Lymphadenectomy Benefits Small Cell Carcinoma of Ovary: A Population-Based Analysis
Abstract
1. Introduction
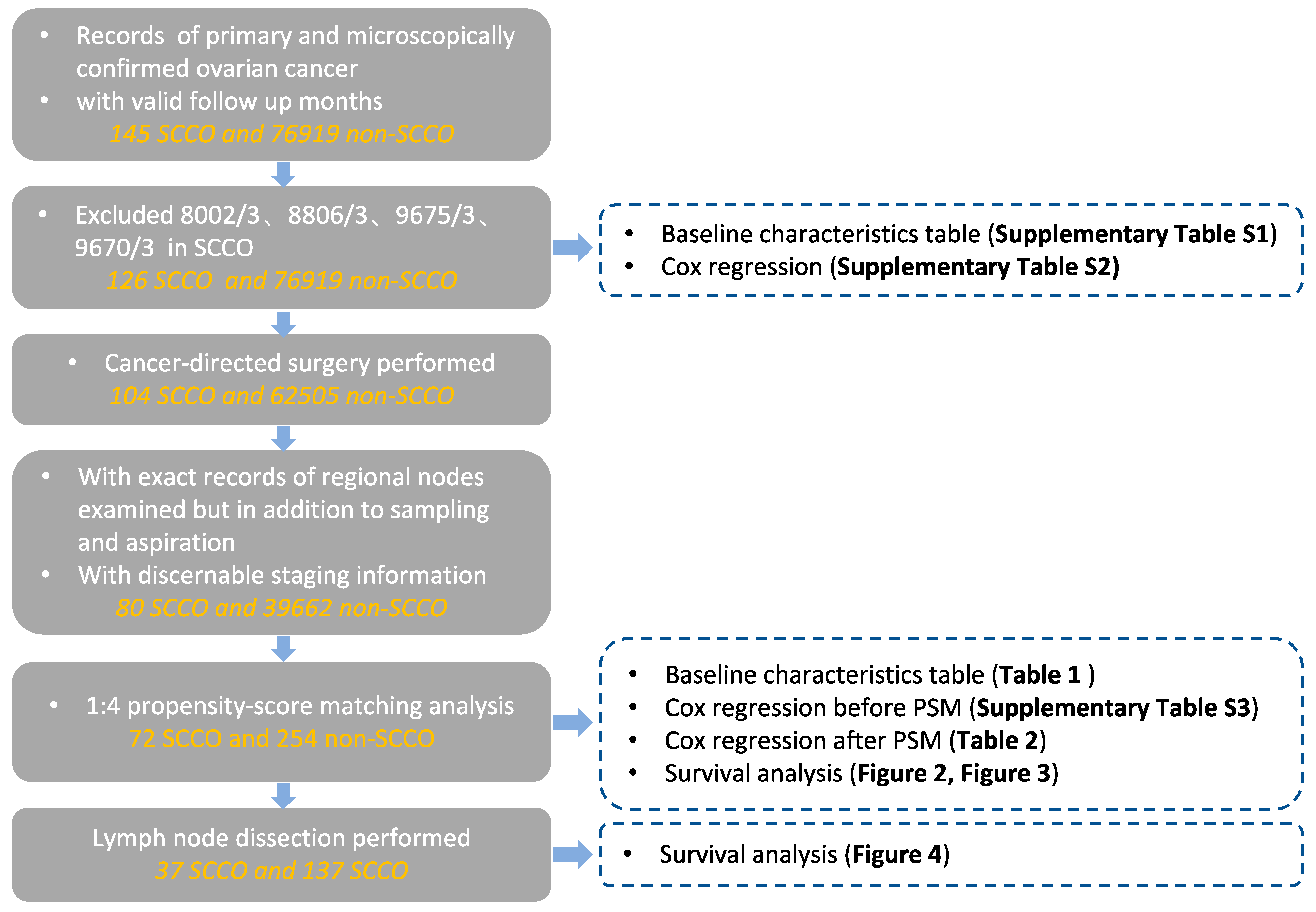
2. Materials and Methods
3. Results
3.1. Patients and Characteristics
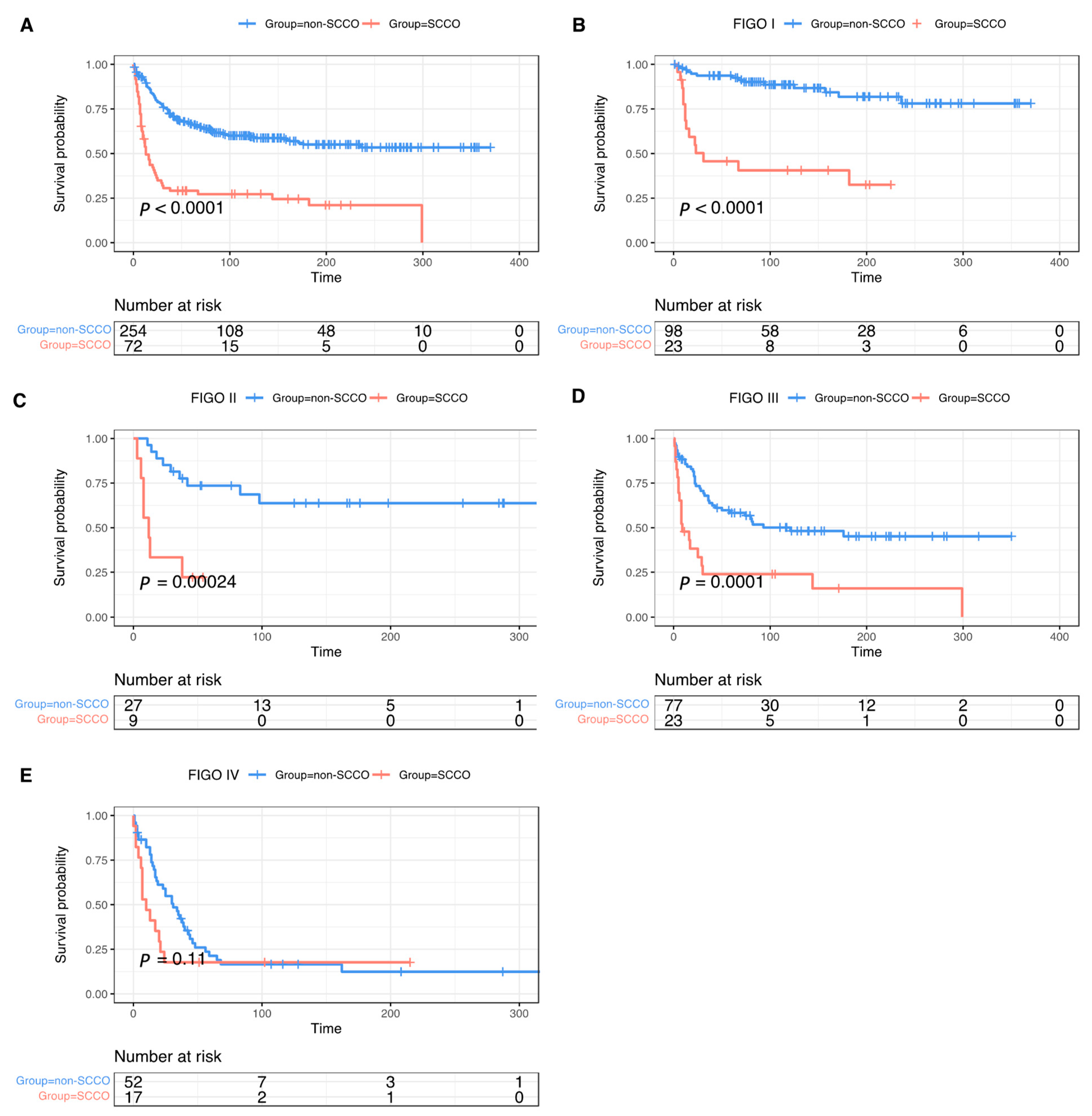
3.2. Survival and Prognostic Analysis
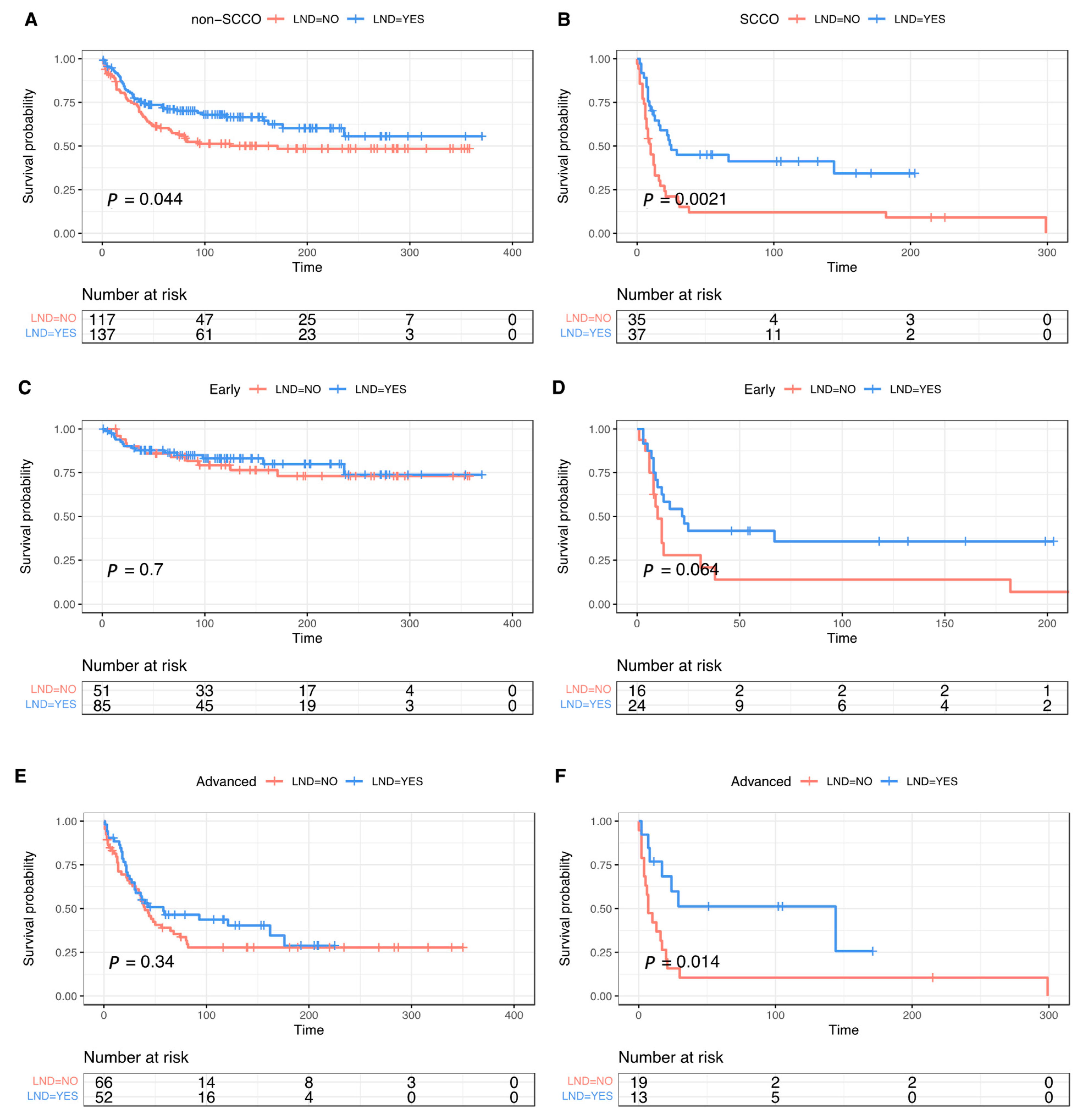
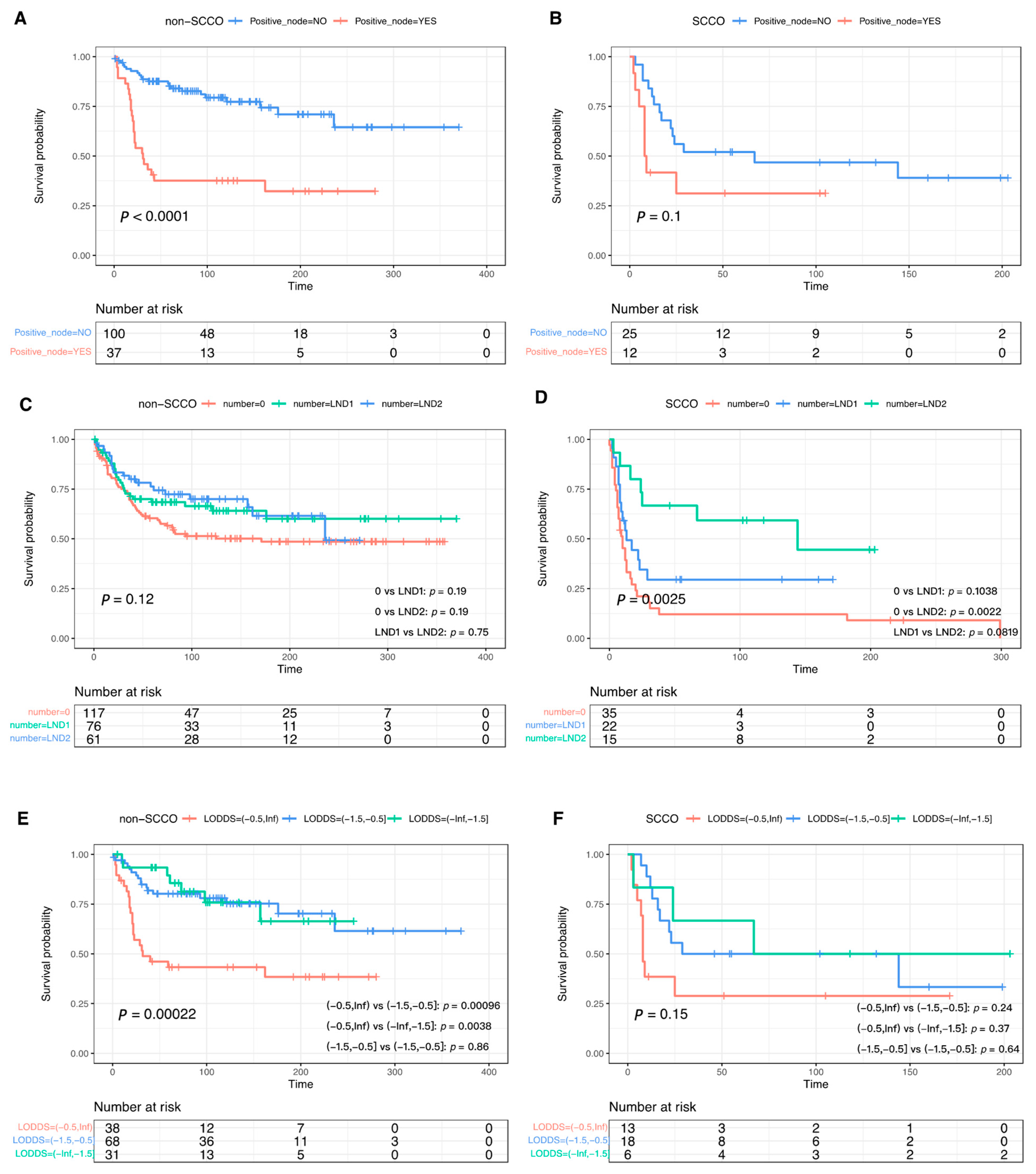
3.3. The Effect of Lymphadenectomy and Role of Lymph Node Metastasis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patibandla, J.R.; Fehniger, J.E.; Levine, D.A.; Jelinic, P. Small Cell Cancers of the Female Genital Tract: Molecular and Clinical Aspects. Gynecol. Oncol. 2018, 149, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Young, R.H.; Oliva, E.; Scully, R.E. Small Cell Carcinoma of the Ovary, Hypercalcemic Type. A Clinicopathological Analysis of 150 Cases. Am. J. Surg. Pathol. 1994, 18, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, L.; Carrot-Zhang, J.; Albrecht, S.; Fahiminiya, S.; Hamel, N.; Tomiak, E.; Grynspan, D.; Saloustros, E.; Nadaf, J.; Rivera, B.; et al. Germline and Somatic SMARCA4 Mutations Characterize Small Cell Carcinoma of the Ovary, Hypercalcemic Type. Nat. Genet. 2014, 46, 438–443. [Google Scholar] [CrossRef]
