Optimal Choice as First-Line Therapy for Patients with Triple-Negative Breast Cancer: A Bayesian Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
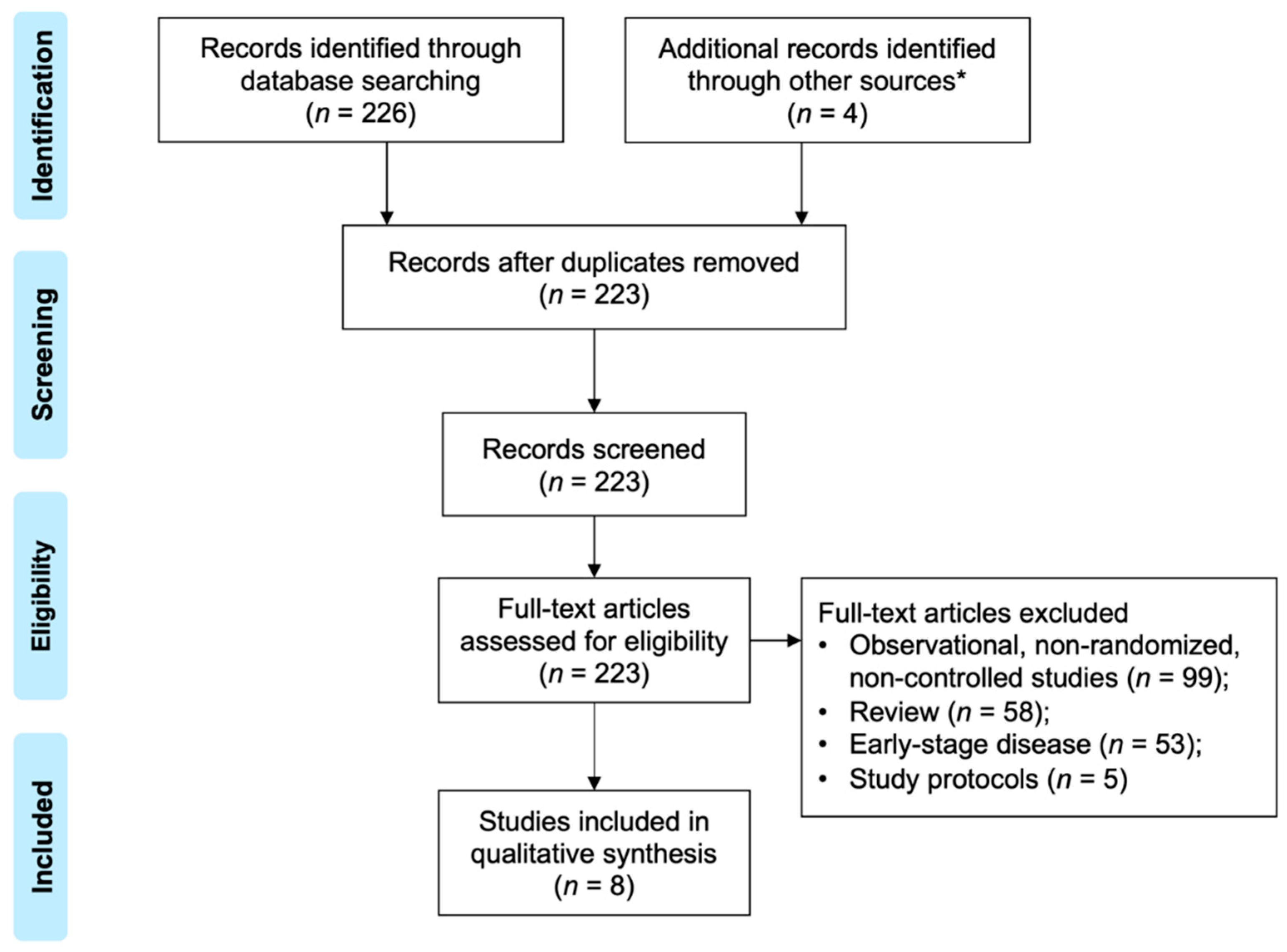
2.1. Study Selection and Outcome Measures
2.2. Data Extraction and Quality Assessment
2.3. Statistical Analysis
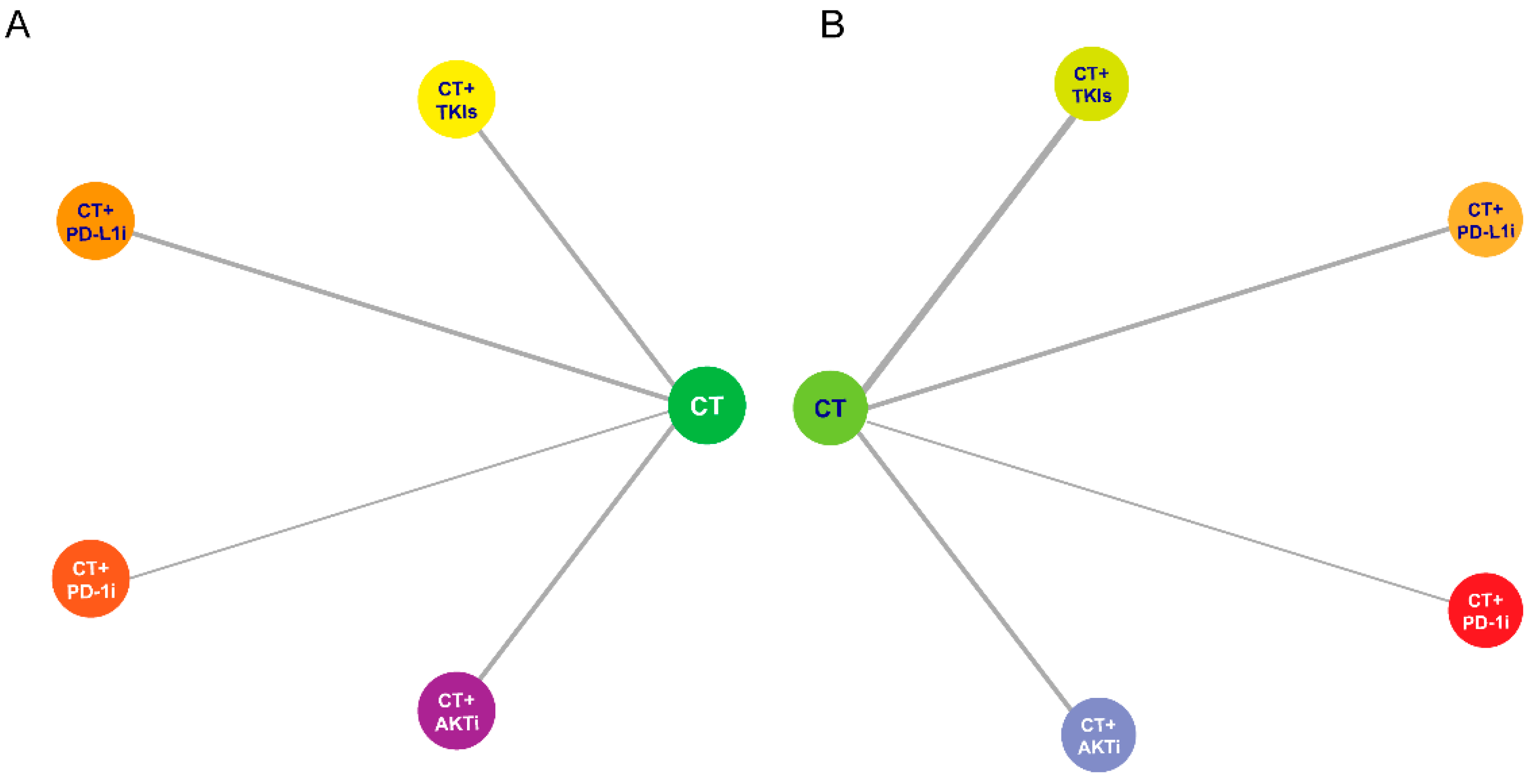
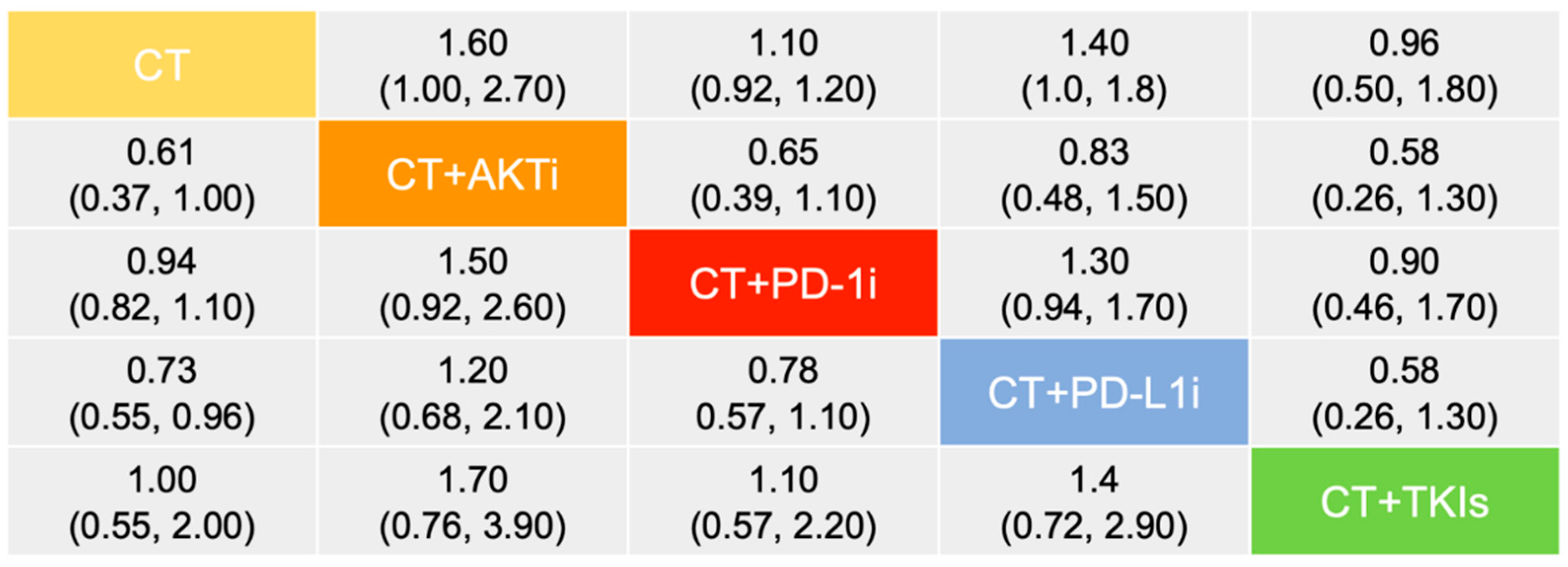
3. Results
3.1. Efficacy
3.2. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Kiely, B.E.; Soon, Y.Y.; Tattersall, M.H.; Stockler, M.R. How Long Have I Got? Estimating Typical, Best-Case, and Worst-Case Scenarios for Patients Starting First-Line Chemotherapy for Metastatic Breast Cancer: A Systematic Review of Recent Randomized Trials. J. Clin. Oncol. 2011, 29, 456–463. [Google Scholar] [CrossRef]
- Kassam, F.; Enright, K.; Dent, R.; Dranitsaris, G.; Myers, J.; Flynn, C.; Fralick, M.; Kumar, R.; Clemons, M. Survival Outcomes for Patients with Metastatic Triple-Negative Breast Cancer: Implications for Clinical Practice and Trial Design. Clin. Breast Cancer 2009, 9, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Z.; Liu, Y.; Xiao, Y.; Hu, X.; Jiang, L.; Zuo, W.-J.; Ma, D.; Ding, J.; Zhu, X.; Zou, J.; et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: The FUTURE trial. Cell Res. 2020, 31, 178–186. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.; Kim, S.; Zvirbule, Ž.; Eniu, A.; Mebis, J.; Sohn, J.; Wongchenko, M.; Chohan, S.; Amin, R.; Yan, Y.; et al. A phase II randomized trial of cobimetinib plus chemotherapy, with or without atezolizumab, as first-line treatment for patients with locally advanced or metastatic triple-negative breast cancer (COLET): Primary analysis. Ann. Oncol. 2021, 32, 652–660. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Abraham, J.; Chan, S.; Wheatley, D.; Brunt, A.M.; Nemsadze, G.; Baird, R.D.; Park, Y.H.; Hall, P.S.; Perren, T.; et al. Capivasertib Plus Paclitaxel Versus Placebo Plus Paclitaxel As First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial. J. Clin. Oncol. 2020, 38, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Dent, R.; Im, S.-A.; Espié, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef]
