Biomarker Discordances and Alterations Observed in Breast Cancer Treated with Neoadjuvant Chemotherapy: Causes, Frequencies, and Clinical Significances
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection and Pathological Evaluations
2.3. Data Evaluation and Statistical Analysis
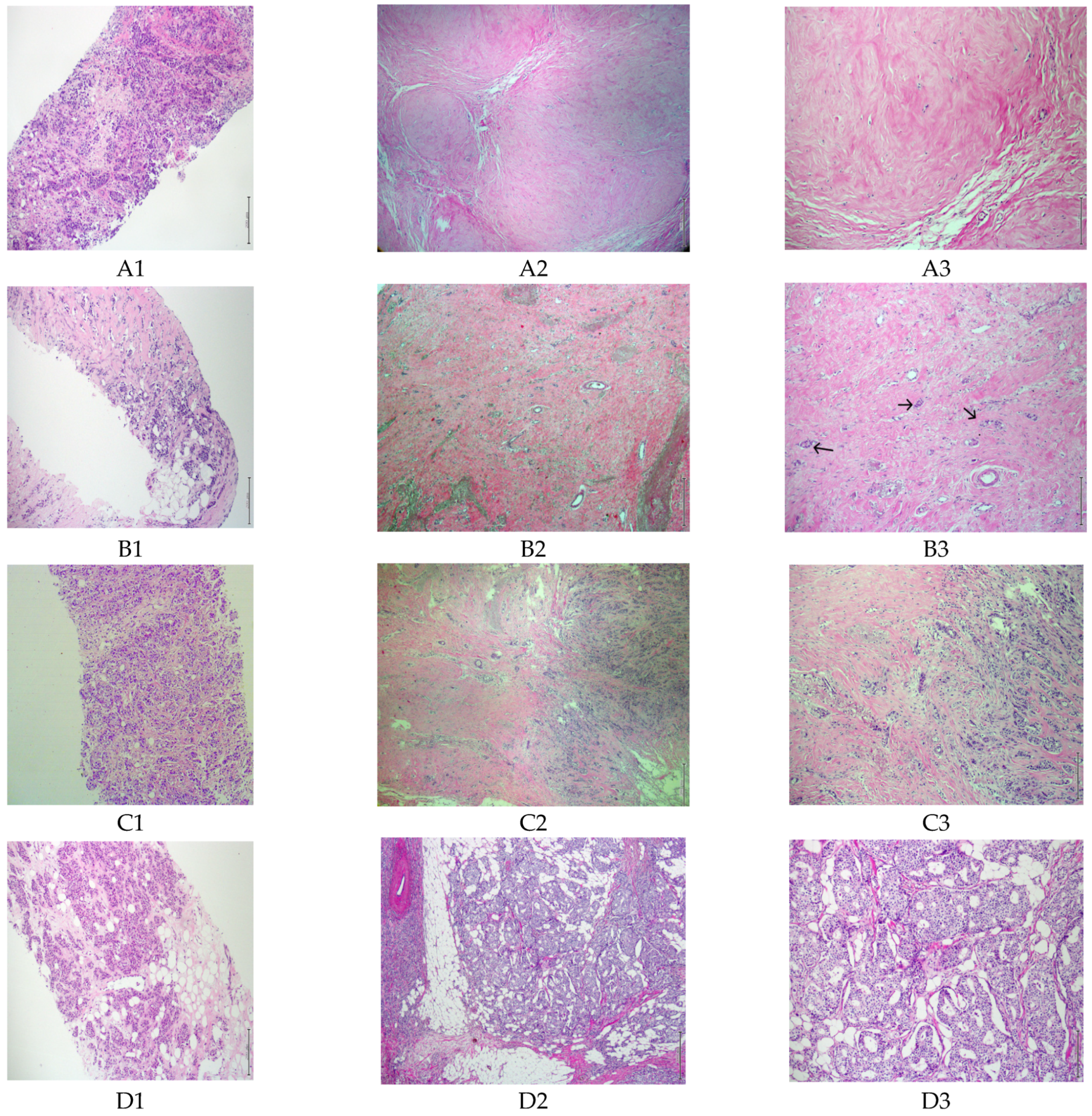
3. Results
3.1. Patients and Clinicopathological Characteristics
3.2. Estrogen Receptor
3.3. Progesterone Receptor
3.4. Hormone Receptor
3.5. Human Epidermal Growth Factor Receptor-2
3.6. Molecular Subtype Changes
3.7. Tumor Grade, Ki-67 Proliferation Index, and ER and PR Expression Levels
3.8. Tumor Grade, Ki-67 Proliferation Index, and ER and PR Expression Levels of Partially Responsive and Unresponsive Patients
3.8.1. Partially Responsive Patients
3.8.2. Unresponsive Patients
4. Discussion
4.1. ER, PR, and HER2 Discordance without NACTx
4.2. ER, PR, and HER2 Discordance after NACTx
4.3. Discordance with Clinical Significance
4.4. Molecular Subtype Changes
4.5. Changes in ER and PR Expression Levels, Tumor Grade, and Ki-67 Proliferation Index before and after NACTx
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HR | Hormone receptor |
| LA | Luminal A |
| FISH | Fluorescence in situ hybridization |
| pCR | Pathological complete response |
| NACTx | Neoadjuvant chemotherapy |
| TN | Triple negative |
| HER2 | Human epidermal growth factor receptor 2 |
| IHC | Immunohistochemical staining |
| MRD | Minimal residual disease |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jayasekera, J.; Mandelblatt, J.S. Systematic Review of the Cost Effectiveness of Breast Cancer Prevention, Screening, and Treatment Interventions. J. Clin. Oncol. 2020, 38, 332–350. [Google Scholar] [CrossRef] [PubMed]
- Klimberg, V.S.; Rivere, A. Ultrasound image-guided core biopsy of the breast. Chin. Clin. Oncol. 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S. Pathological examination of breast cancer biomarkers: Current status in Japan. Breast Cancer 2016, 23, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Seferina, S.C.; Nap, M.; van den Berkmortel, F.; Wals, J.; Voogd, A.C.; Tjan-Heijnen, V.C. Reliability of receptor assessment on core needle biopsy in breast cancer patients. Tumour Biol. 2013, 34, 987–994. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Li, X.; Wang, Q.; Zhou, J.; Shen, J.; Luo, L.; Lu, Y.; Wang, L. Effects of core needle biopsy and subsequent neoadjuvant chemotherapy on molecular alterations and outcome in breast cancer. Onco Targets Ther. 2018, 11, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Xian, Z.; Quinones, A.K.; Tozbikian, G.; Zynger, D.L. Breast cancer biomarkers before and after neoadjuvant chemotherapy: Does repeat testing impact therapeutic management? Hum. Pathol. 2017, 62, 215–221. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, S.; Smit, V.T.; Dekker, T.J.; Nortier, J.W.; Kroep, J.R. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat. Rev. 2011, 37, 422–430. [Google Scholar] [CrossRef]
