Clinical Delays and Comparative Outcomes in Younger and Older Adults with Colorectal Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources
2.2. Eligibility Criteria
2.3. Data Management
2.4. Study Selection
2.5. Data Collection Process
2.6. Outcomes and Definitions
2.7. Risk of Bias Assessment
2.8. Synthesis
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Risk of Bias Assessment
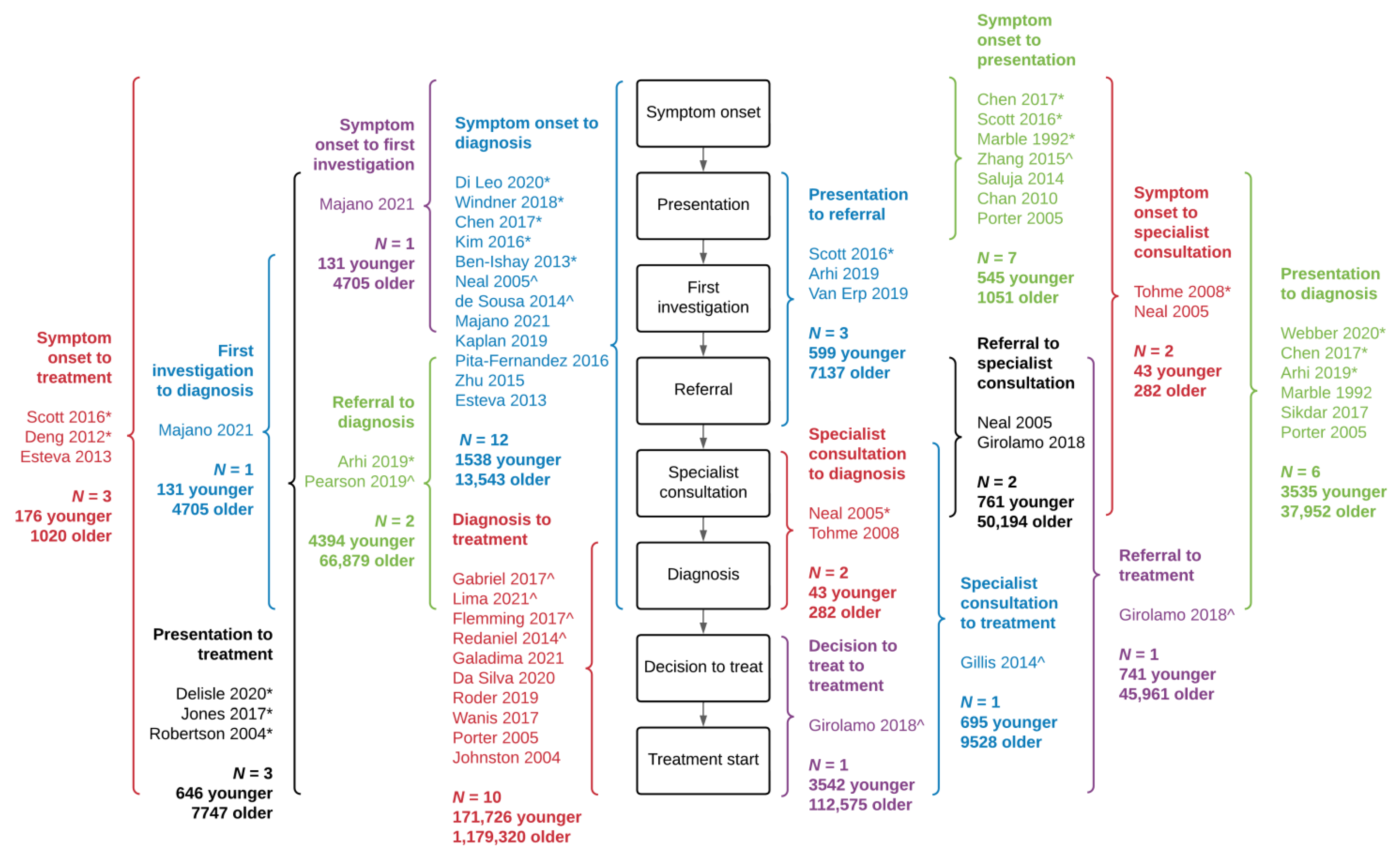
3.4. Delay Measures between Younger and Older Patients
3.5. Interval from Symptom Onset to Diagnosis
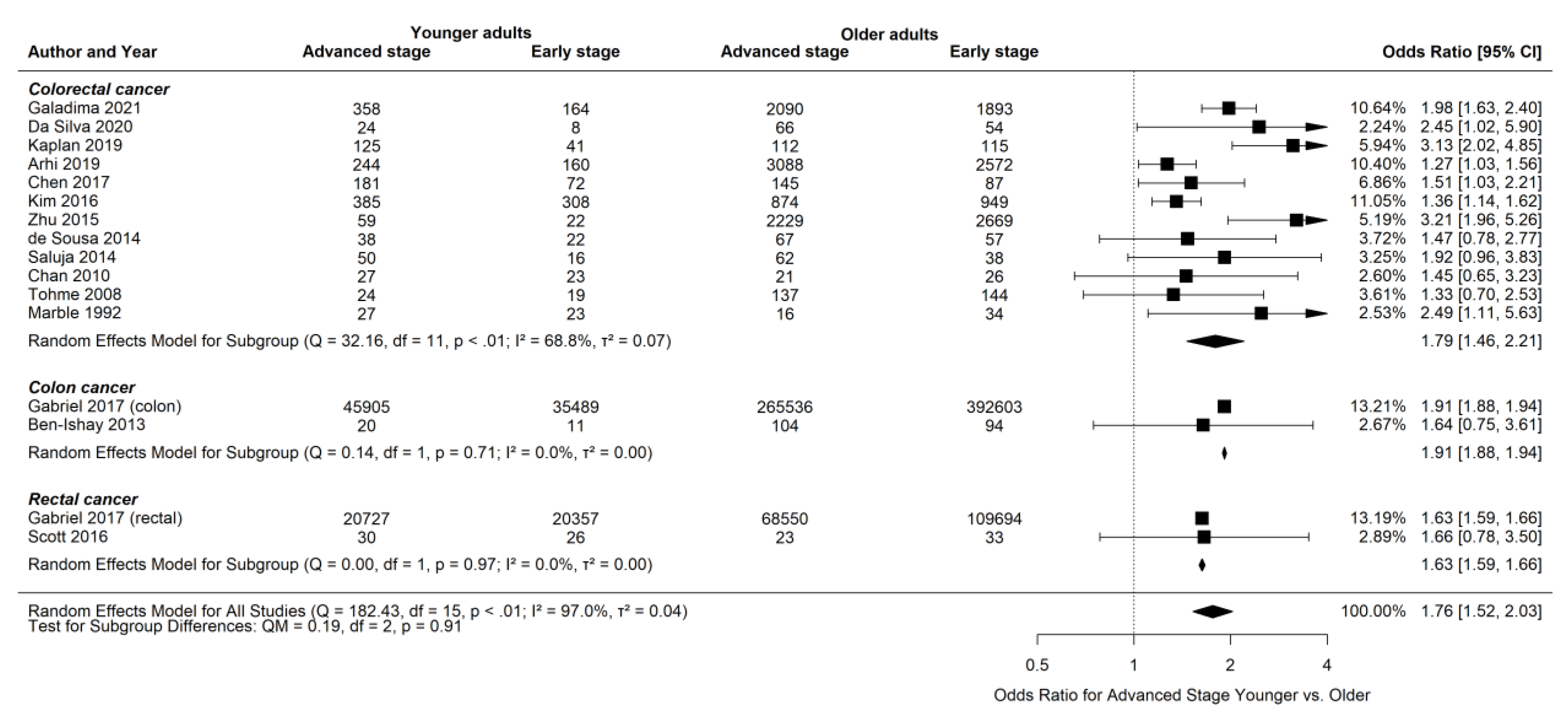
3.6. Advanced Stage at Presentation for Younger and Older Patients
3.7. Interval from to Diagnosis to Treatment Start
3.8. Remaining Delay Intervals
3.9. Impact of Delay on Outcomes between Younger and Older Patients
3.10. Survival and Recurrence for Younger and Older Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bailey, C.E.; Hu, C.-Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, E.; Buzzoni, C.; Zappa, M. Colorectal cancer incidence rates have decreased in central Italy. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2010, 19, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Haggar, F.A.; Preen, D.B.; Pereira, G.; Holman, C.D.J.; Einarsdottir, K. Cancer incidence and mortality trends in Australian adolescents and young adults, 1982–2007. BMC Cancer 2012, 12, 151. [Google Scholar] [CrossRef]
- Patel, P.; De, P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15–49-year-olds in Canada, 1969–2010. Cancer Epidemiol. 2016, 42, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E.; Malvezzi, M. Colorectal Cancer Mortality in Young Adults Is Rising in the United States, Canada, United Kingdom, and Australia but Not in Europe and Asia. Gastroenterology 2021, 160, 1860–1862.e2. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Livingston, E.H.; Yo, C.K. Colorectal cancer in the young. Am. J. Surg. 2004, 187, 343–348. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef]
- Clarke, W.T.; Feuerstein, J.D. Updates in colorectal cancer screening in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2018, 34, 208–216. [Google Scholar] [CrossRef]
- Barrow, P.; Khan, M.; Lalloo, F.; Evans, D.G.; Hill, J. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br. J. Surg. 2013, 100, 1719–1731. [Google Scholar] [CrossRef]
- Siegel, R.L.; Jakubowski, C.D.; Fedewa, S.A.; Davis, A.; Azad, N.S. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2020, 40, e75–e88. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Kim, E.R.; Hong, S.N.; Chang, D.K.; Kim, Y.H. Long-Term Outcome and Prognostic Factors of Sporadic Colorectal Cancer in Young Patients: A Large Institutional-Based Retrospective Study. Medicine 2016, 95, e3641. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, C.; Walters, S.; Gildea, C.; Benitez Majano, S.; Rachet, B.; Morris, M. Can we assess Cancer Waiting Time targets with cancer survival? A population-based study of individually linked data from the National Cancer Waiting Times monitoring dataset in England, 2009–2013. PLoS ONE. 2018, 13, e0201288. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Blackburn, H.N.; Ng, K.; Spiegelman, D.; Irwin, M.L.; Ma, X.; Gross, C.P.; Tabung, F.K.; Giovannucci, E.L.; Kunz, P.L.; et al. Analysis of Survival Among Adults With Early-Onset Colorectal Cancer in the National Cancer Database. JAMA Netw. Open 2021, 4, e2112539. [Google Scholar] [CrossRef]
