Optimization of the Clinical Effectiveness of Radioembolization in Hepatocellular Carcinoma with Dosimetry and Patient-Selection Criteria
Abstract
1. Introduction
2. Clinical Results of SIRT in HCC
3. Clinical Dosimetry in SIRT
4. Personalized Dosimetry in SIRT
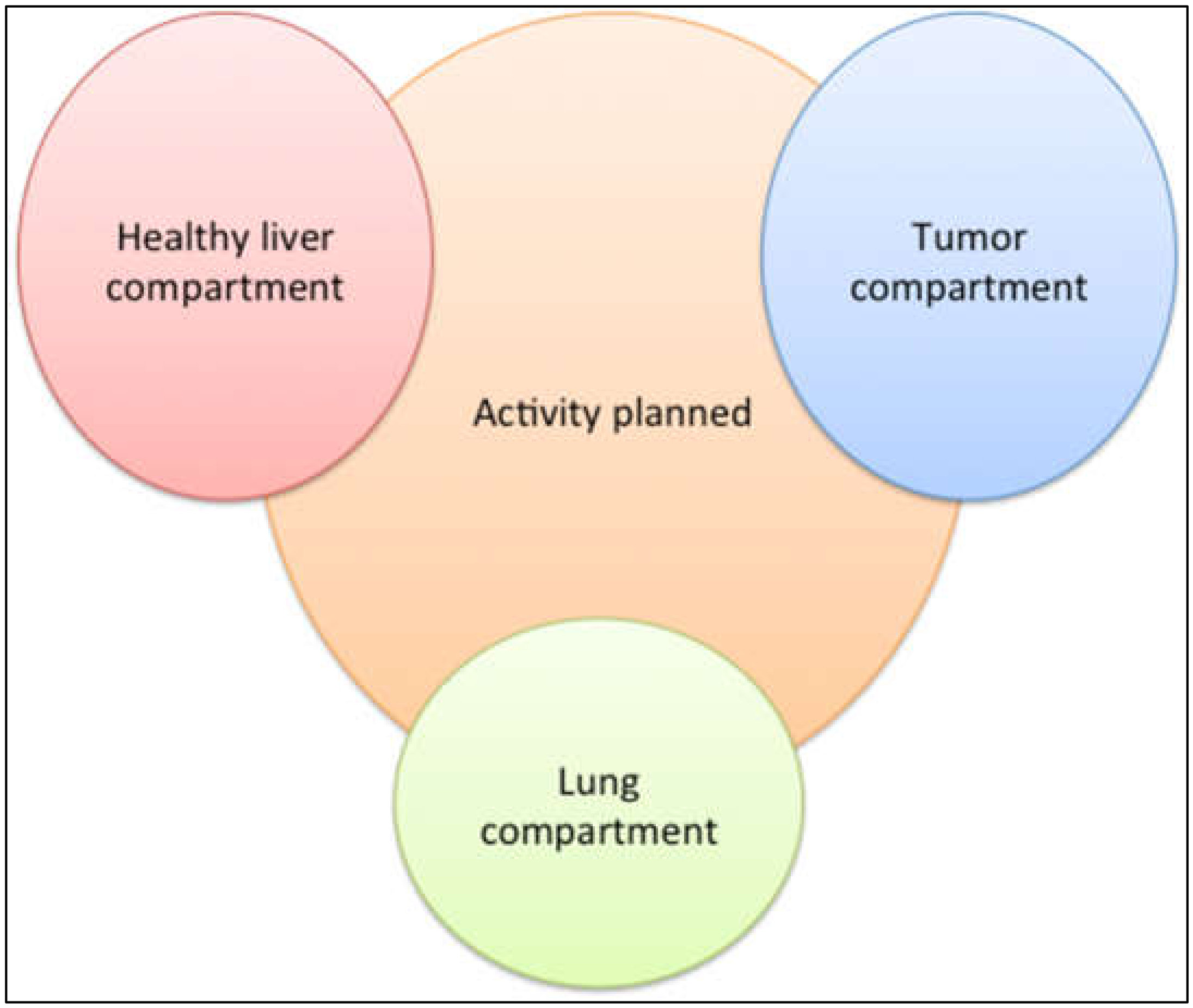
5. Optimization of Tumor Targeting
6. Good HCC Candidates for SIRT
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Padia, S.A.; Lewandowski, R.J.; Johnson, G.E.; Sze, D.Y.; Ward, T.J.; Gaba, R.C.; Baerlocher, M.O.; Gates, V.L.; Riaz, A.; Brown, D.B.; et al. Radioembolization of Hepatic Malignancies: Background, Quality Improvement Guidelines, and Future Directions. J. Vasc. Interv. Radiol. 2017, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, R.J.; Salem, R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin. Interv. Radiol. 2006, 23, 64–72. [Google Scholar] [CrossRef]
- Kennedy, A.S.; Nutting, C.; Coldwell, D.; Gaiser, J.; Drachenberg, C. Pathologic response and microdosimetry of (90)Y microspheres in man: Review of four explanted whole livers. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1552–1563. [Google Scholar] [CrossRef]
- Kennedy, A. Radioembolization of hepatic tumors. J. Gastrointest. Oncol. 2014, 5, 178–189. [Google Scholar] [CrossRef] [PubMed]
- D’Abadie, P.; Hesse, M.; Louppe, A.; Lhommel, R.; Walrand, S.; Jamar, F. Microspheres Used in Liver Radioembolization: From Conception to Clinical Effects. Molecules 2021, 26, 3966. [Google Scholar] [CrossRef] [PubMed]
- SIR-Spheres. Instructions for Use; SIRTeX Medical Limited: North Sydney, Australia, 2019; Available online: https://www.sirtex.com/us/clinicians/instructions-for-use/ (accessed on 9 March 2022).
- Therasphere. Instructionsforuse. 2022. Available online: https://www.bostonscientific.com/en-EU/products/selective-internal-radiation-therapy/therasphere-y90-glass-microspheres.html (accessed on 9 March 2022).
- QuiremSpheres. Instruction for Use. 2022. Available online: https://www.quirem.com/wp-content/uploads/2022/01/LC-8004307-IFU-QuiremSpheres-Multi-Language.pdf (accessed on 9 March 2022).
- Bastiaannet, R.; Kappadath, S.C.; Kunnen, B.; Braat, A.; Lam, M.; de Jong, H. The physics of radioembolization. EJNMMI Phys. 2018, 5, 22. [Google Scholar] [CrossRef]
- Hesse, M.; d’Abadie, P.; Lhommel, R.; Jamar, F.; Walrand, S. Yttrium-90 TOF-PET-Based EUD Predicts Response Post Liver Radioembolizations Using Recommended Manufacturer FDG Reconstruction Parameters. Front. Oncol. 2021, 11, 592529. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system. The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef]
- Couri, T.; Pillai, A. Goals and targets for personalized therapy for HCC. Hepatol. Int. 2019, 13, 125–137. [Google Scholar] [CrossRef]
- Vogel, A.; Martinelli, E.; on behalf of the ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef]