- Jelinic, P.; Mueller, J.J.; Olvera, N.; Dao, F.; Scott, S.N.; Shah, R.; Gao, J.; Schultz, N.; Gonen, M.; Soslow, R.A.; et al. Recurrent SMARCA4 Mutations in Small Cell Carcinoma of the Ovary. Nat. Genet. 2014, 46, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Karnezis, A.N.; Craig, D.W.; Sekulic, A.; Russell, M.L.; Hendricks, W.P.D.; Corneveaux, J.J.; Barrett, M.T.; Shumansky, K.; Yang, Y.; et al. Small Cell Carcinoma of the Ovary, Hypercalcemic Type, Displays Frequent Inactivating Germline and Somatic Mutations in SMARCA4. Nat. Genet. 2014, 46, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Münstedt, K.; Estel, R.; Dreyer, T.; Kurata, A.; Benz, A. Small Cell Ovarian Carcinomas - Characterisation of Two Rare Tumor Entities. Geburtsh Frauenheilk 2013, 73, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, J.H.; Young, R.H.; Scully, R.E. Primary Ovarian Small Cell Carcinoma of Pulmonary Type. A Clinicopathologic, Immunohistologic, and Flow Cytometric Analysis of 11 Cases. Am. J. Surg. Pathol. 1992, 16, 926–938. [Google Scholar] [CrossRef]
- Terada, S.; Suzuki, T.; Hasegawa, A.; Nakayama, S.; Adachi, H. The Cytoreductive Effect of Radiotherapy for Small Cell Ovarian Carcinoma of the Pulmonary Type: A Case Report and Review of the Literature. Case Rep. Obstet. Gynecol. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Gallotta, V.; Federico, A.; Gaballa, K.; D’Indinosante, M.; Conte, C.; Giudice, M.T.; Naldini, A.; Lodoli, C.; Rotolo, S.; Gallucci, V.; et al. The Role of Robotic Aortic Lymphadenectomy in Gynecological Cancer: Surgical and Oncological Outcome in a Single Institution Experience. J. Surg. Oncol 2018, 119, 355–360. [Google Scholar] [CrossRef]
- Gallotta, V.; Jeong, S.Y.; Conte, C.; Trozzi, R.; Cappuccio, S.; Moroni, R.; Ferrandina, G.; Scambia, G.; Kim, T.-J.; Fagotti, A. Minimally Invasive Surgical Staging for Early Stage Ovarian Cancer: A Long-Term Follow Up. Eur. J. Surg. Oncol. 2021, 47, 1698–1704. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Li, J.; Lu, X. The Individualized Significance of Lymphadenectomy across All Age Groups and Histologies in Malignant Ovarian Germ Cell Tumors. Arch. Gynecol. Obstet. 2020, 302, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-L.; Cheng, H.; Tang, M.-S.; Zhang, H.-L.; Wu, R.-Y.; Yu, Y.; Li, X.; Wang, X.-M.; Mai, J.; Yang, C.-L.; et al. A Novel Nomogram Based on LODDS to Predict the Prognosis of Epithelial Ovarian Cancer. Oncotarget 2017, 8, 8120–8130. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Schneider, D.T.; Planchamp, F.; Baust, K.; Braicu, E.I.; Concin, N.; Godzinski, J.; McCluggage, W.G.; Orbach, D.; Pautier, P.; et al. ESGO–SIOPE Guidelines for the Management of Adolescents and Young Adults with Non-Epithelial Ovarian Cancers. Lancet Oncol. 2020, 21, e360–e368. [Google Scholar] [CrossRef]
