Impact of Adjunct Testosterone on Cancer-Related Fatigue: An Ancillary Analysis from a Controlled Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Sampling for Inflammatory Markers and Amino Acids
2.2. Questionnaires to Assess Fatigue, Mood, and Quality of Life
2.2.1. Fatigue
2.2.2. Mood
2.2.3. Health-Related Quality of Life
2.3. Statistical Analyses
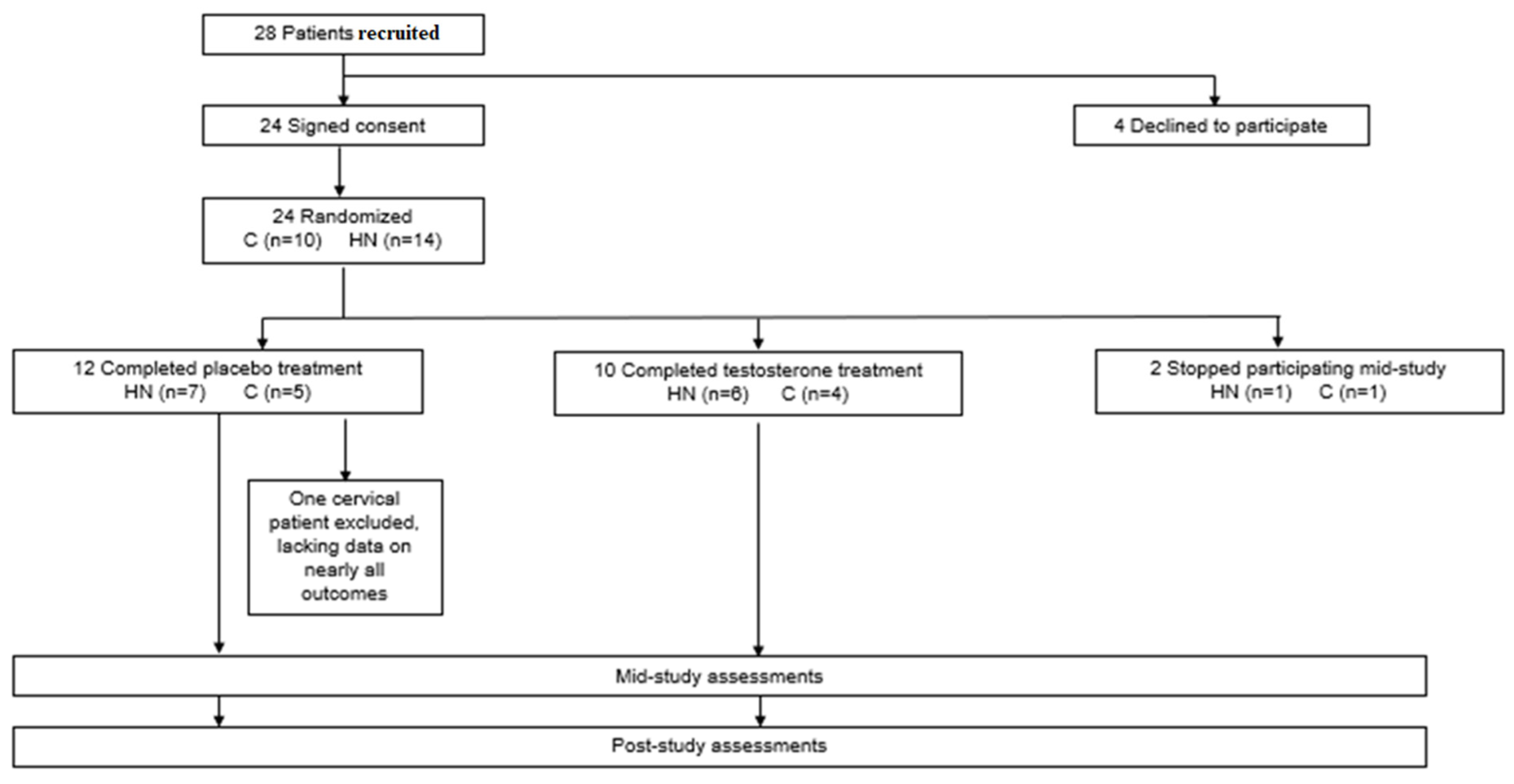
3. Results
3.1. Baseline Demographics
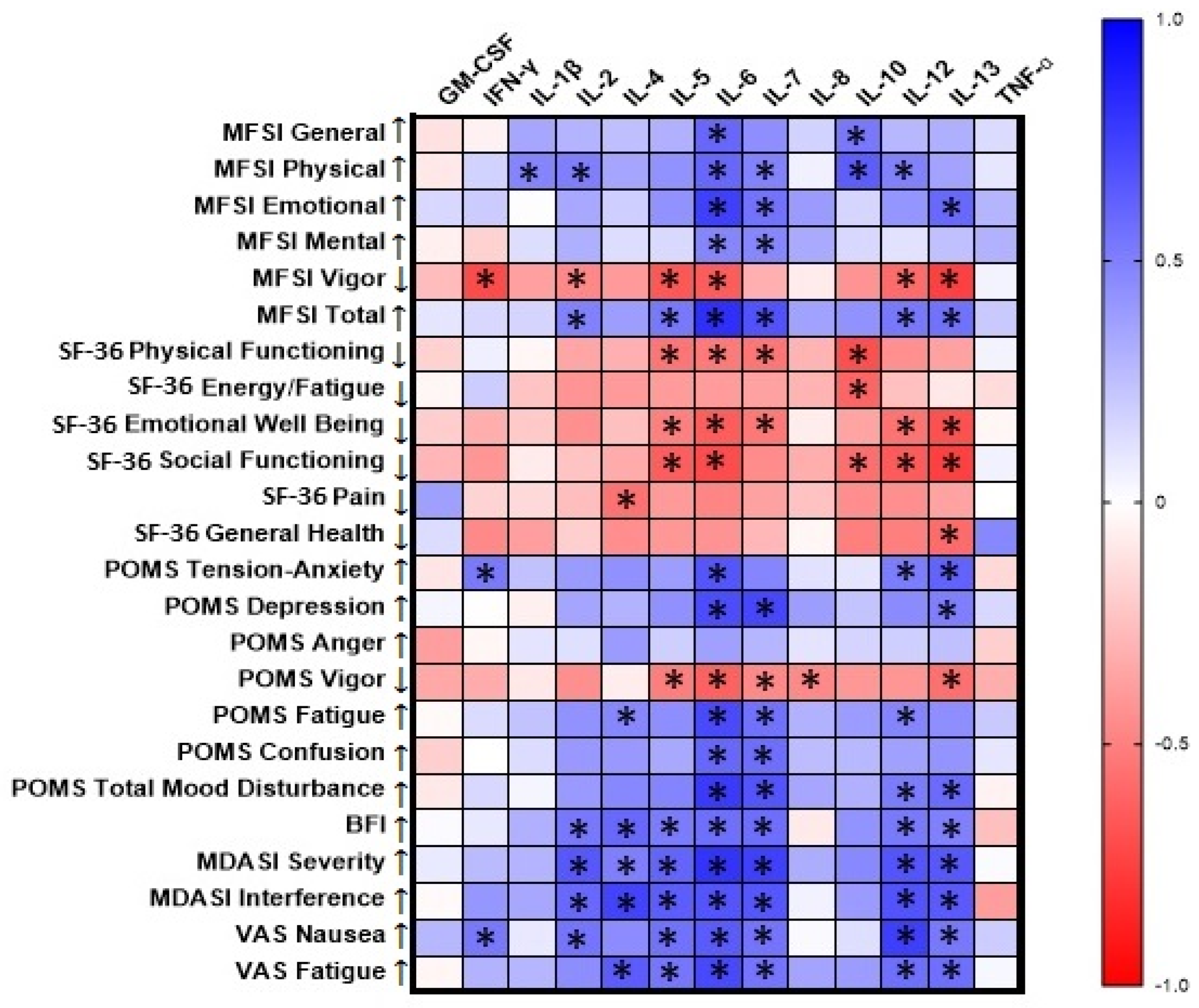
3.2. Correlations of Baseline Cytokine Levels with Questionnaire Scores
3.3. Comparisons of Baseline Questionnaire Scores to Normative Data
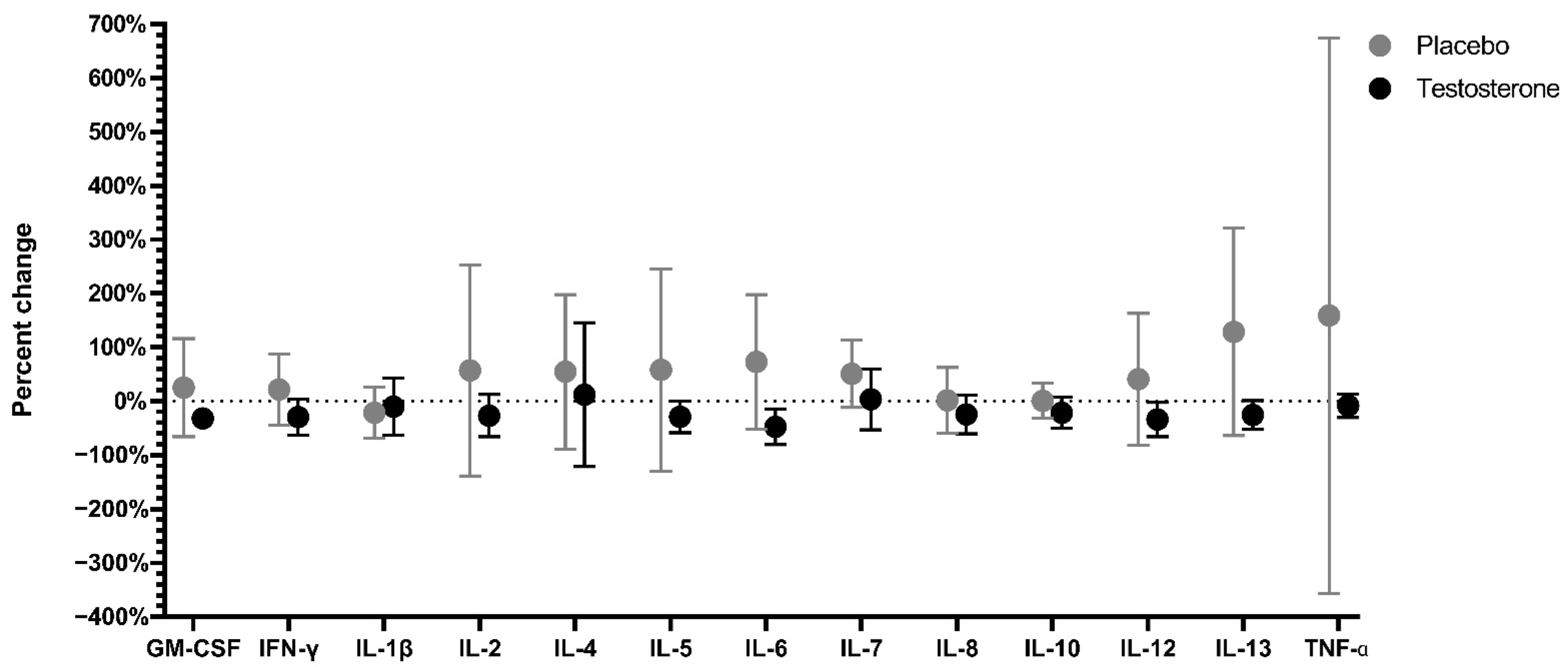
3.4. Serum Cytokine Levels
3.5. Serum Amino Acid Levels
3.6. Effects of Treatment and Time Point on Fatigue, Mood, and Quality of Life
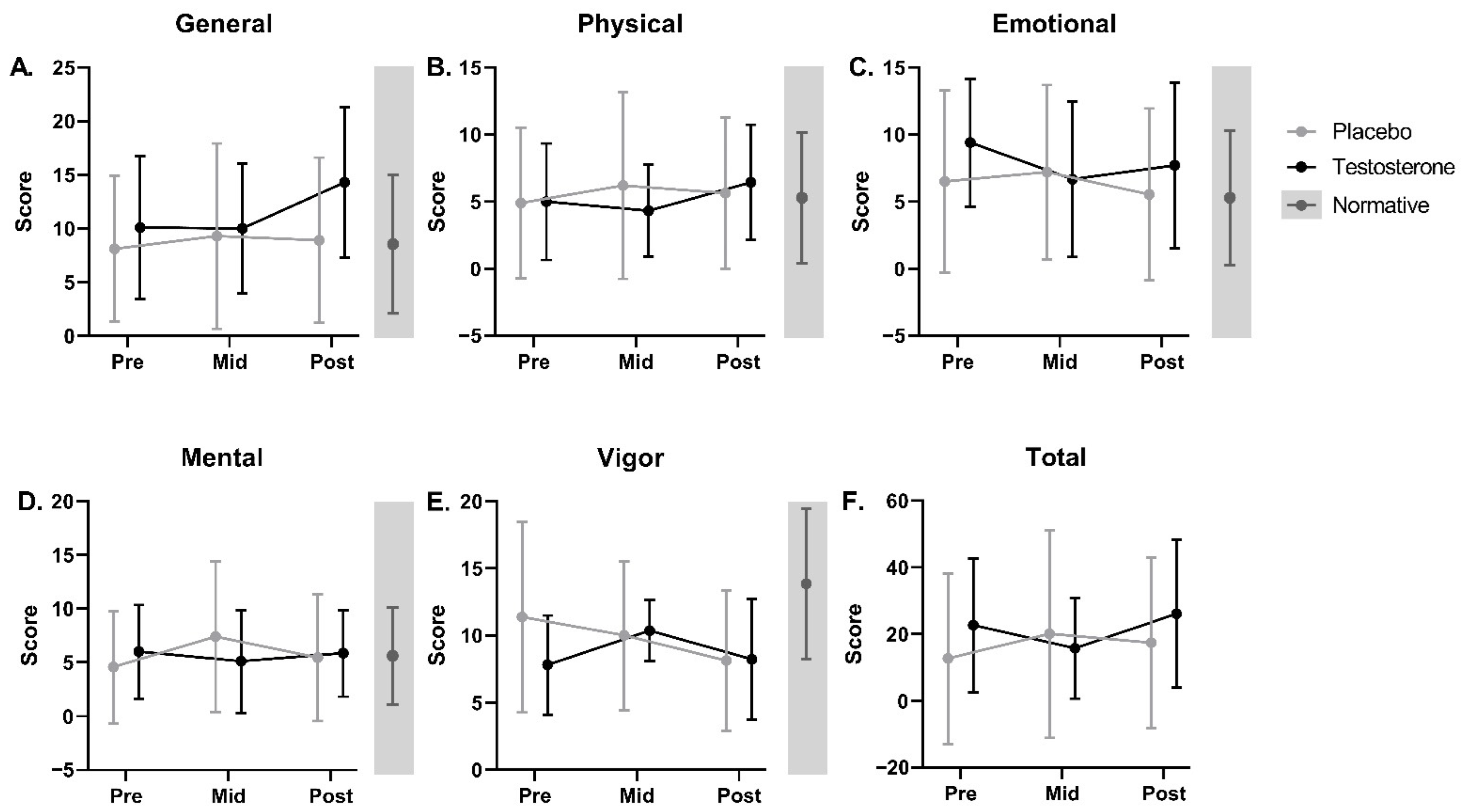
3.6.1. Fatigue
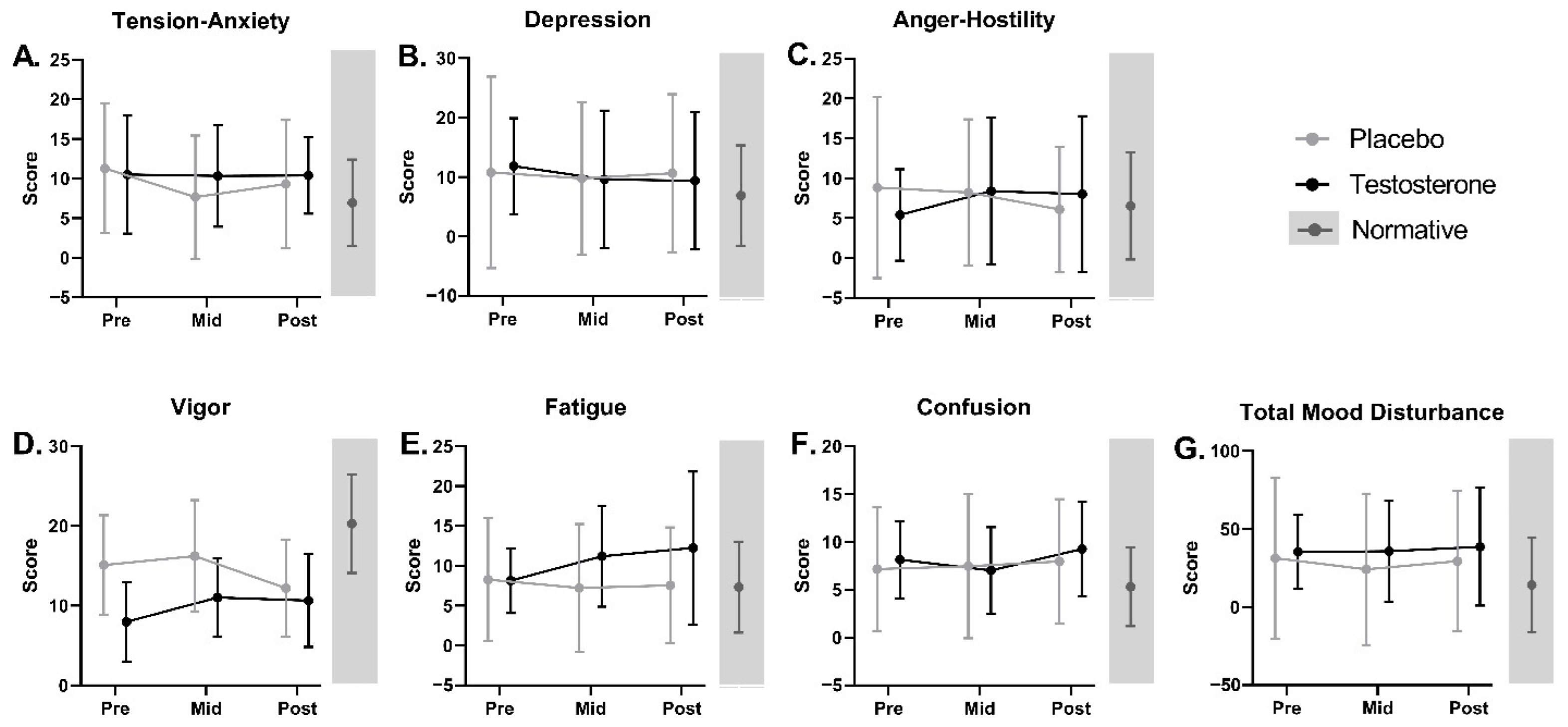
3.6.2. Mood
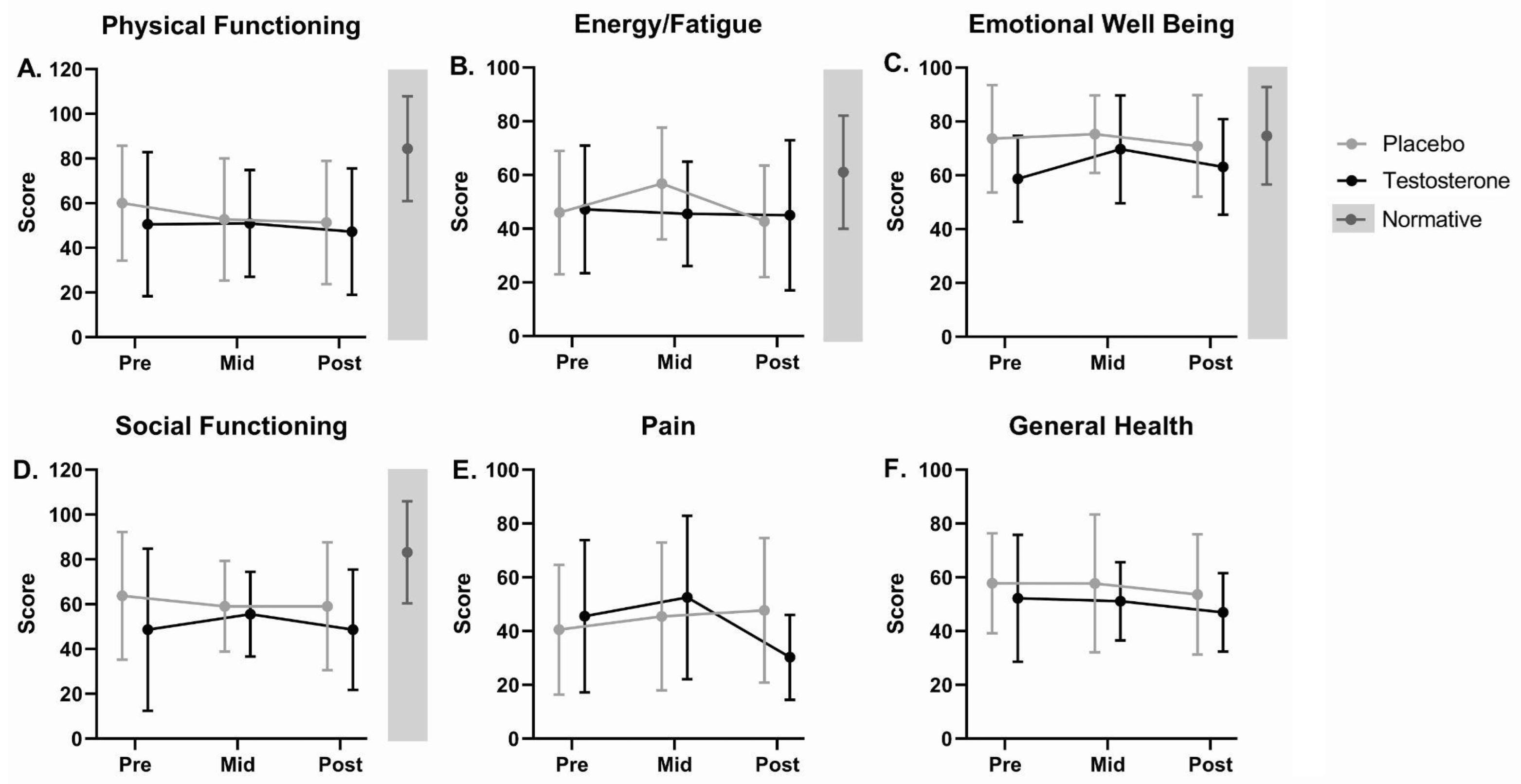
3.6.3. Health-Related Quality of Life
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogelzang, N.J.; Breitbart, W.; Cella, D.; Curt, G.A.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Scherr, S.L.; Portenoy, R.K. Patient, Caregiver, and Oncologist Perceptions of Cancer-Related Fatigue: Results of a Tripart Assessment Survey. The Fatigue Coalition. Semin. Hematol. 1997, 34, 4–12. [Google Scholar]
- Mortimer, J.E.; Barsevick, A.M.; Bennett, C.L.; Berger, A.M.; Cleeland, C.; De Vader, S.R.; Escalante, C.; Gilreath, J.; Hurria, A.; Mendoza, T.R.; et al. Studying Cancer-Related Fatigue: Report of the NCCN Scientific Research Committee. J. Natl. Compr. Cancer Netw. 2010, 8, 1331–1339. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Mendoza, T.R.; Wang, X.S.; Chou, C.; Harle, M.T.; Morrissey, M.; Engstrom, M.C. Assessing Symptom Distress in Cancer Patients: The MD Anderson Symptom Inventory. Cancer 2000, 89, 1634–1646. [Google Scholar] [CrossRef]
- Barsevick, A.M.; Cleeland, C.S.; Manning, D.C.; O’Mara, A.M.; Reeve, B.B.; Scott, J.A.; Sloan, J.A. ASCPRO (Assessing Symptoms of Cancer Using Patient-Reported Outcomes) ASCPRO Recommendations for the Assessment of Fatigue as an Outcome in Clinical Trials. J. Pain Symptom Manag. 2010, 39, 1086–1099. [Google Scholar] [CrossRef]
- Butt, Z.; Rosenbloom, S.K.; Abernethy, A.P.; Beaumont, J.L.; Paul, D.; Hampton, D.; Jacobsen, P.B.; Syrjala, K.L.; Von Roenn, J.H.; Cella, D. Fatigue Is the Most Important Symptom for Advanced Cancer Patients Who Have Had Chemotherapy. J. Natl. Compr. Cancer Netw. 2008, 6, 448–455. [Google Scholar] [CrossRef]
- Hwang, S.S.; Chang, V.T.; Cogswell, J.; Kasimis, B.S. Clinical Relevance of Fatigue Levels in Cancer Patients at a Veterans Administration Medical Center. Cancer 2002, 94, 2481–2489. [Google Scholar] [CrossRef]
- Mendoza, T.R.; Wang, X.S.; Cleeland, C.S.; Morrissey, M.; Johnson, B.A.; Wendt, J.K.; Huber, S.L. The Rapid Assessment of Fatigue Severity in Cancer Patients: Use of the Brief Fatigue Inventory. Cancer 1999, 85, 1186–1196. [Google Scholar] [CrossRef]
- Wang, X.S.; Hao, X.-S.; Wang, Y.; Guo, H.; Jiang, Y.-Q.; Mendoza, T.R.; Cleeland, C.S. Validation Study of the Chinese Version of the Brief Fatigue Inventory (BFI-C). J. Pain Symptom Manag. 2004, 27, 322–332. [Google Scholar] [CrossRef]
- Yun, Y.H.; Wang, X.S.; Lee, J.S.; Roh, J.W.; Lee, C.G.; Lee, W.S.; Lee, K.S.; Bang, S.-M.; Mendoza, T.R.; Cleeland, C.S. Validation Study of the Korean Version of the Brief Fatigue Inventory. J. Pain Symptom Manag. 2005, 29, 165–172. [Google Scholar] [CrossRef]
- Mantovani, G.; Macciò, A.; Madeddu, C.; Gramignano, G.; Lusso, M.R.; Serpe, R.; Massa, E.; Astara, G.; Deiana, L. A Phase II Study with Antioxidants, Both in the Diet and Supplemented, Pharmaconutritional Support, Progestagen, and Anti-Cyclooxygenase-2 Showing Efficacy and Safety in Patients with Cancer-Related Anorexia/Cachexia and Oxidative Stress. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1030–1034. [Google Scholar] [CrossRef]