- Lee, J.J.X.; Tai, D.W.; Choo, S.P. Locoregional therapy in hepatocellular carcinoma: When to start and when to stop and when to revisit. ESMO Open 2021, 6, 100129. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Casadei Gardini, A.; Tamburini, E.; Inarrairaegui, M.; Frassineti, G.L.; Sangro, B. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: A meta-analysis of randomized trials. Onco Targets Ther. 2018, 11, 7315–7321. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163 e1152. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Gandhi, M.; Tan, S.B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef]
- Venerito, M.; Pech, M.; Canbay, A.; Donghia, R.; Guerra, V.; Chatellier, G.; Pereira, H.; Gandhi, M.; Malfertheiner, P.; Chow, P.K.H.; et al. NEMESIS: Noninferiority, Individual-Patient Metaanalysis of Selective Internal Radiation Therapy with (90)Y Resin Microspheres Versus Sorafenib in Advanced Hepatocellular Carcinoma. J. Nucl. Med. 2020, 61, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Pitton, M.B.; Kloeckner, R.; Ruckes, C.; Wirth, G.M.; Eichhorn, W.; Worns, M.A.; Weinmann, A.; Schreckenberger, M.; Galle, P.R.; Otto, G.; et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2015, 38, 352–360. [Google Scholar] [CrossRef]
- Ricke, J.; Klumpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; de Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovic, P.; et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef]
- De La Torre-Alaez, M.; Matilla, A.; Varela, M.; Inarrairaegui, M.; Reig, M.; Lledo, J.; Arenas, J.; Lorente, S.; Testillano, M.; Gomez-Martin, C.; et al. Nivolumab after selective internal radiation therapy using sir spheres resin microspheres in patients with hepatcocellular carcinoma: The NASIR HCC trial. In Proceedings of the International Liver Cancer Association 2020 Virtual Conference, Oral Communication, Virtual Conference, 11–13 September 2020. [Google Scholar]
- Tai, D.; Loke, K.; Gogna, A.; Kaya, N.; Tan, S.; Hennedige, T.; Ng, D.; Irani, F.; Lee, J.; Lim, J.; et al. Radioembolization with Y90-resin micropsheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): A single arm, single centre, phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 1025–1035. [Google Scholar] [CrossRef]
- Lee, Y.H.; Tai, D.; Yip, C.; Choo, S.P.; Chew, V. Combinational Immunotherapy for Hepatocellular Carcinoma: Radiotherapy, Immune Checkpoint Blockade and Beyond. Front. Immunol. 2020, 11, 568759. [Google Scholar] [CrossRef] [PubMed]
- Strigari, L.; Sciuto, R.; Rea, S.; Carpanese, L.; Pizzi, G.; Soriani, A.; Iaccarino, G.; Benassi, M.; Ettorre, G.M.; Maini, C.L. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: Radiobiologic considerations. J. Nucl. Med. 2010, 51, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.L.; Dieudonne, A.; Ronot, M.; Sanchez, M.; Pereira, H.; Chatellier, G.; Garin, E.; Castera, L.; Lebtahi, R.; Vilgrain, V.; et al. Relationship of Tumor Radiation-absorbed Dose to Survival and Response in Hepatocellular Carcinoma Treated with Transarterial Radioembolization with (90)Y in the SARAH Study. Radiology 2020, 296, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Dewaraja, Y.K.; Devasia, T.; Kaza, R.K.; Mikell, J.K.; Owen, D.; Roberson, P.L.; Schipper, M.J. Prediction of Tumor Control in (90)Y Radioembolization by Logit Models with PET/CT-Based Dose Metrics. J. Nucl. Med. 2020, 61, 104–111. [Google Scholar] [CrossRef]