- Ramos, M.; Esteva, M.; Cabeza, E.; Llobera, J.; Ruiz, A. Lack of association between diagnostic and therapeutic delay and stage of colorectal cancer. Eur. J. Cancer 2008, 44, 510–521. [Google Scholar] [CrossRef]
- Ramos, M.; Esteva, M.; Cabeza, E.; Campillo, C.; Llobera, J.; Aguiló, A. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: A review. Eur. J. Cancer 2007, 43, 2467–2478. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Fraser, C.; Murray, A.; Burr, J. Identifying observational studies of surgical interventions in MEDLINE and EMBASE. BMC Med. Res. Methodol. 2006, 6, 41. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Grey Matters: A Practical Tool for Searching Health-Related Grey Literature. CADTH.ca. Published 29 July 2009. Available online: https://www.cadth.ca/resources/finding-evidence/grey-matters (accessed on 4 October 2019).
- Weller, D.; Vedsted, P.; Rubin, G.; Walter, F.M.; Emery, J.; Scott, S.; Campbell, C.; Andersen, R.S.; Hamilton, W.; Olesen, F.; et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer 2012, 106, 1262–1267. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 13 March 2019).
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: http://handbook-5-1.cochrane.org/ (accessed on 28 January 2019).
- Scott, R.B.; Rangel, L.E.; Osler, T.M.; Hyman, N.H. Rectal cancer in patients under the age of 50 years: The delayed diagnosis. Am. J. Surg. 2016, 211, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Wanis, K.N.; Patel, S.V.B.; Brackstone, M. Do Moderate Surgical Treatment Delays Influence Survival in Colon Cancer? Dis. Colon Rectum 2017, 60, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.; Fraser, J.; Peake, M.; Valori, R.; Poirier, V.; Coupland, V.H.; Hiom, S.; McPhail, S.; Moffat, J.; Lyratzopoulos, G.; et al. Establishing population-based surveillance of diagnostic timeliness using linked cancer registry and administrative data for patients with colorectal and lung cancer. Cancer Epidemiol. 2019, 61, 111–118. [Google Scholar] [CrossRef]
- Flemming, J.A.; Nanji, S.; Wei, X.; Webber, C.; Groome, P.; Booth, C.M. Association between the time to surgery and survival among patients with colon cancer: A population-based study. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2017, 43, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ji, M.; Dai, W.; Ye, C.; Hu, Z.; Shi, J.; Zeng, X.; Lin, Y. Clinicopathological characteristics of chinese colorectal cancer patients under 30 years of age: Implication in diagnosis and therapy. Curr. Cancer Drug Targets 2015, 15, 27–34. [Google Scholar] [CrossRef]
- Roder, D.; Karapetis, C.; Olver, I.; Keefe, D.; Padbury, R.; Moore, J.; Joshi, R.; Wattchow, D.; Worthley, D.L.; Miller, C.L.; et al. Time from diagnosis to treatment of colorectal cancer in a South Australian clinical registry cohort: How it varies and relates to survival. BMJ Open 2019, 9, e031421. [Google Scholar] [CrossRef]
- Arhi, C.S.; Ziprin, P.; Bottle, A.; Burns, E.M.; Aylin, P.; Darzi, A. Colorectal cancer patients under the age of 50 experience delays in primary care leading to emergency diagnoses: A population-based study. Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2019, 21, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.A.; Ozaydin, S.; Yerlikaya, H.; Karaagac, M.; Gumus, M.; Cil, T.; Arslan, Y.; Ozdemir, N.; Sakin, A.; Bilici, M.; et al. Clinicopathologic and Prognostic Differences between Three Different Age Groups (Child/Adolescent, Young Adults, and Adults) of Colorectal Cancer Patients: A Multicentre Study. Oncol. Res. Treat. 2019, 42, 516–522. [Google Scholar] [CrossRef]