- Chiyoda, T.; Sakurai, M.; Satoh, T.; Nagase, S.; Mikami, M.; Katabuchi, H.; Aoki, D. Lymphadenectomy for Primary Ovarian Cancer: A Systematic Review and Meta-Analysis. J. Gynecol. Oncol. 2020, 31, e67. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.-W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. In NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®); Version 1.2022; NCCN: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- Nasioudis, D.; Chapman-Davis, E.; Frey, M.K.; Caputo, T.A.; Witkin, S.S.; Holcomb, K. Small Cell Carcinoma of the Ovary: A Rare Tumor With a Poor Prognosis. Int. J. Gynecol. Cancer 2018, 28, 932–938. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-Epithelial Ovarian Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 18, iv1–iv18. [Google Scholar] [CrossRef]
- Blanc-Durand, F.; Lefeuvre-Plesse, C.; Ray-Coquard, I.; Chaltiel, D.; Floquet, A.; Meriaux, É.; Berton, D.; Bello-Roufai, D.; Guillemet, C.; Dupre, P.-F.; et al. Dose-Intensive Regimen Treatment for Small-Cell Carcinoma of the Ovary of Hypercalcemic Type (SCCOHT). Gynecol. Oncol. 2020, 159, 129–135. [Google Scholar] [CrossRef]