- D’Abadie, P.; Hesse, M.; Jamar, F.; Lhommel, R.; Walrand, S. (90)Y TOF-PET based EUD reunifies patient survival prediction in resin and glass microspheres radioembolization of HCC tumours. Phys. Med. Biol. 2018, 63, 245010. [Google Scholar] [CrossRef]
- Chiesa, C.; Maccauro, M.; Romito, R.; Spreafico, C.; Pellizzari, S.; Negri, A.; Sposito, C.; Morosi, C.; Civelli, E.; Lanocita, R.; et al. Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with (90)Y microspheres: The experience of the National Tumor Institute of Milan. Q. J. Nucl. Med. Mol. Imaging 2011, 55, 168–197. [Google Scholar]
- Garin, E.; Lenoir, L.; Rolland, Y.; Edeline, J.; Mesbah, H.; Laffont, S.; Poree, P.; Clement, B.; Raoul, J.L.; Boucher, E. Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microspheres: Preliminary results. J. Nucl. Med. 2012, 53, 255–263. [Google Scholar] [CrossRef]
- Garin, E.; Rolland, Y.; Pracht, M.; Le Sourd, S.; Laffont, S.; Mesbah, H.; Haumont, L.A.; Lenoir, L.; Rohou, T.; Brun, V.; et al. High impact of macroaggregated albumin-based tumour dose on response and overall survival in hepatocellular carcinoma patients treated with (90) Y-loaded glass microsphere radioembolization. Liver. Int. 2017, 37, 101–110. [Google Scholar] [CrossRef]
- Kappadath, S.C.; Mikell, J.; Balagopal, A.; Baladandayuthapani, V.; Kaseb, A.; Mahvash, A. Hepatocellular Carcinoma Tumor Dose Response After (90)Y-radioembolization With Glass Microspheres Using (90)Y-SPECT/CT-Based Voxel Dosimetry. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 451–461. [Google Scholar] [CrossRef]
- Allimant, C.; Kafrouni, M.; Delicque, J.; Ilonca, D.; Cassinotto, C.; Assenat, E.; Ursic-Bedoya, J.; Pageaux, G.P.; Mariano-Goulart, D.; Aho, S.; et al. Tumor Targeting and Three-Dimensional Voxel-Based Dosimetry to Predict Tumor Response, Toxicity, and Survival after Yttrium-90 Resin Microsphere Radioembolization in Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2018, 29, 1662–1670 e1664. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.T.; Alessio, A.M.; Johnson, G.E.; Vaidya, S.; Kwan, S.W.; Monsky, W.; Wilson, A.E.; Lewis, D.H.; Padia, S.A. Prospective Trial Using Internal Pair-Production Positron Emission Tomography to Establish the Yttrium-90 Radioembolization Dose Required for Response of Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol Phys. 2018, 101, 358–365. [Google Scholar] [CrossRef] [PubMed]
- D’Abadie, P.; Walrand, S.; Hesse, M.; Annet, L.; Borbath, I.; Van den Eynde, M.; Lhommel, R.; Jamar, F. Prediction of tumor response and patient outcome after radioembolization of hepatocellular carcinoma using 90Y-PET-computed tomography dosimetry. Nucl. Med. Commun. 2021, 42, 747–754. [Google Scholar] [CrossRef]
- Son, M.H.; Ha, L.N.; Bang, M.H.; Bae, S.; Giang, D.T.; Thinh, N.T.; Paeng, J.C. Diagnostic and prognostic value of (99m)Tc-MAA SPECT/CT for treatment planning of (90)Y-resin microsphere radioembolization for hepatocellular carcinoma: Comparison with planar image. Sci. Rep. 2021, 11, 3207. [Google Scholar] [CrossRef] [PubMed]
