Acute Lymphoblastic Leukemia Developing in a Patient with 46, XY Pure Gonadal Dysgenesis (Swyer Syndrome) with Malignant Gonadal Germ Cell Tumor: A Case Report and Literature Review
Abstract
:1. Introduction
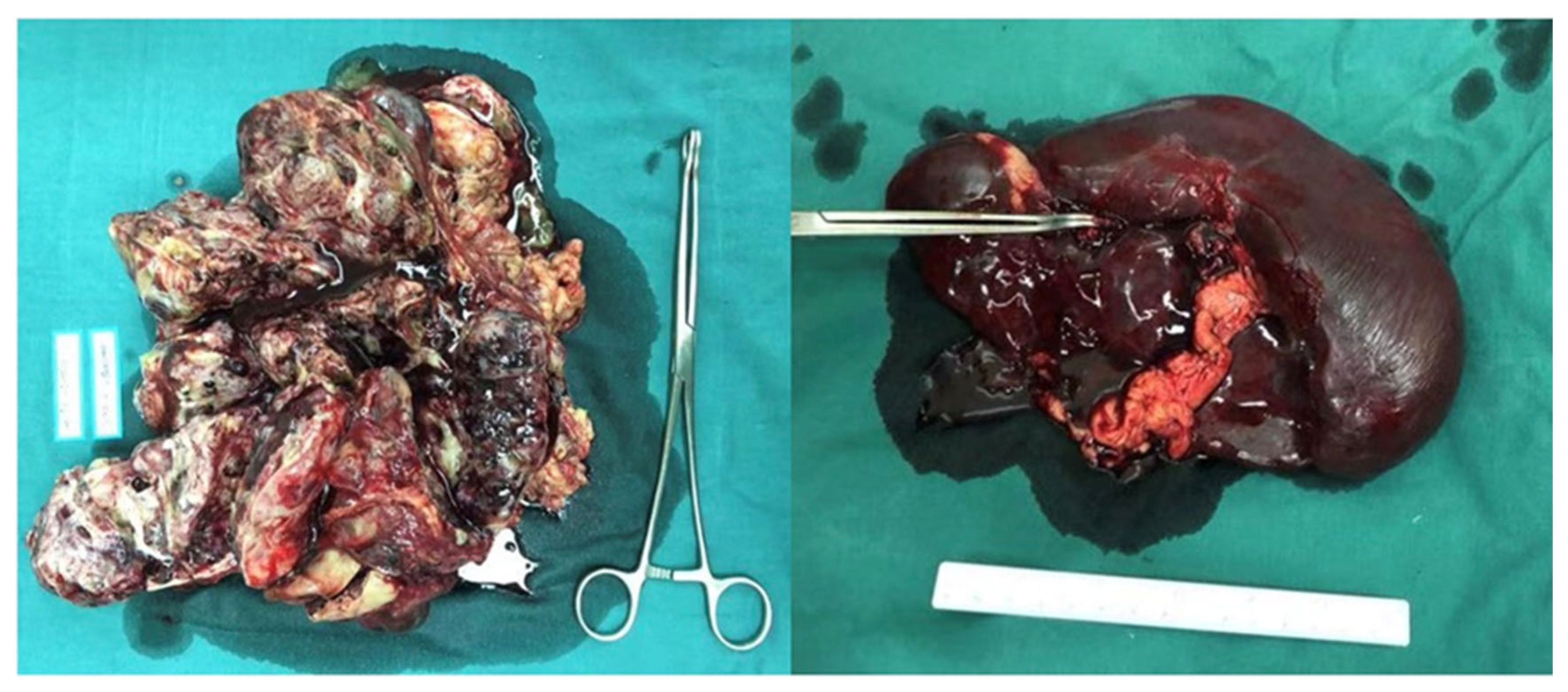
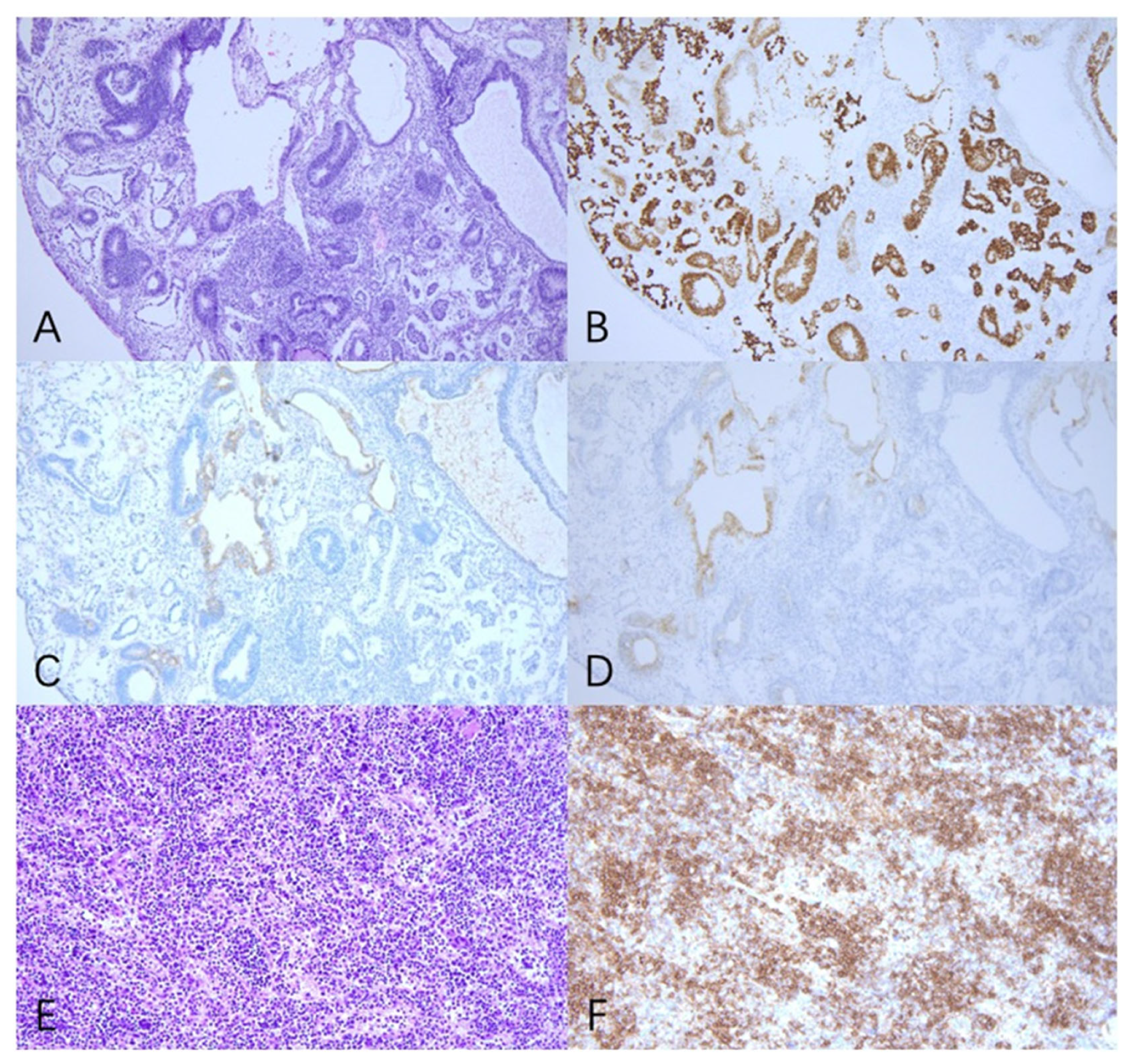
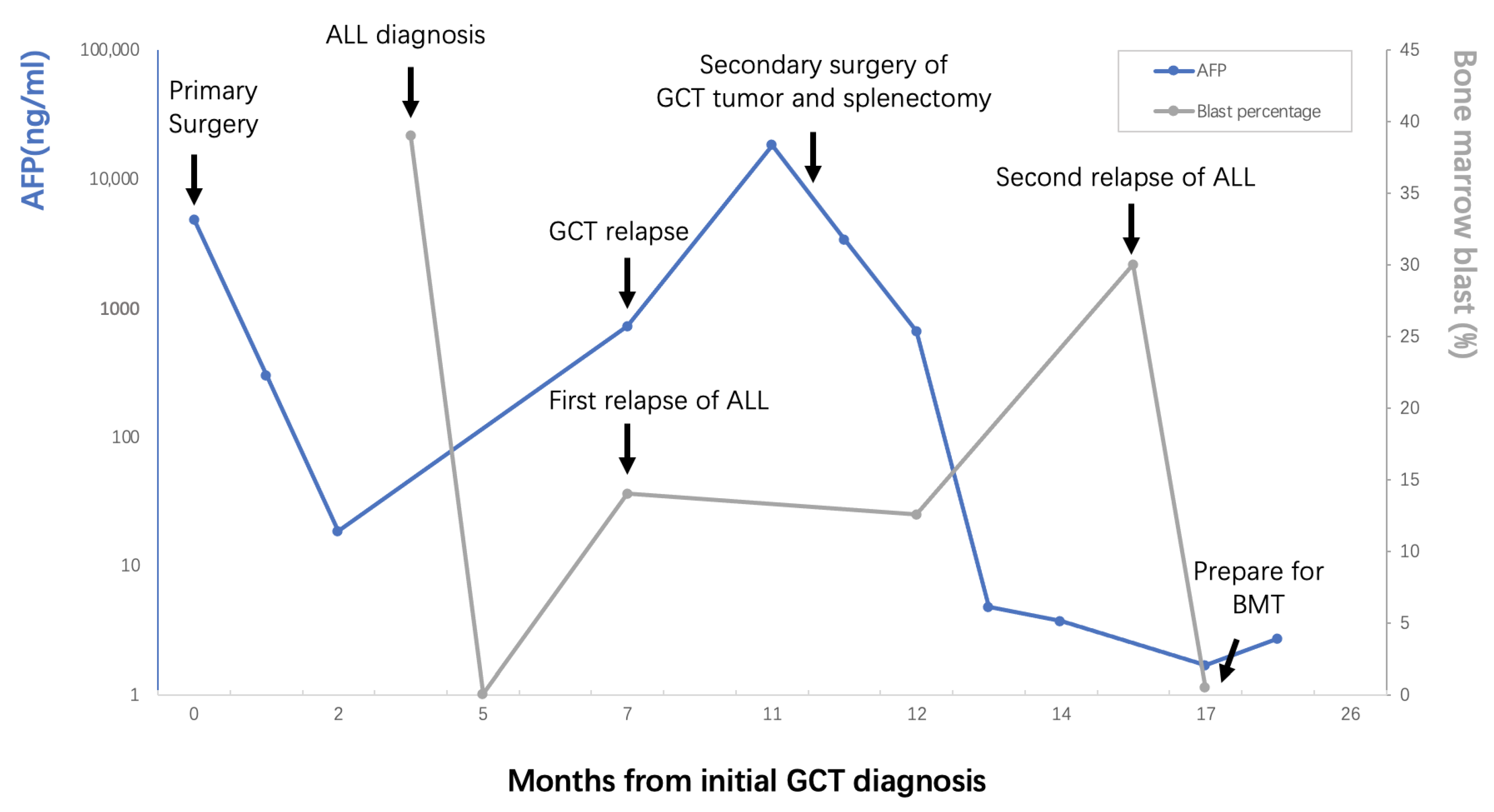
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pauls, K.; Franke, F.E.; Buttner, R.; Zhou, H. Gonadoblastoma: Evidence for a stepwise progression to dysgerminoma in a dysgenetic ovary. Virchows Arch. 2005, 447, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv1–iv18. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [Green Version]
- King, T.F.; Conway, G.S. Swyer syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 504–510. [Google Scholar] [CrossRef] [Green Version]
- McCann-Crosby, B.; Mansouri, R.; Dietrich, J.E.; McCullough, L.B.; Sutton, V.R.; Austin, E.G.; Schlomer, B.; Roth, D.R.; Karaviti, L.; Gunn, S.; et al. State of the art review in gonadal dysgenesis: Challenges in diagnosis and management. Int. J. Pediatr. Endocrinol. 2014, 2014, 4. [Google Scholar] [CrossRef]
- Andersen, M.K.; Christiansen, D.H.; Jensen, B.A.; Ernst, P.; Hauge, G.; Pedersen-Bjergaard, J. Therapy-related acute lymphoblastic leukaemia with MLL rearrangements following DNA topoisomerase II inhibitors, an increasing problem: Report on two new cases and review of the literature since 1992. Br. J. Haematol. 2001, 114, 539–543. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Freund, M.; Schmoll, H.J.; Rieder, H.; Fonatsch, C. Secondary lymphoblastic leukemia following treatment of a malignant germ cell tumour. Ann. Oncol. 1992, 3, 772. [Google Scholar] [CrossRef]