- Wang, X.S. Pathophysiology of Cancer-Related Fatigue. Clin. J. Oncol. Nurs. 2008, 12, 11–20. [Google Scholar] [CrossRef]
- Yang, T.-Y.; Chen, M.-L.; Li, C.-C. Effects of an Aerobic Exercise Programme on Fatigue for Patients with Breast Cancer Undergoing Radiotherapy. J. Clin. Nurs. 2015, 24, 202–211. [Google Scholar] [CrossRef]
- Bower, J.E. Cancer-Related Fatigue: Links with Inflammation in Cancer Patients and Survivors. Brain Behav. Immun. 2007, 21, 863–871. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Bennett, G.J.; Dantzer, R.; Dougherty, P.M.; Dunn, A.J.; Meyers, C.A.; Miller, A.H.; Payne, R.; Reuben, J.M.; Wang, X.S.; et al. Are the Symptoms of Cancer and Cancer Treatment Due to a Shared Biologic Mechanism? A Cytokine-Immunologic Model of Cancer Symptoms. Cancer 2003, 97, 2919–2925. [Google Scholar] [CrossRef]
- Schubert, C.; Hong, S.; Natarajan, L.; Mills, P.J.; Dimsdale, J.E. The Association between Fatigue and Inflammatory Marker Levels in Cancer Patients: A Quantitative Review. Brain Behav. Immun. 2007, 21, 413–427. [Google Scholar] [CrossRef]
- Barsevick, A.; Frost, M.; Zwinderman, A.; Hall, P.; Halyard, M. GENEQOL Consortium I’m so Tired: Biological and Genetic Mechanisms of Cancer-Related Fatigue. Qual. Life Res. 2010, 19, 1419–1427. [Google Scholar] [CrossRef]
- Al-Majid, S.; McCarthy, D.O. Cancer-Induced Fatigue and Skeletal Muscle Wasting: The Role of Exercise. Biol. Res. Nurs. 2001, 2, 186–197. [Google Scholar] [CrossRef]
- Berger, A.M.; Wielgus, K.; Hertzog, M.; Fischer, P.; Farr, L. Patterns of Circadian Activity Rhythms and Their Relationships with Fatigue and Anxiety/Depression in Women Treated with Breast Cancer Adjuvant Chemotherapy. Support. Care Cancer 2010, 18, 105–114. [Google Scholar] [CrossRef]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef]
- Barton, D. Journey to Oz in Search of a Remedy for Fatigue. Cancer J 2014, 20, 15–17. [Google Scholar] [CrossRef]
- Escalante, C.P.; Meyers, C.; Reuben, J.M.; Wang, X.; Qiao, W.; Manzullo, E.; Alvarez, R.H.; Morrow, P.K.; Gonzalez-Angulo, A.M.; Wang, X.S.; et al. A Randomized, Double-Blind, 2-Period, Placebo-Controlled Crossover Trial of a Sustained-Release Methylphenidate in the Treatment of Fatigue in Cancer Patients. Cancer J. 2014, 20, 8–14. [Google Scholar] [CrossRef]
- Hovey, E.; de Souza, P.; Marx, G.; Parente, P.; Rapke, T.; Hill, A.; Bonaventura, A.; Michele, A.; Craft, P.; Abdi, E.; et al. Phase III, Randomized, Double-Blind, Placebo-Controlled Study of Modafinil for Fatigue in Patients Treated with Docetaxel-Based Chemotherapy. Support. Care Cancer 2014, 22, 1233–1242. [Google Scholar] [CrossRef]
- Auret, K.A.; Schug, S.A.; Bremner, A.P.; Bulsara, M. A Randomized, Double-Blind, Placebo-Controlled Trial Assessing the Impact of Dexamphetamine on Fatigue in Patients with Advanced Cancer. J. Pain Symptom Manag. 2009, 37, 613–621. [Google Scholar] [CrossRef]
- Bruera, E.; Valero, V.; Driver, L.; Shen, L.; Willey, J.; Zhang, T.; Palmer, J.L. Patient-Controlled Methylphenidate for Cancer Fatigue: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Clin. Oncol. 2006, 24, 2073–2078. [Google Scholar] [CrossRef]
- Bruera, E.; Yennurajalingam, S.; Palmer, J.L.; Perez-Cruz, P.E.; Frisbee-Hume, S.; Allo, J.A.; Williams, J.L.; Cohen, M.Z. Methylphenidate and/or a Nursing Telephone Intervention for Fatigue in Patients with Advanced Cancer: A Randomized, Placebo-Controlled, Phase II Trial. J. Clin. Oncol. 2013, 31, 2421–2427. [Google Scholar] [CrossRef]
- Butler, J.M.; Case, L.D.; Atkins, J.; Frizzell, B.; Sanders, G.; Griffin, P.; Lesser, G.; McMullen, K.; McQuellon, R.; Naughton, M.; et al. A Phase III, Double-Blind, Placebo-Controlled Prospective Randomized Clinical Trial of d-Threo-Methylphenidate HCl in Brain Tumor Patients Receiving Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1496–1501. [Google Scholar] [CrossRef]
- Kerr, C.W.; Drake, J.; Milch, R.A.; Brazeau, D.A.; Skretny, J.A.; Brazeau, G.A.; Donnelly, J.P. Effects of Methylphenidate on Fatigue and Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Pain Symptom Manag. 2012, 43, 68–77. [Google Scholar] [CrossRef]