- Finn, R.S.; Press, M.F.; Dering, J.; Arbushites, M.; Koehler, M.; Oliva, C.; Williams, L.S.; Di Leo, A. Estrogen Receptor, Progesterone Receptor, Human Epidermal Growth Factor Receptor 2 (HER2), and Epidermal Growth Factor Receptor Expression and Benefit from Lapatinib in a Randomized Trial of Paclitaxel with Lapatinib or Placebo As First-Line Treatment in HER2-Negative or Unknown Metastatic Breast Cancer. J. Clin. Oncol. 2009, 27, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 21, 44–59. [Google Scholar] [CrossRef]
- Dent, R.; Kim, S.-B.; Oliveira, M.; Barrios, C.; O’Shaughnessy, J.; Isakoff, S.J.; Saji, S.; Freitas-Junior, R.; Philco, M.; Bondarenko, I.; et al. Abstract GS3-04: Double-blind placebo (PBO)-controlled randomized phase III trial evaluating first-line ipatasertib (IPAT) combined with paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered locally advanced unresectable or metastatic triple-negative breast cancer (aTNBC): Primary results from IPATunity130 Cohort A. Cancer Res. 2021, 81, GS3-04. [Google Scholar]
- Dent, R.; Oliveira, M.; Isakoff, S.J.; Im, S.-A.; Espié, M.; Blau, S.; Tan, A.R.; Saura, C.; Wongchenko, M.J.; Xu, N.; et al. Final results of the double-blind placebo-controlled randomized phase 2 LOTUS trial of first-line ipatasertib plus paclitaxel for inoperable locally advanced/metastatic triple-negative breast cancer. Breast Cancer Res. Treat. 2021, 189, 377–386. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Ye, J.; Ji, X.; Dennis, P.; Abdullah, H.; Mukhopadhyay, P. Relationship between Progression-Free Survival, Objective Response Rate, and Overall Survival in Clinical Trials of PD-1/PD-L1 Immune Checkpoint Blockade: A Meta-Analysis. Clin. Pharmacol. Ther. 2020, 108, 1274–1288. [Google Scholar] [CrossRef]