- Dawood, S.; Gonzalez-Angulo, A.M. Biomarker discordance pre and post neoadjuvant chemotherapy in breast cancer. Cancer Biomark. 2012, 12, 241–250. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Romero, A.; García-Sáenz, J.A.; Fuentes-Ferrer, M.; López Garcia-Asenjo, J.A.; Furió, V.; Román, J.M.; Moreno, A.; de la Hoya, M.; Díaz-Rubio, E.; Martín, M.; et al. Correlation between response to neoadjuvant chemotherapy and survival in locally advanced breast cancer patients. Ann. Oncol. 2013, 24, 655–661. [Google Scholar] [CrossRef]
- Chávez-MacGregor, M.; González-Angulo, A.M. Breast cancer, neoadjuvant chemotherapy and residual disease. Clin. Transl. Oncol. 2010, 12, 461–467. [Google Scholar] [CrossRef]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.B.; Fahey, V.D.; Feleppa, F.; Buchanan, M.R. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J. Clin. Oncol. 2005, 23, 5148–5154. [Google Scholar] [CrossRef] [PubMed]
- Richter-Ehrenstein, C.; Müller, S.; Noske, A.; Schneider, A. Diagnostic accuracy and prognostic value of core biopsy in the management of breast cancer: A series of 542 patients. Int. J. Surg. Pathol. 2009, 17, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Burge, C.N.; Chang, H.R.; Apple, S.K. Do the histologic features and results of breast cancer biomarker studies differ between core biopsy and surgical excision specimens? Breast 2006, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Usami, S.; Moriya, T.; Amari, M.; Suzuki, A.; Ishida, T.; Sasano, H.; Ohuchi, N. Reliability of prognostic factors in breast carcinoma determined by core needle biopsy. Jpn. J. Clin. Oncol. 2007, 37, 250–255. [Google Scholar] [CrossRef]
- Arnedos, M.; Nerurkar, A.; Osin, P.; A’Hern, R.; Smith, I.E.; Dowsett, M. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann. Oncol. 2009, 20, 1948–1952. [Google Scholar] [CrossRef]
- Taucher, S.; Rudas, M.; Mader, R.M.; Gnant, M.; Dubsky, P.; Roka, S.; Bachleitner, T.; Kandioler, D.; Steger, G.; Mittlböck, M.; et al. Prognostic markers in breast cancer: The reliability of HER2/neu status in core needle biopsy of 325 patients with primary breast cancer. Wien. Klin. Wochenschr. 2004, 116, 26–31. [Google Scholar] [CrossRef]
- Cavaliere, A.; Sidoni, A.; Scheibel, M.; Bellezza, G.; Brachelente, G.; Vitali, R.; Bucciarelli, E. Biopathologic profile of breast cancer core biopsy: Is it always a valid method? Cancer Lett. 2005, 218, 117–121. [Google Scholar] [CrossRef]