- Lower, E.E.; Fleishman, S.; Cooper, A.; Zeldis, J.; Faleck, H.; Yu, Z.; Manning, D. Efficacy of Dexmethylphenidate for the Treatment of Fatigue after Cancer Chemotherapy: A Randomized Clinical Trial. J. Pain Symptom Manag. 2009, 38, 650–662. [Google Scholar] [CrossRef]
- Mar Fan, H.G.; Clemons, M.; Xu, W.; Chemerynsky, I.; Breunis, H.; Braganza, S.; Tannock, I.F. A Randomised, Placebo-Controlled, Double-Blind Trial of the Effects of d-Methylphenidate on Fatigue and Cognitive Dysfunction in Women Undergoing Adjuvant Chemotherapy for Breast Cancer. Support. Care Cancer 2008, 16, 577–583. [Google Scholar] [CrossRef]
- Moraska, A.R.; Sood, A.; Dakhil, S.R.; Sloan, J.A.; Barton, D.; Atherton, P.J.; Suh, J.J.; Griffin, P.C.; Johnson, D.B.; Ali, A.; et al. Phase III, Randomized, Double-Blind, Placebo-Controlled Study of Long-Acting Methylphenidate for Cancer-Related Fatigue: North Central Cancer Treatment Group NCCTG-N05C7 Trial. J. Clin. Oncol. 2010, 28, 3673–3679. [Google Scholar] [CrossRef]
- Roth, A.J.; Nelson, C.; Rosenfeld, B.; Scher, H.; Slovin, S.; Morris, M.; O’Shea, N.; Arauz, G.; Breitbart, W. Methylphenidate for Fatigue in Ambulatory Men with Prostate Cancer. Cancer 2010, 116, 5102–5110. [Google Scholar] [CrossRef]
- Paulsen, O.; Klepstad, P.; Rosland, J.H.; Aass, N.; Albert, E.; Fayers, P.; Kaasa, S. Efficacy of Methylprednisolone on Pain, Fatigue, and Appetite Loss in Patients with Advanced Cancer Using Opioids: A Randomized, Placebo-Controlled, Double-Blind Trial. J. Clin. Oncol. 2014, 32, 3221–3228. [Google Scholar] [CrossRef]
- Yennurajalingam, S.; Bruera, E. Role of Corticosteroids for Fatigue in Advanced Incurable Cancer: Is It a “wonder Drug” or “Deal with the Devil”. Curr. Opin. Support Palliat. Care 2014, 8, 346–351. [Google Scholar] [CrossRef]
- Andrews, R.C.; Walker, B.R. Glucocorticoids and Insulin Resistance: Old Hormones, New Targets. Clin. Sci. 1999, 96, 513–523. [Google Scholar] [CrossRef]
- Björntorp, P.; Rosmond, R. Obesity and Cortisol. Nutrition 2000, 16, 924–936. [Google Scholar] [CrossRef]
- Darmon, P.; Dadoun, F.; Boullu-Ciocca, S.; Grino, M.; Alessi, M.-C.; Dutour, A. Insulin Resistance Induced by Hydrocortisone Is Increased in Patients with Abdominal Obesity. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E995–E1002. [Google Scholar] [CrossRef]
- Bodine, S.C.; Furlow, J.D. Glucocorticoids and Skeletal Muscle. Adv. Exp. Med. Biol. 2015, 872, 145–176. [Google Scholar]
- Patil, C.G.; Lad, S.P.; Katznelson, L.; Laws, E.R., Jr. Brain Atrophy and Cognitive Deficits in Cushing’s Disease. Neurosurg. Focus 2007, 23, E11. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Glucocorticoids and Hippocampal Atrophy in Neuropsychiatric Disorders. Arch. Gen. Psychiatry 2000, 57, 925–935. [Google Scholar] [CrossRef]
- Alt, C.A.; Gore, E.M.; Montagnini, M.L.; Ng, A.V. Muscle Endurance, Cancer-Related Fatigue, and Radiotherapy in Prostate Cancer Survivors. Muscle Nerve 2011, 43, 415–424. [Google Scholar] [CrossRef]
- Arnold, M.E.; Taylor, N.F. Exercise for Patients with Cancer: Reducing Disease-Related Fatigue. Future Oncol. 2011, 7, 165–167. [Google Scholar] [CrossRef]
- McNeely, M.L.; Courneya, K.S. Exercise Programs for Cancer-Related Fatigue: Evidence and Clinical Guidelines. J. Natl. Compr. Cancer Netw. 2010, 8, 945–953. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Weert, E.; May, A.; Korstjens, I.; Post, W.J.; van der Schans, C.P.; van den Borne, B.; Mesters, I.; Ros, W.J.; Hoekstra-Weebers, J.E. Cancer-Related Fatigue and Rehabilitation: A Randomized Controlled Multicenter Trial Comparing Physical Training Combined with Cognitive-Behavioral Therapy with Physical Training Only and with no Intervention. Phys. Rev. D Part. Fields 2010, 90, 1413–1425. [Google Scholar]