- Windner, Z.; Crengle, S.; de Graaf, B.; Samaranayaka, A.; Derrett, S. New Zealanders’ experiences and pathways to a diagnosis of bowel cancer: A cross-sectional descriptive study of a younger cohort. N. Z. Med. J. 2018, 131, 30–39. [Google Scholar]
- Gabriel, E.; Ostapoff, K.; Attwood, K.; Al-Sukhni, E.; Boland, P.; Nurkin, S. Disparities in the Age-Related Rates of Colorectal Cancer in the United States. Am. Surg. 2017, 83, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, K.C.; Dickinson, J.; Winget, M. Factors associated with mode of colorectal cancer detection and time to diagnosis: A population level study. BMC Health Serv. Res. 2017, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.W.; Sundaram, V.; Chew, T.A.; Ladabaum, U. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated With Longer Duration of Symptoms or Time to Diagnosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2017, 15, 728–737.e3. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Ferrans, C.E.; Polite, B.N.; Brewer, K.C.; Maker, A.V.; Pauls, H.A.; Rauscher, G.H. Examining racial disparities in colon cancer clinical delay in the Colon Cancer Patterns of Care in Chicago study. Ann. Epidemiol. 2017, 27, 731–738.e1. [Google Scholar] [CrossRef] [PubMed]
- Pita-Fernández, S.; González-Sáez, L.; López-Calviño, B.; Seoane-Pillado, T.; Rodríguez-Camacho, E.; Pazos-Sierra, A.; González-Santamaría, P.; Pértega-Díaz, S. Effect of diagnostic delay on survival in patients with colorectal cancer: A retrospective cohort study. BMC Cancer 2016, 16, 664. [Google Scholar] [CrossRef]
- Zhang, J.-K.; Fang, L.-L.; Wu, X.-M.; Liu, J.-C.; Zhang, C.-D.; Dai, D.-Q. Factors associated with delaying medical assessment of patients and impacting the prognosis of rectal cancer. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2015, 24, 391–399. [Google Scholar] [CrossRef]
- Saluja, S.S.; Manipadam, J.M.; Mishra, P.K.; Sachdeva, S.; Solanki, N.; Shah, H. Young onset colorectal cancer: How does it differ from its older counterpart? Indian J. Cancer 2014, 51, 565–569. [Google Scholar] [CrossRef]
- Redaniel, M.T.; Martin, R.M.; Blazeby, J.M.; Wade, J.; Jeffreys, M. The association of time between diagnosis and major resection with poorer colorectal cancer survival: A retrospective cohort study. BMC Cancer 2014, 14, 642. [Google Scholar] [CrossRef][Green Version]
- Gillis, A.; Dixon, M.; Smith, A.; Law, C.; Coburn, N.G. A patient-centred approach toward surgical wait times for colon cancer: A population-based analysis. Can. J. Surg. J. Can. Chir. 2014, 57, 94–100. [Google Scholar] [CrossRef]
- De Sousa, J.B.; Souza, C.S.; Fernandes, M.B.; Durães, L.D.C.; De Almeida, R.M.; Dos Santos, A.C.N.; Da Silva, E.F.; De Oliveira, P.G. Do young patients have different clinical presentation of colorectal cancer causing delay in diagnosis? Int. J. Color. Dis. 2014, 29, 519–527. [Google Scholar] [CrossRef]
- Ben-Ishay, O.; Brauner, E.; Peled, Z.; Othman, A.; Person, B.; Kluger, Y. Diagnosis of colon cancer differs in younger versus older patients despite similar complaints. Isr. Med. Assoc. J. IMAJ 2013, 15, 284–287. [Google Scholar]