- Downie, P.A.; Vogelzang, N.J.; Moldwin, R.L.; Le Beau, M.M.; Anastasi, J.; Allen, R.J.; Myers, S.E.; Larson, R.A.; Smith, S.D. Establishment of a leukemia cell line with i(12p) from a patient with a mediastinal germ cell tumor and acute lymphoblastic leukemia. Cancer Res. 1994, 54, 4999–5004. [Google Scholar]
- Larsen, M.; Evans, W.K.; Shepherd, F.A.; Phillips, M.J.; Bailey, D.; Messner, H. Acute lymphoblastic leukemia. Possible origin from a mediastinal germ cell tumor. Cancer 1984, 53, 441–444. [Google Scholar] [CrossRef]
- Okamura, A.; Wakahashi, K.; Ishii, S.; Katayama, Y.; Yamamoto, K.; Matsui, T. Possible alternative strategy for stage I imatinib-sensitive testicular seminoma; lessons from a case associated with Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann. Oncol. 2010, 21, 1129–1130. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Gilbert, E.; Lynch, C.F.; Hall, P.; Storm, H.; Holowaty, E.; Pukkala, E.; Langmark, F.; Kaijser, M.; Andersson, M.; et al. Risk of leukemia among survivors of testicular cancer: A population-based study of 42,722 patients. Ann. Epidemiol. 2008, 18, 416–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.C.; Luedke, D.W.; Sapiente, R.A.; Naidu, R.G. Acute lymphocytic leukemia developing in a male with germ cell carcinoma: A case report. Med. Pediatr. Oncol. 