) | 0.540 |
| COPD | 4 (6) | 3 (8) | 1 (4) | 0.628 |
| Smoking | 6 (9) | 4 (11) | 2 (7) | 0.692 |
| Chronic renal insufficiency | 7 (11) | 2 (5) | 5 (18) | 0.224 |
| ASA classification 1 | ||||
| I | 1 (2) | 0 (0) | 1 (5) | |
| II | 21 (44) | 9 (35) | 12 (55) | |
| III | 25 (52) | 16 (62) | 9 (41) | |
| IV | 1 (2) | 1 (4) | 0 | |
| Etiology | ||||
| Complications of pancreatic surgeries | 49 (75) | 24 (65) | 25 (89) | 0.024 * |
| Pancreatitis | 12 (19) | 9 (24) | 3 (11) | 0.161 |
| Spontaneous bleeding | 4 (6) | 4 (11) | 0 |
| Malignancy | Total n (%) |
|---|---|
| Pancreatic cancer | 31 (48) |
| Head | 22 (34) |
| body | 4 (6) |
| tail | 3 (5) |
| 1 IPMN | 2 (3) |
| Liver & bile duct | 11 (17) |
| Gallbladder cancer | 1 (2) |
| Klatskin tumor | 2 (3) |
| Cholangiocarcinoma | 6 (9) |
| Hepatocellular carcinoma | 2 (3) |
| Stomach and duodenum | 5 (8) |
| 2 OGJA | 2 (3) |
| 3 DLBCL | 1 (2) |
| 4 SRCC | 1 (2) |
| Duodenal cancer | 1 (2) |
| Others | 4 (6) |
| Uterine cancer | 1 (2) |
| Breast cancer | 1 (2) |
| Bladder cancer | 2 (3) |
| Previous surgical procedures | 49 (75) |
| Pancreatectomy | 34 (52) |
| Hepatectomy | 6 (9) |
| Bile duct resection | 3 (5) |
| Gastrectomy | 6 (9) |
| Splenectomy | 12 (19) |
| Previous complications | |
| Anastomotic insufficiency | 21 (32) |
| Pancreatic fistula | 11 (17) |
| Pancreatic cyst | 3 (5) |
| Eroded Vessels | Total 65 (100) | 1 EVT 37 (57) | 2 OS 28 (43) |
|---|---|---|---|
| Branches of the celiac artery | 60 (92) | 34 (92) | 26 (93) |
| Celiac trunk | 2 (3) | 0 | 2 (7) |
| Splenic artery | 13 (20) | 5 (14) | 8 (29) |
| Common hepatic artery | 18 (28) | 9 (24) | 9 (32) |
| The left branch of the hepatic artery proper | 1 (2) | 1 (3) | 0 |
| The right branch of the hepatic artery proper | 9 (14) | 7 (19) | 2 (7) |
| Left gastric artery | 3 (5) | 2 (5) | 1 (4) |
| Light gastric artery | 3 (5) | 2 (5) | 1 (4) |
| Gastroduodenal artery | 10 (15) | 8 (22) | 2 (7) |
| Superior mesenteric artery | 3 (5) | 3 (8) | 0 |
| Inferior phrenic artery | 2 (3) | 0 | 2 (7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruder, L.; Schawe, L.; Gebauer, B.; Frese, J.P.; de Bucourt, M.; Beyer, K.; Pratschke, J.; Greiner, A.; Omran, S. Evaluation of Open Surgical and Endovascular Treatment Options for Visceral Artery Erosions after Pancreatitis and Pancreatic Surgery. Curr. Oncol. 2022, 29, 2472-2482. https://doi.org/10.3390/curroncol29040201
Bruder L, Schawe L, Gebauer B, Frese JP, de Bucourt M, Beyer K, Pratschke J, Greiner A, Omran S. Evaluation of Open Surgical and Endovascular Treatment Options for Visceral Artery Erosions after Pancreatitis and Pancreatic Surgery. Current Oncology. 2022; 29(4):2472-2482. https://doi.org/10.3390/curroncol29040201
Chicago/Turabian StyleBruder, Leon, Larissa Schawe, Bernhard Gebauer, Jan Paul Frese, Maximilian de Bucourt, Katharina Beyer, Johann Pratschke, Andreas Greiner, and Safwan Omran. 2022. "Evaluation of Open Surgical and Endovascular Treatment Options for Visceral Artery Erosions after Pancreatitis and Pancreatic Surgery" Current Oncology 29, no. 4: 2472-2482. https://doi.org/10.3390/curroncol29040201
APA StyleBruder, L., Schawe, L., Gebauer, B., Frese, J. P., de Bucourt, M., Beyer, K., Pratschke, J., Greiner, A., & Omran, S. (2022). Evaluation of Open Surgical and Endovascular Treatment Options for Visceral Artery Erosions after Pancreatitis and Pancreatic Surgery. Current Oncology, 29(4), 2472-2482. https://doi.org/10.3390/curroncol29040201