- Esteva, M.; Leiva, A.; Ramos, M.; Pita-Fernández, S.; González-Luján, L.; Casamitjana, M.; Sánchez, M.A.; Pértega-Díaz, S.; Ruiz, A.; Gonzalez-Santamaría, P.; et al. Factors related with symptom duration until diagnosis and treatment of symptomatic colorectal cancer. BMC Cancer 2013, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.X.; An, W.; Gao, J.; Yin, J.; Cai, Q.C.; Yang, M.; Hong, S.Y.; Fu, X.X.; Da Yu, E.; Xu, X.D.; et al. Factors influencing diagnosis of colorectal cancer: A hospital-based survey in China. J. Dig. Dis. 2012, 13, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Dassanayake, B.; Deen, R.; Wickramarachchi, R.; Kumarage, S.; Samita, S.; Deen, K. Young patients with colorectal cancer have poor survival in the first twenty months after operation and predictable survival in the medium and long-term: Analysis of survival and prognostic markers. World J. Surg. Oncol. 2010, 8, 82. [Google Scholar] [CrossRef]
- Tohmé, C.; Labaki, M.; Hajj, G.; Abboud, B.; Noun, R.; Sarkis, R. Colorectal cancer in young patients: Presentation, clinicopathological characteristics and outcome. J. Med. Liban. 2008, 56, 208–214. [Google Scholar] [PubMed]
- Porter, G.A.; Inglis, K.M.; Wood, L.A.; Veugelers, P.J. Access to care and satisfaction in colorectal cancer patients. World J. Surg. 2005, 29, 1444–1451. [Google Scholar] [CrossRef]
- Neal, R.D.; Allgar, V.L. Sociodemographic factors and delays in the diagnosis of six cancers: Analysis of data from the ‘National Survey of NHS Patients: Cancer’. Br. J. Cancer. 2005, 92, 1971. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.M.; MacGarvie, V.L.; Elliott, D.; Dewar, R.A.D.; MacIntyre, M.M.; Nolan, M.C. Radiotherapy wait times for patients with a diagnosis of invasive cancer, 1992–2000. Clin. Investig. Med. Med. Clin. Exp. 2004, 27, 142–156. [Google Scholar]
- Robertson, R.; Campbell, N.C.; Smith, S.; Donnan, P.T.; Sullivan, F.; Duffy, R.; Ritchie, L.D.; Millar, D.; Cassidy, J.; Munro, A. Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br. J. Cancer 2004, 90, 1479–1485. [Google Scholar] [CrossRef][Green Version]
- Marble, K.; Banerjee, S.; Greenwald, L. Colorectal carcinoma in young patients. J. Surg. Oncol. 1992, 51, 179–182. [Google Scholar] [CrossRef]
- Da Silva, F.M.M.; Duarte, R.P.; Leão, C.C.A.; Vissoci, C.M.; Alvarenga, A.L.A.T.; Ramos, A.B.S.; Goulart, A.E.C. Colorectal cancer in patients under age 50: A five-year experience. Rev. Colégio Bras. Cir. 2020, 47, e20202406. [Google Scholar] [CrossRef] [PubMed]
- Delisle, M.; Helewa, R.M.; Ward, M.A.R.; Hochman, D.J.; Park, J.; McKay, A. The Association Between Wait Times for Colorectal Cancer Treatment and Health Care Costs: A Population-Based Analysis. Dis. Colon Rectum 2020, 63, 160–171. [Google Scholar] [CrossRef]
- Di Leo, M.; Zuppardo, R.A.; Puzzono, M.; Ditonno, I.; Mannucci, A.; Antoci, G.; Raucci, A.R.; Patricelli, M.G.; Elmore, U.; Tamburini, A.M.; et al. Risk factors and clinical characteristics of early-onset colorectal cancer vs. late-onset colorectal cancer: A case-case study. Eur. J. Gastroenterol. Hepatol. 2020, 33, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Galadima, H.I.; Adunlin, G.; Hughes, M.S.; Cropp, C.D.; Lucero, L.; Akpinar-Elci, M. Racial disparities and treatment trends among young-onset colorectal cancer patients: An analysis of a hospital cancer registry. Cancer Epidemiol. 2021, 72, 101911. [Google Scholar] [CrossRef] [PubMed]
- van Erp, N.F.; Helsper, C.W.; Olyhoek, S.M.; Janssen, R.R.T.; Winsveen, A.; Peeters, P.H.M.; de Wit, N.J. Potential for Reducing Time to Referral for Colorectal Cancer Patients in Primary Care. Ann. Fam. Med. 2019, 17, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Webber, C.; Flemming, J.A.; Birtwhistle, R.; Rosenberg, M.; Groome, P.A. Colonoscopy resource availability and its association with the colorectal cancer diagnostic interval: A population-based cross-sectional study. Eur. J. Cancer Care 2020, 29, e13187. [Google Scholar] [CrossRef]