1980, 8, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.; Murali, K.; Ahmad, A.; Hockings, H.; Graham, R.; Liberale, V.; Sarker, S.J.; Ledermann, J.; Berney, D.M.; Shamash, J.; et al. A multicentre retrospective cohort study of ovarian germ cell tumours: Evidence for chemotherapy de-escalation and alignment of paediatric and adult practice. Eur. J. Cancer 2019, 113, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, S.S.; Bornstein, S.G.; Christopherson, W.A.; Surti, U. Associated leukemia and mixed germ cell tumor in a patient with gonadal dysgenesis. Int. J. Gynaecol. Obs. 1991, 35, 83–88. [Google Scholar] [CrossRef]
- Cudennec, C.; Nicolas, J.F. Blood formation in a clonal cell line of mouse teratocarcinoma. J. Embryol. Exp. Morphol. 1977, 38, 203–210. [Google Scholar] [CrossRef]
- Suzuki, N.; Yamazaki, S.; Yamaguchi, T.; Okabe, M.; Masaki, H.; Takaki, S.; Otsu, M.; Nakauchi, H. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol. Ther. 2013, 21, 1424–1431. [Google Scholar] [CrossRef] [Green Version]
- Leonard, J.T.; Raess, P.W.; Dunlap, J.; Hayes-Lattin, B.; Tyner, J.W.; Traer, E. Functional and genetic screening of acute myeloid leukemia associated with mediastinal germ cell tumor identifies MEK inhibitor as an active clinical agent. J. Hematol. Oncol. 2016, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Singla, N.; Lafin, J.T.; Ghandour, R.A.; Kaffenberger, S.; Amatruda, J.F.; Bagrodia, A. Genetics of testicular germ cell tumors. Curr. Opin. Urol. 2019, 29, 344–349. [Google Scholar] [CrossRef]