- Nie, R.; Chen, F.; Yuan, S.; Luo, Y.; Chen, S.; Chen, Y.; Chen, X.; Chen, Y.; Li, Y.; Zhou, Z. Evaluation of objective response, disease control and progression-free survival as surrogate end-points for overall survival in anti-programmed death-1 and anti-programmed death ligand 1 trials. Eur. J. Cancer 2019, 106, 1–11. [Google Scholar] [CrossRef]
- Duan, J.; Cui, L.; Zhao, X.; Bai, H.; Cai, S.; Wang, G.; Zhao, Z.; Zhao, J.; Chen, S.; Song, J.; et al. Use of Immunotherapy with Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients with Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Carretero-González, A.; Otero, I.; Lora, D.; Carril-Ajuria, L.; Castellano, D.; de Velasco, G. Efficacy and safety of anti-PD-1/PD-L1 combinations versus standard of care in cancer: A systematic review and meta-analysis. Oncoimmunology 2021, 10, 1878599. [Google Scholar] [CrossRef]
- de Marinis, F.; Laktionov, K.; Poltoratskiy, A.; Egorova, I.; Hochmair, M.; Passaro, A.; Migliorino, M.R.; Metro, G.; Gottfried, M.; Tsoi, D.; et al. Afatinib in EGFR TKI-naïve patients with locally advanced or metastatic EGFR mutation-positive non-small cell lung cancer: Interim analysis of a Phase 3b study. Lung Cancer 2021, 152, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.; Redman, M.; Lilenbaum, R.; Politi, K.; Stinchcombe, T.; Horn, L.; Chen, E.H.; Mashru, S.H.; Gettinger, S.N.; Melnick, M.A.; et al. Randomized Trial of Afatinib Plus Cetuximab Versus Afatinib Alone for First-Line Treatment of -Mutant Non-Small-Cell Lung Cancer: Final Results from SWOG S1403. J. Clin. Oncol. 2020, 38, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, Y.; Ouyang, Q.; Lu, J.; Zhang, Q.; Yang, S.; Wang, J.; Huang, H.; Liu, H.; Shao, Z.; et al. Randomized and dose-escalation trials of recombinant human serum albumin /granulocyte colony-stimulating factor in patients with breast cancer receiving anthracycline-containing chemotherapy. BMC Cancer 2021, 21, 341. [Google Scholar] [CrossRef]