- Mueller-Holzner, E.; Fink, V.; Frede, T.; Marth, C. Immunohistochemical determination of HER2 expression in breast cancer from core biopsy specimens: A reliable predictor of HER2 status of the whole tumor. Breast Cancer Res. Treat. 2001, 69, 13–19. [Google Scholar] [CrossRef]
- Sutela, A.; Vanninen, R.; Sudah, M.; Berg, M.; Kiviniemi, V.; Rummukainen, J.; Kataja, V.; Kärjä, V. Surgical specimen can be replaced by core samples in assessment of ER, PR and HER-2 for invasive breast cancer. Acta Oncol. 2008, 47, 38–46. [Google Scholar] [CrossRef]
- Apple, S.K.; Lowe, A.C.; Rao, P.N.; Shintaku, I.P.; Moatamed, N.A. Comparison of fluorescent in situ hybridization HER-2/neu results on core needle biopsy and excisional biopsy in primary breast cancer. Mod. Pathol. 2009, 22, 1151–1159. [Google Scholar] [CrossRef]
- Cahill, R.A.; Walsh, D.; Landers, R.J.; Watson, R.G. Preoperative profiling of symptomatic breast cancer by diagnostic core biopsy. Ann. Surg. Oncol. 2006, 13, 45–51. [Google Scholar] [CrossRef]
- Rubovszky, G.; Horváth, Z. Recent Advances in the Neoadjuvant Treatment of Breast Cancer. J. Breast Cancer 2017, 20, 119–131. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Wu, Y.; Scaltriti, M.; Meric-Bernstam, F.; Hunt, K.K.; Dawood, S.; Esteva, F.J.; Buzdar, A.U.; Chen, H.; Eksambi, S.; et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin. Cancer Res. 2009, 15, 7381–7388. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.; Doliny, P.; Reis, I.; Silva, O.; Gomez-Fernandez, C.; Velez, P.; Pauletti, G.; Powell, J.E.; Pegram, M.D.; Slamon, D.J. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J. Clin. Oncol. 2006, 24, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.N.; You, F.; Schnitt, S.J.; Witkiewicz, A.; Lu, X.; Sgroi, D.; Ryan, P.D.; Come, S.E.; Burstein, H.J.; Lesnikoski, B.A.; et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin. Cancer Res. 2007, 13, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Kombak, F.E.; Şahin, H.; Mollamemişoğlu, H.; Önem, İ.; Kaya, H.; Buğdaycı, O.; Arıbal, E. Concordance of immunohistochemistry between core needle biopsy and surgical resection of breast cancer. Turk. J. Med. Sci. 2017, 47, 1791–1796. [Google Scholar] [CrossRef]
- Hukkinen, K.; Kivisaari, L.; Heikkilä, P.S.; Von Smitten, K.; Leidenius, M. Unsuccessful preoperative biopsies, fine needle aspiration cytology or core needle biopsy, lead to increased costs in the diagnostic workup in breast cancer. Acta Oncol. 2008, 47, 1037–1045. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Zengel, B.; Kocatepe Çavdar, D.; Yılmaz, C.; Durusoy, R. Prognostic Value of Receptor Change After Neoadjuvant Chemotherapy in Breast Cancer Patients. Eur. J. Breast Health 2022, 18, 167–171. [Google Scholar] [CrossRef]
- Tao, M.; Chen, S.; Zhang, X.; Zhou, Q. Ki-67 labeling index is a predictive marker for a pathological complete response to neoadjuvant chemotherapy in breast cancer: A meta-analysis. Medicine 2017, 96, e9384. [Google Scholar] [CrossRef]