- Travis, L.B.; Andersson, M.; Gospodarowicz, M.; van Leeuwen, F.E.; Bergfeldt, K.; Lynch, C.F.; Curtis, R.E.; Kohler, B.A.; Wiklund, T.; Storm, H.; et al. Treatment-associated leukemia following testicular cancer. J. Natl. Cancer Inst. 2000, 92, 1165–1171. [Google Scholar] [CrossRef] [Green Version]
- Pedersen-Bjergaard, J.; Daugaard, G.; Hansen, S.W.; Philip, P.; Larsen, S.O.; Rorth, M. Increased risk of myelodysplasia and leukaemia after etoposide, cisplatin, and bleomycin for germ-cell tumours. Lancet Lond. Engl. 1991, 338, 359–363. [Google Scholar] [CrossRef]
- Spencer Chapman, M.; May, P.C.; Olavarria, E.; Nadal Melsio, E. Three distinct hematological malignancies from a single germ cell tumor: A case report. J. Med. Case Rep. 2020, 14, 222. [Google Scholar] [CrossRef] [PubMed]
| Author/Year of Publication | Case | Age | Histologic Type | Stage | Time | Reason of All | Outcome |
|---|---|---|---|---|---|---|---|
| Johnson et al. 1980 [13] | 1 | 23 | Mixed GCT (SE, EC, MT, IT) | - | 11 m | GCT | Died from infection |
| Larsen et al. 1984 [10] | 1 | 16 | GCT | - | 5 m | GCT | Died from infection |
| Bokemeyer et al. 1992 [8] | 1 | 42 | SE | IIIB | 16 m | Chemotherapy | Died from infection |
| Downie et al. 1994 [9] | 1 | 26 | YST | - | 5 m | GCT | Died from intracranial hemorrhage |
| Andersen et al. 2001 [7] | 1 | 25 | SE | 18 m | Chemotherapy | - | |
| Howard et al. 2008 [12] | 10 | - | 6 SE, 4 non-SE | - | >12 m | radiotherapy and chemotherapy | - |
| Okamura et al. 2010 [11] | 1 | 42 | SE | I | 0 m | GCT | Received CBT and alive |