- Brown, J.C.; Huedo-Medina, T.B.; Pescatello, L.S.; Pescatello, S.M.; Ferrer, R.A.; Johnson, B.T. Efficacy of Exercise Interventions in Modulating Cancer-Related Fatigue among Adult Cancer Survivors: A Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 123–133. [Google Scholar] [CrossRef]
- George, S.M.; Alfano, C.M.; Neuhouser, M.L.; Smith, A.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R. Better Postdiagnosis Diet Quality Is Associated with Less Cancer-Related Fatigue in Breast Cancer Survivors. J. Cancer Surviv. 2014, 8, 680–687. [Google Scholar] [CrossRef]
- Maskarinec, G.; Hullar, M.A.J.; Monroe, K.R.; Shepherd, J.A.; Hunt, J.; Randolph, T.W.; Wilkens, L.R.; Boushey, C.J.; Le Marchand, L.; Lim, U.; et al. Fecal Microbial Diversity and Structure Are Associated with Diet Quality in the Multiethnic Cohort Adiposity Phenotype Study. J. Nutr. 2019, 149, 1575–1584. [Google Scholar] [CrossRef]
- Ma, E.; Maskarinec, G.; Lim, U.; Boushey, C.J.; Wilkens, L.R.; Setiawan, V.W.; Le Marchand, L.; Randolph, T.W.; Jenkins, I.C.; Curtis, K.R.; et al. Long-Term Association between Diet Quality and Characteristics of the Gut Microbiome in the Multiethnic Cohort Study. Br. J. Nutr 2021, 128, 93–102. [Google Scholar] [CrossRef]
- Chan, A.; Shwe, M.; Koh, S.A.Q.; Zhu, C.; Chan, Y.S.; Tan, S.H.; Ang, X.J.; Lee, W.Q.; Khine, W.W.T.; Lee, Y.K.; et al. Gut Microbiome Alterations in Breast Cancer Survivors with Cancer-Related Fatigue. J. Clin. Orthod. 2018, 36, e22178. [Google Scholar] [CrossRef]
- Xiao, C.; Fedirko, V.; Beitler, J.; Bai, J.; Peng, G.; Zhou, C.; Gu, J.; Zhao, H.; Lin, I.-H.; Chico, C.E.; et al. The Role of the Gut Microbiome in Cancer-Related Fatigue: Pilot Study on Epigenetic Mechanisms. Support. Care Cancer 2021, 29, 3173–3182. [Google Scholar] [CrossRef]
- Topkan, E.; Yavuz, A.A.; Ozyilkan, O. Cancer Cachexia: Pathophysiologic Aspects and Treatment Options. Asian Pac. J. Cancer Prev. 2007, 8, 445–451. [Google Scholar]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Malnutrition, Fatigue, Frailty, Vulnerability, Sarcopenia and Cachexia: Overlap of Clinical Features. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 213–219. [Google Scholar] [CrossRef]
- Wright, T.J.; Dillon, E.L.; Durham, W.J.; Chamberlain, A.; Randolph, K.M.; Danesi, C.; Horstman, A.M.; Gilkison, C.R.; Willis, M.; Richardson, G.; et al. A Randomized Trial of Adjunct Testosterone for Cancer-Related Muscle Loss in Men and Women. J. Cachexia Sarcopenia Muscle 2018, 9, 482–496. [Google Scholar] [CrossRef]
- Bianchi, V.E. The Anti-Inflammatory Effects of Testosterone. J. Endocr. Soc. 2019, 3, 91–107. [Google Scholar] [CrossRef]
- Malkin, C.J.; Pugh, P.J.; Jones, R.D.; Kapoor, D.; Channer, K.S.; Jones, T.H. The Effect of Testosterone Replacement on Endogenous Inflammatory Cytokines and Lipid Profiles in Hypogonadal Men. J. Clin. Endocrinol. Metab. 2004, 89, 3313–3318. [Google Scholar] [CrossRef]
- Kalinchenko, S.Y.; Tishova, Y.A.; Mskhalaya, G.J.; Gooren, L.J.G.; Giltay, E.J.; Saad, F. Effects of Testosterone Supplementation on Markers of the Metabolic Syndrome and Inflammation in Hypogonadal Men with the Metabolic Syndrome: The Double-Blinded Placebo-Controlled Moscow Study. Clin. Endocrinol. 2010, 73, 602–612. [Google Scholar] [CrossRef]
- Dhindsa, S.; Ghanim, H.; Batra, M.; Kuhadiya, N.D.; Abuaysheh, S.; Sandhu, S.; Green, K.; Makdissi, A.; Hejna, J.; Chaudhuri, A.; et al. Insulin Resistance and Inflammation in Hypogonadotropic Hypogonadism and Their Reduction after Testosterone Replacement in Men with Type 2 Diabetes. Diabetes Care 2016, 39, 82–91. [Google Scholar] [CrossRef]
- Baracos, V.E. Management of Muscle Wasting in Cancer-Associated Cachexia: Understanding Gained from Experimental Studies. Cancer 2001, 92, 1669–1677. [Google Scholar] [CrossRef]
- Stein, K.D.; Martin, S.C.; Hann, D.M.; Jacobsen, P.B. A Multidimensional Measure of Fatigue for Use with Cancer Patients. Cancer Pract. 1998, 6, 143–152. [Google Scholar] [CrossRef]