- Majano, S.B.; Rachet, B.; Lyratzopoulos, G.; Renzi, C.; de Wit, N.J. Do presenting symptoms, use of pre-diagnostic endoscopy and risk of emergency cancer diagnosis vary by comorbidity burden and type in patients with colorectal cancer? Br. J. Cancer 2021, 126, 652–663. [Google Scholar] [CrossRef]
- Lima, M.A.N.; Villela, D.A.M. Fatores sociodemográficos e clínicos associados ao tempo para o início do tratamento de câncer de cólon e reto no Brasil, 2006–2015. Cad. Saúde Pública Online 2021, 37, e00214919. [Google Scholar] [CrossRef]
- Eaglehouse, Y.L.; Georg, M.W.; Shriver, C.D.; Zhu, K. Racial Comparisons in Timeliness of Colon Cancer Treatment in an Equal-Access Health System. J. Natl. Cancer Inst. 2020, 112, 410–417. [Google Scholar] [CrossRef]
- Bergin, R.J.; Thomas, R.J.S.; Whitfield, K.; White, V. Concordance between Optimal Care Pathways and colorectal cancer care: Identifying opportunities to improve quality and reduce disparities. J. Eval. Clin. Pract. 2020, 26, 918–926. [Google Scholar] [CrossRef]
- Neal, R.D. Do diagnostic delays in cancer matter? Br. J. Cancer 2009, 101 (Suppl 2), S9–S12. [Google Scholar] [CrossRef]
- Kaltenmeier, C.; Shen, C.; Medich, D.S.; Geller, D.A.; Bartlett, D.L.; Tsung, A.; Tohme, S. Time to Surgery and Colon Cancer Survival in the United States. Ann. Surg. 2021, 274, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.D.; Tharmanathan, P.; France, B.; Din, N.U.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R.; et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112 (Suppl 1), S92–S107. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, R.J. Timing and Delays in Breast Cancer Evaluation and Treatment. Ann. Surg. Oncol. 2018, 25, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Connell, L.C.; Mota, J.M.; Braghiroli, M.I.; Hoff, P.M. The Rising Incidence of Younger Patients With Colorectal Cancer: Questions About Screening, Biology, and Treatment. Curr. Treat. Options Oncol. 2017, 18, 23. [Google Scholar] [CrossRef]
- Walter, F.; Webster, A.; Scott, S.; Emery, J. The Andersen Model of Total Patient Delay: A systematic review of its application in cancer diagnosis. J. Health Serv. Res. Policy 2012, 17, 110–118. [Google Scholar] [CrossRef]
- Bartlett, V.L.; Dhruva, S.S.; Shah, N.D.; Ryan, P.; Ross, J.S. Feasibility of Using Real-World Data to Replicate Clinical Trial Evidence. JAMA Netw. Open 2019, 2, e1912869. [Google Scholar] [CrossRef]

| Study | Characteristic | |||||||
|---|---|---|---|---|---|---|---|---|
| Definition of Young | No. Younger | No. Older | Country | Study Type | Data Source | Years of Study | Number of Sites | |
| Colon and Rectal Cancer | ||||||||
| Lima 2021 [61] | <50 | 14,675 | 64,472 | Brazil | Retrospective cohort | Cancer registry/health administrative data | 2006–2015 | Population-based |
| Majano 2021 [60] | <45 | 131 | 4705 | UK | Retrospective cohort | Cancer registry/health administrative data | 2011–2015 | Population-based |
| Galadima 2021 [57] | <50 | 522 | 3983 | USA | Retrospective cohort | Cancer registry/health administrative data | 2008–2016 | Population-based |
| Delisle 2020 [55] | <50 | 519 | 6417 | Canada | Retrospective cohort | Cancer registry/health administrative data | 2004–2014 | Population-based |
| Di Leo 2020 [56] | <50 | 54 | 494 | Italy | Retrospective cohort | Primary data collection | 2015–2018 | 1 |
| Da Silva 2020 [54] | <50 | 39 | 145 | Brazil | Retrospective cohort | Primary data collection | 2013–2018 | 1 |