| Variables | Mean or Subgroups | n (%) |
|---|---|---|
| Age, years | Mean ± SD (range) | 50.3 ± 9.6 (28–69) |
| Menopausal status | Pre-menopausal | 45 (44.1) |
| Peri-menopausal | 8 (7.8) | |
| Post-menopausal | 49 (48.0) | |
| Clinical stage | Early | 54 (52.9) |
| Locally advanced | 37 (36.3) | |
| Inflammatory | 5 (4.9) | |
| Oligo-metastatic | 6 (5.9) | |
| Tumor size, mm | Mean ± SD (range) | 29 ± 13 (5–85) |
| cT | T1 | 11 (10.8) |
| T2 | 68 (66.7) | |
| T3 | 4 (3.9) | |
| T4 | 19 (18.6) | |
| cN | N0 | 6 (5.9) |
| N1 | 66 (64.7) | |
| N2 | 26 (25.5) | |
| N3 | 4 (3.9) | |
| Histology | IDC | 90 (88.2) |
| ILC | 6 (5.9) | |
| Mixed | 2 (2.0) | |
| Other | 4 (3.9) | |
| Centricity | Unicentric | 90 (88.2) |
| Multicentric | 12 (11.8) | |
| Solitary tumor vs. multiple tumors | Solitary | 45 (44.1) |
| Multiple | 57 (55.9) | |
| Molecular subtype | Luminal A | 16 (15.7) |
| LB-HER2(−) | 54 (52.9) | |
| LB-HER2(+) | 12 (11.8) | |
| HER2 enriched | 3 (2.9) | |
| Triple negative | 17 (16.7) | |
| NACTx regimen | EC-w paclitaxel | 32 (31.4) |
| DD AC-w paclitaxel | 39 (38.2) | |
| DD AC-docetaxel | 14 (13.7) | |
| AC-taxane | 6 (5.9) | |
| FEC-docetaxel | 1 (1.0) | |
| Only anthracycline based | 6 (5.9) | |
| Only taxane based | 4 (3.9) | |
| Anti-HER2 drug(s) | Not received | 88 (86.3) |
| Trastuzumab | 10 (9.8) | |
| Trastuzumab + pertuzumab | 4 (3.9) | |
| Pathological response status | Partially responsive to NACTx | 71 (69.6) |
| Unresponsive to NACTx | 31 (30.4) |
| Tru-Cut Bx | Post-NACTx Residual Tumor, n (%) | Total n (%) | Concordance | Discordance | p-Value w | Clinical Outcome | ||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive to (−) | Negative to (+) | |||||
| ER Status | 95.1% | 4.9% | 0.180 | 1.2% | 19% | |||
| ER(+) | 80 (98.8) | 1 (1.2) | 81 (79.4) | Adjuvant ET | Adjuvant ET | |||
| ER(−) | 4 (19.0) | 17 (81.0) | 21 (20.6) | |||||
| Total | 84 (82.4) | 18 (17.6) | 102 (100.0) | |||||
| PR Status | 97.1% | 2.9% | 0.083 | 3.9% | 0% | |||
| PR(+) | 73 (96.1) | 3 (3.9) | 76 (74.5) | Adjuvant ET | Adjuvant ET | |||
| PR(−) | 0 (0.0) | 26 (100.0) | 26 (25.5) | |||||
| Total | 73 (71.6) | 29 (28.4) | 102 (100.0) | |||||
| HR Status | 96.1% | 3.9% | 0.317 | 1.2% | 15% * | |||
| HR(+) | 81 (98.8) | 1 (1.2) | 82 (80.4) | Adjuvant ET | Adjuvant ET | |||
| HR(−) | 3 (15.0) * | 17 (85.0) | 20 (19.6) | |||||
| Total | 84 (82.4) | 18 (17.6) | 102 (100.0) | |||||
| HER2 Status | 89.2% | 10.8% | 0.763 | 40% | 5.7% * | |||
| HER2(+) | 9 (60.0) | 6 (40.0) | 15 (14.7) | Adjuvant Trastuzumab | Adjuvant Trastuzumab | |||
| HER2(−) | 5 (5.7) * | 82 (94.3) | 87 (85.3) | |||||
| Total | 14 (13.7) | 88 (86.3) | 102 (100.0) | |||||