- Chan, A.; Yo, T.E.; Wang, X.J.; Ng, T.; Chae, J.-W.; Yeo, H.L.; Shwe, M.; Gan, Y.X. Minimal Clinically Important Difference of the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) for Fatigue Worsening in Asian Breast Cancer Patients. J. Pain Symptom Manag. 2018, 55, 992–997.e2. [Google Scholar] [CrossRef]
- Cordero, E.D.; Loredo, J.S.; Murray, K.E.; Dimsdale, J.E. Characterizing Fatigue: The Effects of Ethnicity and Acculturation. J. Appl. Biobehav. Res. 2012, 17, 59–78. [Google Scholar] [CrossRef]
- Asvat, Y.; Malcarne, V.L.; Sadler, G.R.; Jacobsen, P.B. Validity of the Multidimensional Fatigue Symptom Inventory-Short Form in an African-American Community-Based Sample. Ethn. Health 2014, 19, 631–644. [Google Scholar] [CrossRef]
- Curran, S.L.; Andrykowski, M.A.; Studts, J.L. Short Form of the Profile of Mood States (POMS-SF): Psychometric Information. Psychol. Assess. 1995, 7, 80–83. [Google Scholar] [CrossRef]
- Nyenhuis, D.L.; Yamamoto, C.; Luchetta, T.; Terrien, A.; Parmentier, A. Adult and Geriatric Normative Data and Validation of the Profile of Mood States. J. Clin. Psychol. 1999, 55, 79–86. [Google Scholar] [CrossRef]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The RAND 36-Item Health Survey 1.0. Health Econ. 1993, 2, 217–227. [Google Scholar] [CrossRef]
- Ware, J.E.; Snow, K.K.; Kosinski, M.; Gandek, B. SF-36 Health Survey: Manual and Interpretation Guide; The Health Institute: Scarborough, ON, Canada, 1993. [Google Scholar]
- Ringash, J.; Bezjak, A.; O’Sullivan, B.; Redelmeier, D.A. Interpreting Differences in Quality of Life: The FACT-H&N in Laryngeal Cancer Patients. Qual. Life Res. 2004, 13, 725–733. [Google Scholar]
- Hornsby, B.W.Y.; Kipp, A.M. Subjective Ratings of Fatigue and Vigor in Adults with Hearing Loss Are Driven by Perceived Hearing Difficulties Not Degree of Hearing Loss. Ear Hear. 2016, 37, e1–e10. [Google Scholar] [CrossRef]
- Yavuzsen, T.; Davis, M.P.; Ranganathan, V.K.; Walsh, D.; Siemionow, V.; Kirkova, J.; Khoshknabi, D.; Lagman, R.; LeGrand, S.; Yue, G.H. Cancer-Related Fatigue: Central or Peripheral? J. Pain Symptom Manag. 2009, 38, 587–596. [Google Scholar] [CrossRef]
- Davis, M.P.; Walsh, D. Mechanisms of Fatigue. J. Support. Oncol. 2010, 8, 164–174. [Google Scholar]
- Taylor, J.L.; Todd, G.; Gandevia, S.C. Evidence for a Supraspinal Contribution to Human Muscle Fatigue. Clin. Exp. Pharmacol. Physiol. 2006, 33, 400–405. [Google Scholar] [CrossRef]
- Taylor, J.L.; Gandevia, S.C. A Comparison of Central Aspects of Fatigue in Submaximal and Maximal Voluntary Contractions. J. Appl. Physiol. 2008, 104, 542–550. [Google Scholar] [CrossRef]
- Bigland-Ritchie, B.; Jones, D.A.; Hosking, G.P.; Edwards, R.H. Central and Peripheral Fatigue in Sustained Maximum Voluntary Contractions of Human Quadriceps Muscle. Clin. Sci. Mol. Med. 1978, 54, 609–614. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue in Neurological Disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue and Basal Ganglia. J. Neurol. Sci. 2000, 179, 34–42. [Google Scholar] [CrossRef]
- Fernandez, C.; Firdous, S.; Jehangir, W.; Behm, B.; Mehta, Z.; Berger, A.; Davis, M. Cancer-Related Fatigue: Perception of Effort or Task Failure? Am. J. Hosp. Palliat. Care 2020, 37, 34–40. [Google Scholar] [CrossRef]
- Kozłowski, L.; Zakrzewska, I.; Tokajuk, P.; Wojtukiewicz, M.Z. Concentration of Interleukin-6 (IL-6), Interleukin-8 (IL-8) and Interleukin-10 (IL-10) in Blood Serum of Breast Cancer Patients. Rocz. Akad. Med. Bialymst. 2003, 48, 82–84. [Google Scholar]
- Adler, H.L.; McCURDY, M.A.; Kattan, M.W.; Timme, T.L.; Scardino, P.T.; Thompson, T.C. Elevated Levels of Circulating Interleukin-6 and Transforming Growth Factor-Beta1 in Patients with Metastatic Prostatic Carcinoma. J. Urol. 1999, 161, 182–187. [Google Scholar] [CrossRef]
- Miura, T.; Mitsunaga, S.; Ikeda, M.; Shimizu, S.; Ohno, I.; Takahashi, H.; Furuse, J.; Inagaki, M.; Higashi, S.; Kato, H.; et al. Characterization of Patients with Advanced Pancreatic Cancer and High Serum Interleukin-6 Levels. Pancreas 2015, 44, 756–763. [Google Scholar] [CrossRef]