- Nodari, G.; Popoff, R.; Riedinger, J.M.; Lopez, O.; Pellegrinelli, J.; Dygai-Cochet, I.; Tabouret-Viaud, C.; Presles, B.; Chevallier, O.; Gehin, S.; et al. Impact of contouring methods on pre-treatment and post-treatment dosimetry for the prediction of tumor control and survival in HCC patients treated with selective internal radiation therapy. EJNMMI Res. 2021, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Kafrouni, M.; Allimant, C.; Fourcade, M.; Vauclin, S.; Delicque, J.; Ilonca, A.D.; Guiu, B.; Manna, F.; Molinari, N.; Mariano-Goulart, D.; et al. Retrospective Voxel-Based Dosimetry for Assessing the Ability of the Body-Surface-Area Model to Predict Delivered Dose and Radioembolization Outcome. J. Nucl. Med. 2018, 59, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- D’Abadie, P.; Walrand, S.; Hesse, M.; Amini, N.; Lhommel, R.; Sawadogo, K.; Jamar, F. Accurate non-tumoral 99mTc-MAA absorbed dose prediction to plan optimized activities in liver radioembolization using resin microspheres. Phys. Med. 2021, 89, 250–257. [Google Scholar] [CrossRef]
- Garin, E.; Palard, X.; Rolland, Y. Personalised Dosimetry in Radioembolisation for HCC: Impact on Clinical Outcome and on Trial Design. Cancers 2020, 12, 1557. [Google Scholar] [CrossRef]
- Chiesa, C.; Mira, M.; Bhoori, S.; Bormolini, G.; Maccauro, M.; Spreafico, C.; Cascella, T.; Cavallo, A.; De Nile, M.C.; Mazzaglia, S.; et al. Radioembolization of hepatocarcinoma with (90)Y glass microspheres: Treatment optimization using the dose-toxicity relationship. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3018–3032. [Google Scholar] [CrossRef]
- Chiesa, C.; Sjogreen-Gleisner, K.; Walrand, S.; Strigari, L.; Flux, G.; Gear, J.; Stokke, C.; Gabina, P.M.; Bernhardt, P.; Konijnenberg, M. EANM dosimetry committee series on standard operational procedures: A unified methodology for (99m)Tc-MAA pre- and (90)Y peri-therapy dosimetry in liver radioembolization with (90)Y microspheres. EJNMMI Phys. 2021, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Abouchaleh, N.; Gabr, A.; Ali, R.; Al Asadi, A.; Mora, R.A.; Kallini, J.R.; Mouli, S.; Riaz, A.; Lewandowski, R.J.; Salem, R. (90)Y Radioembolization for Locally Advanced Hepatocellular Carcinoma with Portal Vein Thrombosis: Long-Term Outcomes in a 185-Patient Cohort. J. Nucl. Med. 2018, 59, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Zu, Q.; Schenning, R.C.; Jahangiri, Y.; Tomozawa, Y.; Kolbeck, K.J.; Kaufman, J.A.; Al-Hakim, R.; Naugler, W.E.; Nabavizadeh, N.; Kardosh, A.; et al. Yttrium-90 Radioembolization for BCLC Stage C Hepatocellular Carcinoma Comparing Child-Pugh A Versus B7 Patients: Are the Outcomes Equivalent? Cardiovasc. Intervent. Radiol. 2020, 43, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef]
- Garin, E.; Rolland, Y.; Edeline, J.; Icard, N.; Lenoir, L.; Laffont, S.; Mesbah, H.; Breton, M.; Sulpice, L.; Boudjema, K.; et al. Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J. Nucl. Med. 2015, 56, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, C.; Sposito, C.; Vaiani, M.; Cascella, T.; Bhoori, S.; Morosi, C.; Lanocita, R.; Romito, R.; Chiesa, C.; Maccauro, M.; et al. Development of a prognostic score to predict response to Yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J. Hepatol. 2018, 68, 724–732. [Google Scholar] [CrossRef]