- Kamgar, M.; Greenwald, M.K.; Assad, H.; Hastert, T.A.; McLaughlin, E.M.; Reding, K.W.; Paskett, E.D.; Bea, J.W.; Shadyab, A.H.; Neuhouser, M.L.; et al. Prevalence and predictors of peripheral neuropathy after breast cancer treatment. Cancer Med. 2021, 10, 6666–6676. [Google Scholar] [CrossRef]
- Jones, R.H.; Casbard, A.; Carucci, M.; Cox, C.; Butler, R.; Alchami, F.; Madden, T.-A.; Bale, C.; Bezecny, P.; Joffe, J.; et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020, 21, 345–357. [Google Scholar] [CrossRef]
- Chien, A.J.; Tripathy, D.; Albain, K.S.; Symmans, W.F.; Rugo, H.S.; Melisko, M.E.; Wallace, A.M.; Schwab, R.; Helsten, T.; Forero-Torres, A.; et al. MK-2206 and Standard Neoadjuvant Chemotherapy Improves Response in Patients with Human Epidermal Growth Factor Receptor 2–Positive and/or Hormone Receptor–Negative Breast Cancers in the I-SPY 2 Trial. J. Clin. Oncol. 2020, 38, 1059–1069. [Google Scholar] [CrossRef]
- Chen, T.W.; Razak, A.R.; Bedard, P.L.; Siu, L.L.; Hansen, A.R. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann. Oncol. 2015, 26, 1824–1829. [Google Scholar] [CrossRef]
- Sridhar, S.; Blais, N.; Tran, B.; Reaume, M.; North, S.; Stockler, M.; Chi, K.; Fleshner, N.; Liu, G.; Robinson, J.; et al. Efficacy and Safety of nab-Paclitaxel vs Paclitaxel on Survival in Patients with Platinum-Refractory Metastatic Urothelial Cancer: The Canadian Cancer Trials Group BL.12 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1751–1758. [Google Scholar] [CrossRef]