- Knüpfer, H.; Preiss, R. Significance of Interleukin-6 (IL-6) in Breast Cancer (Review). Breast Cancer Res. Treat. 2007, 102, 129–135. [Google Scholar] [CrossRef]
- Bossola, M.; Di Stasio, E.; Giungi, S.; Rosa, F.; Tazza, L. Fatigue Is Associated with Serum Interleukin-6 Levels and Symptoms of Depression in Patients on Chronic Hemodialysis. J. Pain Symptom Manag. 2015, 49, 578–585. [Google Scholar] [CrossRef]
- Pereira, J.R.; Santos, L.V.D.; Santos, R.M.S.; Campos, A.L.F.; Pimenta, A.L.; de Oliveira, M.S.; Bacheti, G.G.; Rocha, N.P.; Teixeira, A.L.; Christo, P.P.; et al. IL-6 Serum Levels Are Elevated in Parkinson’s Disease Patients with Fatigue Compared to Patients without Fatigue. J. Neurol. Sci. 2016, 370, 153–156. [Google Scholar] [CrossRef]
- Späth-Schwalbe, E.; Hansen, K.; Schmidt, F.; Schrezenmeier, H.; Marshall, L.; Burger, K.; Fehm, H.L.; Born, J. Acute Effects of Recombinant Human Interleukin-6 on Endocrine and Central Nervous Sleep Functions in Healthy Men. J. Clin. Endocrinol. Metab. 1998, 83, 1573–1579. [Google Scholar] [CrossRef]
- Bagnall-Moreau, C.; Chaudhry, S.; Salas-Ramirez, K.; Ahles, T.; Hubbard, K. Chemotherapy-Induced Cognitive Impairment Is Associated with Increased Inflammation and Oxidative Damage in the Hippocampus. Mol. Neurobiol. 2019, 56, 7159–7172. [Google Scholar] [CrossRef]
- Stasi, R.; Abriani, L.; Beccaglia, P.; Terzoli, E.; Amadori, S. Cancer-Related Fatigue: Evolving Concepts in Evaluation and Treatment. Cancer 2003, 98, 1786–1801. [Google Scholar] [CrossRef]
- Singer, S.; Kuhnt, S.; Zwerenz, R.; Eckert, K.; Hofmeister, D.; Dietz, A.; Giesinger, J.; Hauss, J.; Papsdorf, K.; Briest, S.; et al. Age- and Sex-Standardised Prevalence Rates of Fatigue in a Large Hospital-Based Sample of Cancer Patients. Br. J. Cancer 2011, 105, 445–451. [Google Scholar] [CrossRef]
- Ganz, P.A.; Moinpour, C.M.; Pauler, D.K.; Kornblith, A.B.; Gaynor, E.R.; Balcerzak, S.P.; Gatti, G.S.; Erba, H.P.; McCoy, S.; Press, O.W.; et al. Health Status and Quality of Life in Patients with Early-Stage Hodgkin’s Disease Treated on Southwest Oncology Group Study 9133. J. Clin. Oncol. 2003, 21, 3512–3519. [Google Scholar] [CrossRef]
- Hermelink, K.; Untch, M.; Lux, M.P.; Kreienberg, R.; Beck, T.; Bauerfeind, I.; Münzel, K. Cognitive Function during Neoadjuvant Chemotherapy for Breast Cancer: Results of a Prospective, Multicenter, Longitudinal Study. Cancer 2007, 109, 1905–1913. [Google Scholar] [CrossRef]
- Lange, M.; Giffard, B.; Noal, S.; Rigal, O.; Kurtz, J.-E.; Heutte, N.; Lévy, C.; Allouache, D.; Rieux, C.; Le Fel, J.; et al. Baseline Cognitive Functions among Elderly Patients with Localised Breast Cancer. Eur. J. Cancer 2014, 50, 2181–2189. [Google Scholar] [CrossRef]
- Menning, S.; de Ruiter, M.B.; Veltman, D.J.; Koppelmans, V.; Kirschbaum, C.; Boogerd, W.; Reneman, L.; Schangen, S.B. Multimodal MRI and Cognitive Function in Patients with Breast Cancer Prior to Adjuvant Treatment—The Role of Fatigue. NeuroImage: Clinical 2015, 7, 547–554. [Google Scholar] [CrossRef]
- Bond, S.M.; Dietrich, M.S.; Murphy, B.A. Neurocognitive Function in Head and Neck Cancer Patients Prior to Treatment. Support. Care Cancer 2012, 20, 149–157. [Google Scholar] [CrossRef]
- Ahles, T.A.; Saykin, A.J.; McDonald, B.C.; Furstenberg, C.T.; Cole, B.F.; Hanscom, B.S.; Mulrooney, T.J.; Schwartz, G.N.; Kaufman, P.A. Cognitive Function in Breast Cancer Patients Prior to Adjuvant Treatment. Breast Cancer Res. Treat. 2008, 110, 143–152. [Google Scholar] [CrossRef]
- Wefel, J.S.; Saleeba, A.K.; Buzdar, A.U.; Meyers, C.A. Acute and Late Onset Cognitive Dysfunction Associated with Chemotherapy in Women with Breast Cancer. Cancer 2010, 116, 3348–3356. [Google Scholar] [CrossRef]
- Li, J.; Yu, L.; Long, Z.; Li, Y.; Cao, F. Perceived Cognitive Impairment in Chinese Patients with Breast Cancer and Its Relationship with Post-Traumatic Stress Disorder Symptoms and Fatigue. Psychooncology 2015, 24, 676–682. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, Mechanisms, and Management of Cancer-Related Cognitive Impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef]