- De Graaf, W.; van Lienden, K.P.; Dinant, S.; Roelofs, J.J.; Busch, O.R.; Gouma, D.J.; Bennink, R.J.; van Gulik, T.M. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J. Gastrointest. Surg. 2010, 14, 369–378. [Google Scholar] [CrossRef]
- Braat, M.; de Jong, H.W.; Seinstra, B.A.; Scholten, M.V.; van den Bosch, M.; Lam, M. Hepatobiliary scintigraphy may improve radioembolization treatment planning in HCC patients. EJNMMI Res. 2017, 7, 2. [Google Scholar] [CrossRef][Green Version]
- Bennink, R.J.; Cieslak, K.P.; van Delden, O.M.; van Lienden, K.P.; Klumpen, H.J.; Jansen, P.L.; van Gulik, T.M. Monitoring of Total and Regional Liver Function after SIRT. Front. Oncol. 2014, 4, 152. [Google Scholar] [CrossRef]
- Tong, A.K.; Kao, Y.H.; Too, C.W.; Chin, K.F.; Ng, D.C.; Chow, P.K. Yttrium-90 hepatic radioembolization: Clinical review and current techniques in interventional radiology and personalized dosimetry. Br. J. Radiol. 2016, 89, 20150943. [Google Scholar] [CrossRef]
- Louie, J.D.; Kothary, N.; Kuo, W.T.; Hwang, G.L.; Hofmann, L.V.; Goris, M.L.; Iagaru, A.H.; Sze, D.Y. Incorporating cone-beam CT into the treatment planning for yttrium-90 radioembolization. J. Vasc. Interv. Radiol. 2009, 20, 606–613. [Google Scholar] [CrossRef] [PubMed]
- D’Abadie, P.; Walrand, S.; Goffette, P.; Amini, N.; Maanen, A.V.; Lhommel, R.; Jamar, F. Antireflux catheter improves tumor targeting in liver radioembolization with resin microspheres. Diagn. Interv. Radiol. 2021, 27, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Imaoka, S.; Hasegawa, Y.; Nakano, S.; Ishikawa, O.; Ohigashi, H.; Taniguchi, K.; Koyama, H.; Iwanaga, T.; Terasawa, T. Changes in distribution of hepatic blood flow induced by intra-arterial infusion of angiotensin II in human hepatic cancer. Cancer 1985, 55, 311–316. [Google Scholar] [CrossRef]
- Macedo, M.P.; Lautt, W.W. Shear-induced modulation of vasoconstriction in the hepatic artery and portal vein by nitric oxide. Am. J. Physiol. 1998, 274, G253–G260. [Google Scholar] [CrossRef]
- Walrand, S.; Hesse, M.; d’Abadie, P.; Jamar, F. Hepatic Arterial Buffer Response in Liver Radioembolization and Potential Use for Improved Cancer Therapy. Cancers 2021, 13, 1537. [Google Scholar] [CrossRef]
- Lautt, W.W. Hepatic Circulation: Physiology and Pathophysiology. In Colloquium Series on Integrated Systems Physiology: From Molecule to Function to Disease; Morgan & Claypool: San Rafael, CA, USA, 2009. [Google Scholar]
- Biro, G.P.; Douglas, J.R.; Keon, W.J.; Taichman, G.C. Changes in regional blood flow distribution induced by infusions of dopexamine hydrochloride or dobutamine in anesthetized dogs. Am. J. Cardiol. 1988, 62, 30C–36C. [Google Scholar] [CrossRef]