| ER Discordance | Univariate Model | Multivariate Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||||
| Age (years) | 0.934 | 0.847 | - | 1.029 | 0.168 | |||||
| Tumor size (mm) | 0.995 | 0.928 | - | 1.067 | 0.886 | |||||
| Pre-NACTx ER expression (%) | 0.940 | 0.885 | - | 0.999 | 0.046 | 0.940 | 0.885 | - | 0.999 | 0.046 |
| Pre-NACTx Ki-67 proliferation index (%) | 1.039 | 0.996 | - | 1.084 | 0.075 | |||||
| Pre-NACTx tumor grade | 9.584 | 1.057 | - | 86.896 | 0.045 | 0.292 | ||||
| NACTx regimen | 1.079 | 0.640 | - | 1.818 | 0.776 | |||||
| Histology | - | - | - | - | 0.999 | |||||
| Centricity (unicentric vs. multicentric) | - | - | - | - | 0.999 | |||||
| Solitary tumor vs. multiple tumors | 0.837 | 0.134 | - | 5.237 | 0.849 | |||||
| Clinical tumor stage | 0.614 | 0.177 | - | 2.130 | 0.614 | |||||
| Clinical lymph node stage | 1.370 | 0.355 | - | 5.297 | 0.648 | |||||
| PR Discordance | Univariate Model | Multivariate Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||||
| Age (years) | 1.093 | 0.952 | - | 1.254 | 0.209 | |||||
| Tumor size (mm) | 0.833 | 0.707 | - | 0.982 | 0.029 | 0.833 | 0.707 | - | 0.982 | 0.029 |
| Pre-NACTx PR expression (%) | 0.984 | 0.953 | - | 1.016 | 0.317 | |||||
| Pre-NACTx Ki-67 proliferation index (%) | 1.033 | 0.980 | - | 1.090 | 0.230 | |||||
| Pre-NACTx tumor grade (1–3) | 1.377 | 0.177 | - | 10.693 | 0.760 | |||||
| NACTx regimen | 0.813 | 0.323 | - | 2.048 | 0.661 | |||||
| Histology | - | - | - | - | 0.998 | |||||
| Centricity (unicentric vs. multicentric) | 4.000 | 0.335 | - | 47.810 | 0.273 | |||||
| Solitary tumor vs. multiple tumors | 1.600 | 0.140 | - | 18.228 | 0.705 | |||||
| Clinical tumor stage | 2.994 | 0.876 | - | 10.230 | 0.080 | |||||
| Clinical lymph node stage | 0.437 | 0.054 | - | 3.548 | 0.437 | |||||
| HER2 Discordance | Univariate Model | Multivariate Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||||
| Age (years) | 1.053 | 0.985 | - | 1.129 | 0.147 | |||||
| Tumor size (mm) | 1.002 | 0.957 | - | 1.049 | 0.924 | |||||
| Pre-NACTx HER2 score (0–3) | 3.732 | 1.782 | - | 7.818 | <0.001 | 0.806 | ||||
| Pre-NACTx Ki-67 proliferation index (%) | 1.012 | 0.979 | - | 1.046 | 0.467 | |||||
| Pre-NACTx tumor grade (1–3) | 4.683 | 1.322 | - | 16.592 | 0.017 | 0.146 | ||||
| NACTx regimen | 0.747 | 0.436 | - | 1.281 | 0.289 | |||||
| Anti-HER2 therapy | 7.076 | 2.437 | - | 20.542 | <0.001 | 7.076 | 2.437 | - | 20.542 | <0.001 |
| Histology | 0.629 | 0.139 | - | 2.840 | 0.547 | |||||
| Centricity (unicentric vs. multicentric) | 0.727 | 0.085 | - | 6.244 | 0.772 | |||||
| Solitary tumor vs. multiple tumors | 4.031 | 0.825 | - | 19.699 | 0.085 | |||||
| Clinical tumor stage | 1.085 | 0.548 | - | 2.147 | 0.815 | |||||
| Clinical lymph node stage | 0.995 | 0.367 | - | 2.694 | 0.992 | |||||