- Gibson, E.M.; Monje, M. Microglia in Cancer Therapy-Related Cognitive Impairment. Trends Neurosci. 2021, 44, 441–451. [Google Scholar] [CrossRef]
- Briones, T.L.; Woods, J. Dysregulation in Myelination Mediated by Persistent Neuroinflammation: Possible Mechanisms in Chemotherapy-Related Cognitive Impairment. Brain Behav. Immun. 2014, 35, 23–32. [Google Scholar] [CrossRef]
- Lomeli, N.; Lepe, J.; Gupta, K.; Bota, D.A. Cognitive Complications of Cancer and Cancer-Related Treatments—Novel Paradigms. Neurosci. Lett. 2021, 749, 135720. [Google Scholar] [CrossRef]
- Országhová, Z.; Mego, M.; Chovanec, M. Long-Term Cognitive Dysfunction in Cancer Survivors. Front. Mol. Biosci. 2021, 8, 770413. [Google Scholar] [CrossRef]
- Zwielehner, J.; Lassl, C.; Hippe, B.; Pointner, A.; Switzeny, O.J.; Remely, M.; Kitzweger, E.; Ruckser, R.; Haslberger, A.G. Changes in Human Fecal Microbiota Due to Chemotherapy Analyzed by TaqMan-PCR, 454 Sequencing and PCR-DGGE Fingerprinting. PLoS ONE 2011, 6, e28654. [Google Scholar]
- Montassier, E.; Batard, E.; Massart, S.; Gastinne, T.; Carton, T.; Caillon, J.; Le Fresne, S.; Caroff, N.; Hardouin, J.B.; Moreau, P.; et al. 16S RRNA Gene Pyrosequencing Reveals Shift in Patient Faecal Microbiota during High-Dose Chemotherapy as Conditioning Regimen for Bone Marrow Transplantation. Microb. Ecol. 2014, 67, 690–699. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Yooseph, S.; Harkins, D.M.; Moncera, K.J.; Zabokrtsky, K.B.; Torralba, M.G.; Tovchigrechko, A.; Highlander, S.K.; Pieper, R.; Sender, L.; et al. Gastrointestinal Microbial Populations Can Distinguish Pediatric and Adolescent Acute Lymphoblastic Leukemia (ALL) at the Time of Disease Diagnosis. BMC Genom. 2016, 17, 635. [Google Scholar] [CrossRef]
- Urban, R.J.; Pyles, R.B.; Stewart, C.J.; Ajami, N.; Randolph, K.M.; Durham, W.J.; Danesi, C.P.; Dillon, E.L.; Summons, J.R.; Singh, C.K.; et al. Altered Fecal Microbiome Years after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1037–1051. [Google Scholar] [CrossRef]
- Urban, R.J. A Treatable Syndrome in Patients with Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1124–1125. [Google Scholar] [CrossRef]
- Callan, C.; Ladds, E.; Husain, L.; Pattinson, K.; Greenhalgh, T. “I Can’t Cope with Multiple Inputs”: A Qualitative Study of the Lived Experience of “brain Fog” after COVID-19. BMJ Open 2022, 12, e056366. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Cockshell, S.J.; Mathias, J.L. Cognitive Functioning in Chronic Fatigue Syndrome: A Meta-Analysis. Psychol. Med. 2010, 40, 1253–1267. [Google Scholar] [CrossRef]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced Diversity and Altered Composition of the Gut Microbiome in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef]
- Wasser, C.I.; Mercieca, E.-C.; Kong, G.; Hannan, A.J.; McKeown, S.J.; Glikmann-Johnston, Y.; Stout, J.C. Gut Dysbiosis in Huntington’s Disease: Associations among Gut Microbiota, Cognitive Performance and Clinical Outcomes. Brain Commun. 2020, 2, fcaa110. [Google Scholar] [CrossRef]
- Hughes, H.K.; Rose, D.; Ashwood, P. The Gut Microbiota and Dysbiosis in Autism Spectrum Disorders. Curr. Neurol. Neurosci. Rep. 2018, 18, 81. [Google Scholar] [CrossRef]
- Neroni, B.; Evangelisti, M.; Radocchia, G.; Di Nardo, G.; Pantanella, F.; Villa, M.P.; Schippa, S. Relationship between Sleep Disorders and Gut Dysbiosis: What Affects What? Sleep Med. 2021, 87, 1–7. [Google Scholar] [CrossRef]
- Hajjar, J.; Mendoza, T.; Zhang, L.; Fu, S.; Piha-Paul, S.A.; Hong, D.S.; Janku, F.; Karp, D.D.; Ballhausen, A.; Gong, J.; et al. Associations between the Gut Microbiome and Fatigue in Cancer Patients. Sci. Rep. 2021, 11, 5847. [Google Scholar] [CrossRef]

| Total | Placebo | Testosterone | |||
|---|---|---|---|---|---|
| (N = 21) | (N = 11) | (N = 10) | p | ||
| Age (y) | Mean ± SD | 51.9 ± 10.1 | 50.1 ± 11.8 | 53.9 ± 8 | 0.39 |
| Range | 35–71 | 35–71 | 35–61 | ||