- Fitton, A.; Benfield, P. Dopexamine hydrochloride. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in acute cardiac insufficiency. Drugs 1990, 39, 308–330. [Google Scholar] [CrossRef]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonne, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur.J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Rich, N.E.; John, B.V.; Parikh, N.D.; Rowe, I.; Mehta, N.; Khatri, G.; Thomas, S.M.; Anis, M.; Mendiratta-Lala, M.; Hernandez, C.; et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients with Cirrhosis. Hepatology 2020, 72, 1654–1665. [Google Scholar] [CrossRef]
- Sacks, A.; Peller, P.J.; Surasi, D.S.; Chatburn, L.; Mercier, G.; Subramaniam, R.M. Value of PET/CT in the management of primary hepatobiliary tumors, part 2. AJR Am. J. Roentgenol. 2011, 197, W260–W265. [Google Scholar] [CrossRef]
- Sun, D.W.; An, L.; Wei, F.; Mu, L.; Shi, X.J.; Wang, C.L.; Zhao, Z.W.; Li, T.F.; Lv, G.Y. Prognostic significance of parameters from pretreatment (18)F-FDG PET in hepatocellular carcinoma: A meta-analysis. Abdom. Radiol. 2016, 41, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Abuodeh, Y.; Naghavi, A.O.; Ahmed, K.A.; Venkat, P.S.; Kim, Y.; Kis, B.; Choi, J.; Biebel, B.; Sweeney, J.; Anaya, D.A.; et al. Prognostic value of pre-treatment F-18-FDG PET-CT in patients with hepatocellular carcinoma undergoing radioembolization. World J. Gastroenterol. 2016, 22, 10406–10414. [Google Scholar] [CrossRef] [PubMed]
- Jreige, M.; Mitsakis, P.; Van Der Gucht, A.; Pomoni, A.; Silva-Monteiro, M.; Gnesin, S.; Boubaker, A.; Nicod-Lalonde, M.; Duran, R.; Prior, J.O.; et al. (18)F-FDG PET/CT predicts survival after (90)Y transarterial radioembolization in unresectable hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Antkowiak, M.; Gabr, A.; Das, A.; Ali, R.; Kulik, L.; Ganger, D.; Moore, C.; Abecassis, M.; Katariya, N.; Mouli, S.; et al. Prognostic Role of Albumin, Bilirubin, and ALBI Scores: Analysis of 1000 Patients with Hepatocellular Carcinoma Undergoing Radioembolization. Cancers 2019, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Abuodeh, Y.; Jin, W.; Frakes, J.; Friedman, M.; Biebel, B.; Choi, J.; El-Haddad, G.; Kis, B.; Sweeney, J.; et al. Using the Albumin-Bilirubin (ALBI) grade as a prognostic marker for radioembolization of hepatocellular carcinoma. J. Gastrointest. Oncol. 2018, 9, 840–846. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2021, 76, 862–873. [Google Scholar] [CrossRef]
- Lescure, C.; Estrade, F.; Pedrono, M.; Campillo-Gimenez, B.; Le Sourd, S.; Pracht, M.; Palard, X.; Bourien, H.; Muzellec, L.; Uguen, T.; et al. ALBI Score Is a Strong Predictor of Toxicity Following SIRT for Hepatocellular Carcinoma. Cancers 2021, 13, 3794. [Google Scholar] [CrossRef]
- Ali, R.; Gabr, A.; Abouchaleh, N.; Al Asadi, A.; Mora, R.A.; Kulik, L.; Abecassis, M.; Riaz, A.; Salem, R.; Lewandowski, R.J. Survival Analysis of Advanced HCC Treated with Radioembolization: Comparing Impact of Clinical Performance Status Versus Vascular Invasion/Metastases. Cardiovasc. Intervent. Radiol. 2018, 41, 260–269. [Google Scholar] [CrossRef] [PubMed]
| Studies | Groups | Nb of Patients | BCLC Score | Adverse Events (≥Grade 3) | RR | TTP (mo) | PFS (mo) | OS (mo) |
|---|---|---|---|---|---|---|---|---|
| Pitton et al., 2015 [22] | SIRT (resin) | 12 | B: 100% | NA | NA | 12.4 | 6 | 19.7 |
| TACE | 12 | A: 8% B: 92% | NA | NA | 11.2 | 7.2 | 26.3 | |