- Witkowski, L.; Goudie, C.; Foulkes, W.D.; McCluggage, W.G. Small-Cell Carcinoma of the Ovary of Hypercalcemic Type (Malignant Rhabdoid Tumor of the Ovary). Surg. Pathol. Clin. 2016, 9, 215–226. [Google Scholar] [CrossRef]
- Harrison, M.L.; Hoskins, P.; du Bois, A.; Quinn, M.; Rustin, G.J.S.; Ledermann, J.A.; Baron-Hay, S.; Friedlander, M.L. Small Cell of the Ovary, Hypercalcemic Type—Analysis of Combined Experience and Recommendation for Management. A GCIG Study. Gynecol. Oncol. 2006, 100, 233–238. [Google Scholar] [CrossRef]
- Asom, A.S.; Lastra, R.R.; Hasan, Y.; Weinberg, L.; Fleming, G.F.; Kurnit, K.C. Small Cell Carcinoma of the Ovary, Pulmonary Type: A Role for Adjuvant Radiotherapy after Carboplatin and Etoposide? Gynecol. Oncol. Rep. 2022, 39, 100925. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Singh, N.; Gilks, C.B. Key Changes to the World Health Organization (WHO) Classification of Female Genital Tumours Introduced in the 5th Edition (2020). Histopathology 2022, 80, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Tischkowitz, M.; Huang, S.; Banerjee, S.; Hague, J.; Hendricks, W.P.D.; Huntsman, D.G.; Lang, J.D.; Orlando, K.A.; Oza, A.M.; Pautier, P.; et al. Small-Cell Carcinoma of the Ovary, Hypercalcemic Type–Genetics, New Treatment Targets, and Current Management Guidelines. Clin. Cancer Res. 2020, 26, 3908–3917. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.Y.; Karnezis, A.N.; Colborne, S.; Santos, N.D.; Lang, J.D.; Hendricks, W.P.; Orlando, K.A.; Yap, D.; Kommoss, F.; et al. The Histone Methyltransferase EZH2 Is a Therapeutic Target in Small Cell Carcinoma of the Ovary, Hypercalcaemic Type: Targeting EZH2 in SCCOHT. J. Pathol. 2017, 242, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Meehan, B.; Macdonald, E.; Venneti, S.; Wang, X.Q.D.; Witkowski, L.; Jelinic, P.; Kong, T.; Martinez, D.; Morin, G.; et al. CDK4/6 Inhibitors Target SMARCA4-Determined Cyclin D1 Deficiency in Hypercalcemic Small Cell Carcinoma of the Ovary. Nat. Commun. 2019, 10, 558. [Google Scholar] [CrossRef]
- Jelinic, P.; Ricca, J.; Van Oudenhove, E.; Olvera, N.; Merghoub, T.; Levine, D.A.; Zamarin, D. Immune-Active Microenvironment in Small Cell Carcinoma of the Ovary, Hypercalcemic Type: Rationale for Immune Checkpoint Blockade. JNCI J. Natl. Cancer Inst. 2018, 110, 787–790. [Google Scholar] [CrossRef]
| Clinical Parameter | Unmatched Dataset | Matched Dataset (4:1) | ||||
|---|---|---|---|---|---|---|
| Non-SCCO | SCCO | p-Value | Non-SCCO | SCCO | p-Value | |
| (n = 39,662) | (n = 80) | (n = 254) | (n = 72) | |||
| Marital status | ||||||
| Married and other | 31,702 (79.9%) | 43 (53.8%) | <0.001 | 148 (58.3%) | 40 (55.6%) | 0.85 |
| Single | 6762 (17.0%) | 36 (45.0%) | 101 (39.8%) | 31 (43.1%) | ||