| Webber 2020 [59] | <50 | 1902 | 22,059 | Canada | Retrospective cohort | Cancer registry/health administrative data | 2008–2012 | Population-based |
| Van Erp 2019 [58] | <50 | 35 | 274 | Netherlands | Retrospective cohort | Cancer registry/health administrative data | 2007–2011 | Population-based |
| Roder 2019 [30] | <50 | 91 | 1584 | Australia | Retrospective cohort | Cancer registry/health administrative data | 2000–2010 | 4 |
| Arhi 2019 [31] | <50 | 508 | 6807 | UK | Retrospective cohort | Cancer registry/health administrative data | 2006–2013 | Population-based |
| Kaplan 2019 [32] | 20–25 | 141 | 237 | Turkey | Retrospective cohort | Primary data collection | 2003–2015 | 20 |
| Pearson 2019 [27] | <50 | 3886 | 60,072 | UK | Retrospective cohort | Cancer registry/health administrative data | 2014–2015 | Population-based |
| Windner 2018 [33] | <50 | 41 | 55 | New Zealand | Survey study | Primary data collection | - | - |
| Girolamo 2018 [13] | 15–44 | 3542 | 112,575 | UK | Retrospective cohort | Cancer registry/health administrative data | 2009–2013 | Population-based |
| Gabriel 2017 [34] | <50 | 155,090 | 1,058,102 | USA | Retrospective cohort | Cancer registry/health administrative data | 1998–2011 | Population-based |
| Sikdar 2017 [35] | <50 | 822 | 8804 | Canada | Retrospective cohort | Cancer registry/health administrative data | 2004–2010 | Population-based |
| Chen 2017 [36] | <50 | 253 | 232 | USA | Retrospective cohort | Primary data collection | 2008–2014 | 1 |
| Kim 2016 [12] | ≤45 | 693 | 1823 | Republic of Korea | Retrospective cohort | Primary data collection | 2006–2011 | 1 |
| Pita-Fernandez2016 [38] | <50 | - | 942 younger and older | Spain | Retrospective cohort | Primary data collection | 1994–2000 | 1 |
| Zhu 2015 [29] | <30 | 83 | 4911 | China | Retrospective cohort | Primary data collection | 1995–2013 | 1 |
| Saluja 2014 [40] | <40 | 66 | 100 | India | Retrospective cohort | Primary data collection | 2003–2012 | 1 |
| Redaniel 2014 [41] | 15–44 | 921 | 45,590 | UK | Retrospective cohort | Cancer registry/health administrative data | 1996–2009 | Population-based |
| de Sousa 2014 [43] | <50 | 66 | 149 | Brazil | Retrospective cohort | Primary data collection | 2006–2010 | 1 |
| Esteva 2013 [45] | <50 | 45 | 732 | Spain | Cross-sectional study | Primary data collection | 2006–2008 | 5 regions in Spain |
| Deng 2012 [46] | <50 | 75 | 232 | China | Prospective cohort | Primary data collection | 2008–2009 | 1 |
| Chan 2010 [47] | <40 | 53 | 47 | Sri Lanka | Retrospective cohort | Primary data collection | 1996–2008 | 1 |
| Tohme 2008 [48] | <45 | 43 | 282 | Lebanon | Retrospective cohort | Primary data collection | 1995–2005 | 1 |
| Porter 2005 [49] | <50 | - | 110 younger and older | Canada | Prospective cohort | Primary data collection | 2001 | 1 |
| Neal 2005 [50] | <45 | - | 13,244 younger and older | UK | Survey study | Primary data collection | 2002 | Population-based |
| Johnston 2004 [51] | 25-50 | 95 | 503 | Canada | Retrospective cohort | Cancer registry/health administrative data | 1992–2000 | Population-based |
| Robertson 2004 [52] | <50 | 53 | 1018 | UK | Retrospective cohort | Cancer registry/health administrative data | 1997–1998 | Population-based |
| Marble 1992 [53] | <40 | 50 | 50 | USA | Retrospective cohort | Primary data collection | 1935–1988 | 1 |
| Colon cancer | ||||||||
| Flemming 2017 [28] | <50 | 246 | 4080 | Canada | Retrospective cohort | Cancer registry/health administrative data and primary data collection | 2002–2008 | Population-based |