| Body Mass (initial, kg) | Mean ± SD | 65.4 ± 22.6 | 69.4 ± 25.7 | 60.9 ± 19.1 | 0.40 |
| Range | 22.6–130.7 | 44.4–130.7 | 39–101 | ||
| BMI (initial) | Mean ± SD | 23 ± 6.8 | 23.9 ± 6.9 | 22 ± 6.9 | 0.55 |
| Range | 14.5–40.3 | 16.3–40.3 | 14.5–37.6 | ||
| Days in study | Mean ± SD | 47.5 ± 8.8 | 47.8 ± 10 | 47 ± 7.7 | 0.81 |
| Sex—no. (%) | Male | 10 (47.6) | 7 (63.6) | 3 (30) | 0.20 |
| Female | 11 (52.4) | 4 (36.3) | 7 (70) | ||
| Race—no. (%) | Black | 4 (19.1) | 2 (18.2) | 2 (20) | 0.52 |
| Caucasian | 15 (71.4) | 7 (63.6) | 8 (80) | ||
| Hispanic | 2 (9.5) | 2 (18.2) | 0 (0) | ||
| Tumor Stage—no. (%) | IIB | 2 (9.5) | 0 (0) | 2 (20) | 0.71 |
| III | 1 (4.8) | 1 (9.1) | 0 (0) | ||
| IIIB | 5 (23.8) | 3 (27.3) | 2 (20) | ||
| IV | 1 (4.8) | 1 (9.1) | 0 (0) | ||
| IVA | 9 (42.9) | 4 (36.4) | 5 (50) | ||
| IVB | 3 (14.3) | 2 (18.2) | 1 (10) | ||
| Cancer Type—no. (%) | Cervical | 8 (38.1) | 4 (36.4) | 4 (40) | 1 |
| Head/neck | 13 (61.9) | 7 (63.6) | 6 (60) | ||
| Received Treatment—no. (%) | Chemotherapy | 16 (76.2) | 7 (63.6) | 8 (80) | 0.64 |
| Radiation | 19 (90.5) | 10 (90.9) | 9 (90) | 1 |
| Cancer Patients (n) | Normative Data (n) | p | p (adj) | |
|---|---|---|---|---|
| MFSI General | 8.5 ± 6.4 (21) | 7.3 ± 5.9 (176) | 0.413 | 0.966 |
| MFSI Physical | 5.0 ± 4.9 (21) | 4.0 ± 4.4 (176) | 0.390 | 0.966 |
| MFSI Emotional | 7.9 ± 6.0 (21) | 4.0 ± 4.6 (176) | 0.009 | 0.124 |
| MFSI Mental | 5.2 ± 4.8 (21) | 4.2 ± 4.1 (176) | 0.384 | 0.966 |
| MFSI Vigor | 9.7 ± 5.9 (21) | 13.8 ± 5.6 (176) | 0.006 | 0.101 |
| SF-36 Physical Functioning * | 55.5 ± 28.6 (19) | 84.2 ± 23.3 (2474) | <0.001 | 0.006 |
| SF-36 Limitations Physical * | 25.0 ± 36.3 (19) | 81.0 ± 34.0 (2474) | <0.001 | <0.001 |
| SF-36 Limitations Emotional | 56.1 ± 49.8 (19) | 81.3 ± 33.0 (2474) | 0.041 | 0.343 |
| SF-36 Energy-Fatigue | 46.6 ± 22.7 (19) | 60.9 ± 21.0 (2474) | 0.014 | 0.152 |
| SF-36 Emotional Well Being | 66.5 ± 19.3 (19) | 74.7 ± 18.1 (2474) | 0.080 | 0.467 |
| SF-36 Social Functioning * | 56.6 ± 32.4 (19) | 83.3 ± 22.7 (2474) | 0.002 | 0.031 |
| POMS Tension-Anxiety | 11.0 ± 7.7 (19) | 7.0 ± 5.5 (432) | 0.037 | 0.391 |
| POMS Depression | 11.3 ± 12.8 (20) | 7.1 ± 8.4 (432) | 0.162 | 0.829 |
| POMS Anger–Hostility | 7.3 ± 9.2 (20) | 6.6 ± 6.7 (432) | 0.740 | 0.966 |
| POMS Vigor * | 11.9 ± 6.7 (20) | 20.2 ± 6.2 (432) | <0.001 | <0.001 |
| POMS Fatigue | 9.3 ± 7.6 (20) | 7.3 ± 5.7 (432) | 0.259 | 0.933 |
| POMS Confusion | 7.6 ± 5.5 (19) | 5.2 ± 4.1 (432) | 0.076 | 0.579 |
| POMS Total Mood Disturbance | 33.0 ± 41.2 (19) | 12.7 ± 29.6 (432) | 0.047 | 0.440 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGovern, K.A.; Durham, W.J.; Wright, T.J.; Dillon, E.L.; Randolph, K.M.; Danesi, C.P.; Urban, R.J.; Sheffield-Moore, M. Impact of Adjunct Testosterone on Cancer-Related Fatigue: An Ancillary Analysis from a Controlled Randomized Trial. Curr. Oncol. 2022, 29, 8340-8356. https://doi.org/10.3390/curroncol29110658
McGovern KA, Durham WJ, Wright TJ, Dillon EL, Randolph KM, Danesi CP, Urban RJ, Sheffield-Moore M. Impact of Adjunct Testosterone on Cancer-Related Fatigue: An Ancillary Analysis from a Controlled Randomized Trial. Current Oncology. 2022; 29(11):8340-8356. https://doi.org/10.3390/curroncol29110658
Chicago/Turabian StyleMcGovern, Kristen A., William J. Durham, Traver J. Wright, E. Lichar Dillon, Kathleen M. Randolph, Christopher P. Danesi, Randall J. Urban, and Melinda Sheffield-Moore. 2022. "Impact of Adjunct Testosterone on Cancer-Related Fatigue: An Ancillary Analysis from a Controlled Randomized Trial" Current Oncology 29, no. 11: 8340-8356. https://doi.org/10.3390/curroncol29110658
APA StyleMcGovern, K. A., Durham, W. J., Wright, T. J., Dillon, E. L., Randolph, K. M., Danesi, C. P., Urban, R. J., & Sheffield-Moore, M. (2022). Impact of Adjunct Testosterone on Cancer-Related Fatigue: An Ancillary Analysis from a Controlled Randomized Trial. Current Oncology, 29(11), 8340-8356. https://doi.org/10.3390/curroncol29110658