| Tru-Cut Bx | Post- NACTx Residual Tumor | Total, n (%) | ||||
|---|---|---|---|---|---|---|
| Molecular Subtypes | LA | LB-HER2(−) | LB-HER2(+) | HER2 Enriched | TN | |
| LA, n (%) | 9 (56.3) | 6 (37.5) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 16 (100.0) |
| LB-HER2(−), n (%) | 19 (35.2) | 32 (59.3) | 2 (3.7) | 0 (0.0) | 1 (1.9) | 54 (100.0) |
| LB-HER2(+), n (%) | 1 (8.3) | 5 (41.7) | 6 (50.0) | 0 (0.0) | 0 (0.0) | 12 (100.0) |
| HER2 enriched, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 3 (100.0) |
| TN, n (%) | 0 (0.0) | 2 (11.8) | 1 (5.9) | 1 (5.9) | 13 (76.5) | 17 (100.0) |
| Total, n (%) | 29 (28.4) | 45 (44.1) | 10 (9.8) | 4 (3.9) | 14 (13.7) | 102 (100.0) |
| Category | Tru-Cut | Post-op | Stable (n) | Decrease (n) | Increase (n) | p-Value w |
|---|---|---|---|---|---|---|
| Grade, mean | 2.24 ± 0.6 | 2.25 ± 0.6 | 70 | 15 | 17 | 0.724 |
| Ki-67 proliferation index, mean % | 27.6 ± 17.5 | 22.2 ± 18.7 | 13 | 59 | 30 | 0.001 |
| ER, mean % | 62.4 ± 36.2 | 66.1 ± 37.4 | 47 | 20 | 35 | 0.062 |
| PR, mean % | 51.2 ± 39.9 | 42.3 ± 37.0 | 40 | 44 | 18 | 0.001 |
| Category | Partially Responsive (n = 71) | p-Value w | Unresponsive (n = 31) | p-Value w | |
|---|---|---|---|---|---|
| Grade, mean | Tru-cut | 2.3 ± 0.5 | 0.414 | 2.1 ± 0.61 | 0.034 |
| Post-op | 2.2 ± 0.6 | 2.3 ± 0.6 | |||
| Ki-67 proliferation index, mean % | Tru-cut | 27.5 ± 15.8 | 0.001 | 27.8 ± 21.1 | 0.446 |
| Post-op | 20.6 ± 15.1 | 26.1 ± 25.1 | |||
| ER, mean % | Tru-cut | 64.1 ± 35.8 | 0.266 | 58.9 ± 37.5 | 0.124 |
| Post-op | 67.2 ± 35.9 | 63.5 ± 41.1 | |||
| PR, mean % | Tru-cut | 49.9 ± 40.6 | 0.004 | 54.2 ± 38.8 | 0.132 |
| Post-op | 40.5 ± 36.3 | 46.4 ± 38.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, C.; Cavdar, D.K. Biomarker Discordances and Alterations Observed in Breast Cancer Treated with Neoadjuvant Chemotherapy: Causes, Frequencies, and Clinical Significances. Curr. Oncol. 2022, 29, 9695-9710. https://doi.org/10.3390/curroncol29120761
Yilmaz C, Cavdar DK. Biomarker Discordances and Alterations Observed in Breast Cancer Treated with Neoadjuvant Chemotherapy: Causes, Frequencies, and Clinical Significances. Current Oncology. 2022; 29(12):9695-9710. https://doi.org/10.3390/curroncol29120761
Chicago/Turabian StyleYilmaz, Cengiz, and Demet Kocatepe Cavdar. 2022. "Biomarker Discordances and Alterations Observed in Breast Cancer Treated with Neoadjuvant Chemotherapy: Causes, Frequencies, and Clinical Significances" Current Oncology 29, no. 12: 9695-9710. https://doi.org/10.3390/curroncol29120761
APA StyleYilmaz, C., & Cavdar, D. K. (2022). Biomarker Discordances and Alterations Observed in Breast Cancer Treated with Neoadjuvant Chemotherapy: Causes, Frequencies, and Clinical Significances. Current Oncology, 29(12), 9695-9710. https://doi.org/10.3390/curroncol29120761