| Wanis 2017 [26] | <50 | 47 | 861 | Canada | Retrospective cohort | Primary data collection | 2006–2015 | 1 |
| Jones 2017 [37] | <50 | 74 | 312 | USA | Prospective cohort | Primary data collection | 2010–2013 | 9 |
| Gillis 2014 [42] | <50 | 695 | 9528 | Canada | Prospective cohort | Cancer registry/health administrative data | 2002–2008 | Population-based |
| Ben-Ishay 2013 [44] | <50 | 31 | 205 | Israel | Retrospective cohort | Primary data collection | 2000–2009 | 1 |
| Rectal cancer | ||||||||
| Scott 2016 [25] | <50 | 56 | 56 | USA | Case control | Primary data collection | 1997–2007 | 1 |
| Zhang 2015 [39] | <50 | 67 | 566 | China | Prospective cohort | Primary data collection | 2008–2009 | 1 |
| Finding | Studies |
|---|---|
| Delay measures | |
| Longer delays among younger patients (N = 14) | Webber 2020, Delisle 2020, Di Leo 2020, Windner 2018, Chen 2017, Jones 2017, Kim 2016, Scott 2016, Ben-Ishay 2013, Robertson 2004, Arhi 2019, Deng 2012, Tohme 2008, Marble 1992 |
| No significant differences (N = 14) | Majano 2021, Galadima 2021, Da Silva 2020, Van Erp 2019, Roder 2019, Kaplan 2019, Wanis 2017, Pita-Fernandez 2016, Zhu 2015, Saluja 2014, Esteva 2013, Chan 2010, Porter 2005, Johnston 2004 |
| Mixed findings (N = 2) | Neal 2005, Sikdar 2017 |
| Shorter delays among younger patients (N = 9) | Girolamo 2018, Gabriel 2017, Lima 2021, Pearson 2019, Flemming 2017, Zhang 2015, Redaniel 2014, Gillis 2014, de Sousa 2014 |
| Stage at presentation | |
| Worse stage at presentation among younger patients (N = 10) | Galadima 2021, Da Silva 2020, Arhi 2019, Kaplan 2019, Gabriel 2017, Chen 2017, Kim 2016, Zhu 2015, Marble 1992, Pita-Fernandez 2016 |
| No significant difference (N = 7) | Pita-Fernandez 2016, Scott 2016, Saluja 2014, de Sousa 2014, Ben-Ishay 2013, Chan 2010, Tohme 2008 |
| Survival | |
| Worse survival among younger patients (N = 3) | Kim 2016, Marble 1992, Kaplan 2019 |
| No significant difference (N = 7) | Da Silva 2020, Kaplan 2019, Scott 2016, Saluja 2014, de Sousa 2014, Ben-Ishay 2013, Tohme 2008 |
| Better survival among younger patients (N = 6) | Delisle 2020, Girolamo 2018, Gabriel 2017, Flemming 2017, Wanis 2017, Redaniel 2014 |
| Recurrence and disease-free survival | |
| Worse recurrence among younger patients (N = 2) | Kaplan 2019, Kim 2016 |
| No significant difference (N = 3) | Da Silva 2020, de Sousa 2014, Tohme 2008 |
| Study | Outcome | Finding in Younger Adults * | Finding in Older Adults * |
|---|---|---|---|
| Stage | |||
| Kim 2016 [12] | Advanced stage (Stage III/IV) | Longer delay associated with higher odds of advanced stage Symptom onset to diagnosis <1 month: Reference 1–3 months: OR 3.01 (1.77–5.12) >3 months: OR 6.33 (3.05–13.12) | Delay not associated with advanced stage Symptom onset to diagnosis <1 month: Reference 1–3 months: OR 1.28 (0.81–2.02) >3 months: OR 1.46 (0.81–2.62) |
| Chen 2017 [36] | Advanced stage (Stage III/IV) | Advanced stage associated with shorter delays Symptom onset to presentation Stage III/IV vs. Stage I/II: median difference—30 days Presentation to diagnosis Stage III/IV vs. Stage I/II: median difference —0 days Symptom onset to diagnosis Stage III/IV vs. Stage I/II: median difference—40 days | Mixed findings Symptom onset to presentation Stage III/IV vs. Stage I/II: median difference +9 days Presentation to diagnosis Stage III/IV vs. Stage I/II: median difference—14 days Symptom onset to diagnosis Stage III/IV vs. Stage I/II: median difference—5 days |