| Salem et al., 2016 [17] | SIRT (glass) | 24 | A: 75% B: 25% | NA | 87% | >26 * | NA | 18.6 |
| TACE | 21 | A: 81% B: 19% | NA | 74% | 4.8 | NA | 17.7 | |
| SARAH [19] | SIRT (resin) | 237 | C: 100% | 41% | 19% * | NA | 4.1 | 9.9 |
| Sorafenib | 222 | C: 100% | 63% * | 12% | NA | 3.7 | 9.9 | |
| SIRveNIB [20] | SIRT (resin) | 130 | B: 61% C: 39% | 28% | 23% * | 6.1 | 6.3 | 8.8 |
| sorafenib | 162 | B: 54% C: 45% | 51% * | 2% | 5.4 | 5.2 | 10 | |
| SORAMIC [23] | SIRT (resin) + sorafenib | 114 | A: 4% B: 28% C: 68% | 65% * | NA | NA | NA | 14 |
| sorafenib | 174 | A: 2% B: 28% C: 70% | 54% | NA | NA | NA | 11.1 |
| Study | Study Design | Type of Microspheres | Nb of Patients | Correlation with Radiological Response | Correlation with PFS | Correlation with OS |
|---|---|---|---|---|---|---|
| Strigari et al., 2010 [27] | Retrospective | Resin | 73 | ✓ | NA | NA |
| Chiesa et al., 2011 [31] | Retrospective | Glass | 46 | ✓ | NA | NA |
| Garin et al., 2012 [32] | Retrospective | Glass | 36 | ✓ | ✓ | ✓ |
| Garin et al., 2017 [33] | Retrospective | Glass | 85 | ✓ | NA | ✓ |
| Kappadath et al., 2018 [34] | Retrospective | Glass | 34 | ✓ | NA | NA |
| Allimant et al., 2018 [35] | Retrospective | Resin | 38 | ✓ | ✓ | NA |
| Chan et al., 2018 [36] | Prospective | Glass | 27 | ✓ | NA | NA |
| Hermann et al., 2020 [28] | Prospective + | Resin | 121 | ✓ | NA | ✓ |
| Dewaraja et al., 2020 [29] | Retrospective | Glass | 28 | ✓ | NA | NA |
| d’Abadie et al., 2021 [37] | Retrospective | Resin and glass | 45 | ✓ | ✓ | ✓ |
| Son et al., 2021 [38] | Prospective + | Resin | 34 | ✓ | NA | NA |
| Nodari et al., 2021 [39] | Retrospective | Resin and glass | 48 | ✓ | NA | ✓ |
| Garin et al., 2021 [40] | Prospective, randomized, multicenter | Glass | 56 | ✓ | ✓ | ✓ |
| Study | Nb of Patients | Nb of Tumors | Dosimetry Performed with | Criteria for Radiological Response Assessment | TD Threshold for Radiological Response | Median PFS above and under the TD Threshold | Median OS above and under the TD Threshold |
|---|---|---|---|---|---|---|---|
| Chiesa et al., 2011 [31] | 46 | 91 | MAA SPECT/CT | EASL | 257 Gy (Se: 85%, Sp: 70%) | NA | NA |
| Garin et al., 2012 [32] | 36 | 58 | MAA SPECT/CT | EASL | 205 Gy (Se: 100%, Sp: 75%) | 14 mo vs. 5.2 mo * | 18 mo vs. 9 mo * |
| Garin et al., 2017 [33] | 85 | 132 | MAA SPECT/CT | EASL | 205 Gy (Se: 98%, Sp NA) | NA | 21 mo vs. 6.5 mo * |
| Kappadath et al., 2018 [34] | 34 | 53 | 90Y SPECT/CT | modified RECIST 1.1 | 160 Gy (50% response) | NA | NA |
| Chan et al., 2018 [36] | 27 | 38 | 90Y PET/CT | modified RECIST 1.1 | 200 Gy (Se: 66%, Sp: 100%) | NA | NA |
| d’Abadie et al., 2021 [37] | 26 | 73 | 90Y PET/CT | modified RECIST 1.1 | 118 Gy (Se: 93%, Sp: 75%) | 5.5 mo vs. 1.8 mo * | 14.6 mo vs. 5.5 mo * |
| Nodari et al., 2021 [39] | 23 | NA | 90Y PET/CT | NA | 156 Gy (Se and Sp NA) | NA | 23 mo vs. 14 mo * |
| Study | Nb of Patients | Nb of Tumors | Dosimetry Performed with | Criteria for Radiological Response Assessment | TD Thresholdfor Radiological Response | Median PFS above and under the TD Threshold | Median OS above and under the TD Threshold |