- Socinski, M.A.; Bondarenko, I.; Karaseva, N.A.; Makhson, A.M.; Vynnychenko, I.; Okamoto, I.; Hon, J.K.; Hirsh, V.; Bhar, P.; Zhang, H.; et al. Weekly nab-Paclitaxel in Combination with Carboplatin Versus Solvent-Based Paclitaxel Plus Carboplatin as First-Line Therapy in Patients with Advanced Non–Small-Cell Lung Cancer: Final Results of a Phase III Trial. J. Clin. Oncol. 2012, 30, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Egger, S.J.; Chan, M.M.K.; Luo, Q.; Wilcken, N. Platinum-containing regimens for triple-negative metastatic breast cancer. Cochrane Database Syst. Rev. 2020, 2020, CD013750. [Google Scholar] [CrossRef]
- Balko, J.M.; Giltnane, J.M.; Wang, K.; Schwarz, L.J.; Young, C.D.; Cook, R.S.; Owens, P.; Sanders, M.E.; Kuba, M.G.; Sánchez, V.; et al. Molecular Profiling of the Residual Disease of Triple-Negative Breast Cancers after Neoadjuvant Chemotherapy Identifies Actionable Therapeutic Targets. Cancer Discov. 2014, 4, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.; Garber, J.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1-or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wang, J.; Wu, Y.; Xu, H.; Wang, Y.; Xu, B. Optimal Choice as First-Line Therapy for Patients with Triple-Negative Breast Cancer: A Bayesian Network Meta-Analysis. Curr. Oncol. 2022, 29, 9172-9180. https://doi.org/10.3390/curroncol29120718
Han Y, Wang J, Wu Y, Xu H, Wang Y, Xu B. Optimal Choice as First-Line Therapy for Patients with Triple-Negative Breast Cancer: A Bayesian Network Meta-Analysis. Current Oncology. 2022; 29(12):9172-9180. https://doi.org/10.3390/curroncol29120718
Chicago/Turabian StyleHan, Yiqun, Jiayu Wang, Yun Wu, Hangcheng Xu, Yan Wang, and Binghe Xu. 2022. "Optimal Choice as First-Line Therapy for Patients with Triple-Negative Breast Cancer: A Bayesian Network Meta-Analysis" Current Oncology 29, no. 12: 9172-9180. https://doi.org/10.3390/curroncol29120718
APA StyleHan, Y., Wang, J., Wu, Y., Xu, H., Wang, Y., & Xu, B. (2022). Optimal Choice as First-Line Therapy for Patients with Triple-Negative Breast Cancer: A Bayesian Network Meta-Analysis. Current Oncology, 29(12), 9172-9180. https://doi.org/10.3390/curroncol29120718