| Girolamo 2018 [13] ** | Advanced stage (Stage III/IV) | Delay not associated with advanced stage Referral to specialist consultation >2 weeks: OR 1.43 (0.65–3.52) Decision to treat to treatment >31 days: OR 0.76 (0.43–1.39) Referral to treatment >62 days: OR 1.03 (0.68–1.57) | Longed delay associated with lower odds of advanced stage for 2 intervals Referral to specialist consultation >2 weeks: OR 0.84 (0.76–0.92) Decision to treat to treatment >31 days: OR 0.66 (0.61–0.72) Referral to treatment >62 days: OR 0.99 (0.95–1.04) |
| Survival | |||
| Kim 2016 [12] | Overall survival | Longer delay associated with worse survival in adjusted analysis only Symptom onset to diagnosis, unadjusted <1 month: Reference 1–3 months: HR 1.41 (0.86–2.31) >3 months: HR 1.69 (0.99–2.91) Symptom onset to diagnosis, adjusted for sex and tumor differentiation <1 month: Reference1–3 months: HR 1.62 (0.95–2.76) >3 months: HR 2.57 (1.34–4.94) | Delay not associated with survival Symptom onset to diagnosis, unadjusted <1 month: Reference 1–3 months: HR 1.05 (0.59–1.87) >3 months: HR 0.75 (0.41–1.35) |
| Girolamo 2018 [13] | 1-year overall survival | Longer delay associated with improved survival Referral to specialist consultation <2 weeks vs. >2 weeks 88.9% (86.6–91.2) vs. 90.1% (80.9–99.3) Decision to treat to treatment <31 days vs. >31 days 89.8% (88.8–90.8) vs. 94.8% (89.1–100.0) Referral to treatment <62 days vs. >62 days 90.7% (88.3–93.0) vs. 94.0% (90.1–97.9) | Longer delay associated with improved survival Referral to specialist consultation <2 weeks vs. >2 weeks Age 45–54: 89.0% (87.9–90.1) vs. 89.4% (84.9–93.9) Age 55–64: 86.0% (85.3–86.8) vs. 88.5% (85.5–91.5) Age 65–74: 83.1% (82.4–83.7) vs. 86.5% (83.9–89.2) Age 75+: 76.9% (76.3–77.6) vs. 79.7% (76.9–82.4) Decision to treat to treatment <31 days vs. >31 days Age 45–54: 89.0% (87.9–90.1) vs. 91.0% (90.4–91.6) Age 55–64: 86.0% (85.3–86.8) vs. 91.9% (91.6–92.3) Age 65–74: 83.1% (82.4–83.7) vs. 91.0% (90.7–91.3) Age 75+: 76.9% (76.3–77.6) vs. 85.7% (85.3–86.1) Referral to treatment <62 days vs. >62 days Age 45–54: 91.6% (90.5–92.7) vs. 93.9% (92.1–95.8) Age 55–64: 90.3% (89.6–91.1) vs. 91.5% (90.2–92.8) Age 65–74: 89.4% (88.8–90.1) vs. 89.7% (88.7–90.8) Age 75+: 85.4% (84.7–86.1) vs. 90.9% (89.9–91.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelo, M.; Sue-Chue-Lam, C.; Paszat, L.; Scheer, A.S.; Hansen, B.E.; Kishibe, T.; Baxter, N.N. Clinical Delays and Comparative Outcomes in Younger and Older Adults with Colorectal Cancer: A Systematic Review. Curr. Oncol. 2022, 29, 8609-8625. https://doi.org/10.3390/curroncol29110679
Castelo M, Sue-Chue-Lam C, Paszat L, Scheer AS, Hansen BE, Kishibe T, Baxter NN. Clinical Delays and Comparative Outcomes in Younger and Older Adults with Colorectal Cancer: A Systematic Review. Current Oncology. 2022; 29(11):8609-8625. https://doi.org/10.3390/curroncol29110679
Chicago/Turabian StyleCastelo, Matthew, Colin Sue-Chue-Lam, Lawrence Paszat, Adena S. Scheer, Bettina E. Hansen, Teruko Kishibe, and Nancy N. Baxter. 2022. "Clinical Delays and Comparative Outcomes in Younger and Older Adults with Colorectal Cancer: A Systematic Review" Current Oncology 29, no. 11: 8609-8625. https://doi.org/10.3390/curroncol29110679
APA StyleCastelo, M., Sue-Chue-Lam, C., Paszat, L., Scheer, A. S., Hansen, B. E., Kishibe, T., & Baxter, N. N. (2022). Clinical Delays and Comparative Outcomes in Younger and Older Adults with Colorectal Cancer: A Systematic Review. Current Oncology, 29(11), 8609-8625. https://doi.org/10.3390/curroncol29110679