| Newton et al. 2019 [14] | 1 | 14 | DG, YST | IIIA | 24 m | chemotherapy | Died from ALL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Y.; Wang, J.; Yang, J.; Yu, S.; Yin, M.; Li, S.; Yang, J. Acute Lymphoblastic Leukemia Developing in a Patient with 46, XY Pure Gonadal Dysgenesis (Swyer Syndrome) with Malignant Gonadal Germ Cell Tumor: A Case Report and Literature Review. Curr. Oncol. 2022, 29, 9753-9759. https://doi.org/10.3390/curroncol29120766
Zhang X, Zhang Y, Wang J, Yang J, Yu S, Yin M, Li S, Yang J. Acute Lymphoblastic Leukemia Developing in a Patient with 46, XY Pure Gonadal Dysgenesis (Swyer Syndrome) with Malignant Gonadal Germ Cell Tumor: A Case Report and Literature Review. Current Oncology. 2022; 29(12):9753-9759. https://doi.org/10.3390/curroncol29120766
Chicago/Turabian StyleZhang, Xinyue, Ying Zhang, Jinhui Wang, Jie Yang, Shuangni Yu, Min Yin, Sijian Li, and Jiaxin Yang. 2022. "Acute Lymphoblastic Leukemia Developing in a Patient with 46, XY Pure Gonadal Dysgenesis (Swyer Syndrome) with Malignant Gonadal Germ Cell Tumor: A Case Report and Literature Review" Current Oncology 29, no. 12: 9753-9759. https://doi.org/10.3390/curroncol29120766
APA StyleZhang, X., Zhang, Y., Wang, J., Yang, J., Yu, S., Yin, M., Li, S., & Yang, J. (2022). Acute Lymphoblastic Leukemia Developing in a Patient with 46, XY Pure Gonadal Dysgenesis (Swyer Syndrome) with Malignant Gonadal Germ Cell Tumor: A Case Report and Literature Review. Current Oncology, 29(12), 9753-9759. https://doi.org/10.3390/curroncol29120766