|---|---|---|---|---|---|---|---|
| Allimant et al., 2018 [35] | 38 | 42 | 90Y PET/CT | modified RECIST 1.1 | 61 Gy (Se: 76%, Sp: 75%) | 12.1 mo vs. 6.3 mo *+ | NA |
| Hermann et al., 2020 [28] | 121 | NA | MAA SPECT/CT | RECIST 1.1 | 100 Gy (72% response) | NA | 14.1 mo vs. 6.1 mo * |
| d’Abadie et al., 2021 [37] | 19 | 60 | 90Y PET/CT | modified RECIST 1.1 | 61 Gy (Se: 87%, Sp: 64%) | 4.6 mo vs. 1.6 mo * | 16 mo vs. 5.3 mo * |
| Son et al.,2021 [38] | 34 | 45 | MAA SPECT/CT | modified RECIST 1.1 | 125 Gy (Se: 86%, Sp: 75%) | NA | NA |
| Nodari et al., 2021 [39] | 25 | NA | 90Y PET/CT | NA | 98 Gy (Se and Sp NA) | NA | 23 mo vs. 14 mo * |
| Personalized Dosimetry | Standard Dosimetry | |
|---|---|---|
| Number of patients | 28 | 28 |
| Activity planned in GBq, median | 3.6 * | 2.6 |
| Response rate at 3 mo, EASL criteria | 71% * | 36% |
| Curative surgery intent after SIRT | 36% * | 4% |
| REILD | 9% | 10% |
| Overall survival in mo, median | 26.6 + | 10.7 |
| Study | Nb of Patients | Parameter Related to Worse Prognosis | Median Survival (95% CI Interval) |
|---|---|---|---|
| Ali et al., 2018 [76] | 547 | ECOG 2 | 4.3 mo (2.5–7.8) |
| Extrahepatic metastases | 7.4 mo (6.0–9.0) | ||
| PVT | 7.3 mo (6.3–8.0) | ||
| Spreafico et al., 2018 [50] | 120 | Bilirubin > 1.2 mg/dL | 9.5 mo (8.8–10.2) |
| PVT extended to right/left main branch | 8.2 mo (5.7–10.8) | ||
| Tumor burden > 50% liver volume | 6.4 mo (5.2–7.6) | ||
| Abouchaleh et al., 2018 [46] | 185 | ECOG 2 | 2.5 mo (2–4.6) |
| Bilirubin 2–3 mg/dL | 5 mo (2.2–9.7) | ||
| PVT extended to right/left main branch | 7.7 mo (5.3–10.4) | ||
| Antkowiak et al., 2019 [69] | 541 | Bilirubin 2–3 mg/dL | 8 mo (6.7–21) |
| ALBI grade 3 | 6.7 mo (5.7–8.8) | ||
| Zu et al., 2020 [47] | 91 | CHILD B7 | 6 mo (4.4–7.6) |
| Lescure et al., 2021 [75] | 222 | ALBI grade 3 | 8.1 mo (4.1–12.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Abadie, P.; Walrand, S.; Lhommel, R.; Hesse, M.; Borbath, I.; Jamar, F. Optimization of the Clinical Effectiveness of Radioembolization in Hepatocellular Carcinoma with Dosimetry and Patient-Selection Criteria. Curr. Oncol. 2022, 29, 2422-2434. https://doi.org/10.3390/curroncol29040196
d’Abadie P, Walrand S, Lhommel R, Hesse M, Borbath I, Jamar F. Optimization of the Clinical Effectiveness of Radioembolization in Hepatocellular Carcinoma with Dosimetry and Patient-Selection Criteria. Current Oncology. 2022; 29(4):2422-2434. https://doi.org/10.3390/curroncol29040196
Chicago/Turabian Styled’Abadie, Philippe, Stephan Walrand, Renaud Lhommel, Michel Hesse, Ivan Borbath, and François Jamar. 2022. "Optimization of the Clinical Effectiveness of Radioembolization in Hepatocellular Carcinoma with Dosimetry and Patient-Selection Criteria" Current Oncology 29, no. 4: 2422-2434. https://doi.org/10.3390/curroncol29040196
APA Styled’Abadie, P., Walrand, S., Lhommel, R., Hesse, M., Borbath, I., & Jamar, F. (2022). Optimization of the Clinical Effectiveness of Radioembolization in Hepatocellular Carcinoma with Dosimetry and Patient-Selection Criteria. Current Oncology, 29(4), 2422-2434. https://doi.org/10.3390/curroncol29040196