| Unknown | 1198 (3.0%) | 1 (1.3%) | 5 (2.0%) | 1 (1.4%) | ||
| Race | ||||||
| Black | 2564 (6.5%) | 7 (8.8%) | 0.468 | 26 (10.2%) | 7 (9.7%) | 0.89 |
| Other | 3430 (8.6%) | 10 (12.5%) | 37 (14.6%) | 9 (12.5%) | ||
| Unknown | 100 (0.3%) | 0 (0%) | ||||
| White | 33,568 (84.6%) | 63 (78.8%) | 191 (75.2%) | 56 (77.8%) | ||
| Malignancy | ||||||
| ≥2 | 9463 (23.9%) | 10 (12.5%) | 0.0244 | 37 (14.6%) | 10 (13.9%) | 1 |
| 1 | 30,199 (76.1%) | 70 (87.5%) | 217 (85.4%) | 62 (86.1%) | ||
| Grade | ||||||
| I | 3464 (8.7%) | 0 (0%) | <0.001 | |||
| II | 6436 (16.2%) | 0 (0%) | ||||
| III | 13,787 (34.8%) | 20 (25.0%) | 77 (30.3%) | 20 (27.8%) | 0.9 | |
| IV | 5758 (14.5%) | 32 (40.0%) | 79 (31.1%) | 24 (33.3%) | ||
| Unknown | 10,217 (25.8%) | 28 (35.0%) | 98 (38.6%) | 28 (38.9%) | ||
| Laterality | ||||||
| Bilateral | 16,829 (42.4%) | 12 (15.0%) | <0.001 | 50 (19.7%) | 12 (16.7%) | 0.685 |
| Unilateral | 22,833 (57.6%) | 68 (85.0%) | 204 (80.3%) | 60 (83.3%) | ||
| Age | ||||||
| Mean (SD) | 59.1 (14.9) | 37.2 (19.1) | <0.001 | 40.9 (20.3) | 39.4 (18.7) | 0.548 |
| Median [Min, Max] | 60.0 [0, 100] | 32.0 [10.0, 91.0] | 41.0 [2.00, 89.0] | 35.0 [14.0, 91.0] | ||
| Year of diagnosis | ||||||
| 1988–1997 | 12,984 (32.7%) | 22 (27.5%) | 0.601 | 64 (25.2%) | 17 (23.6%) | 0.956 |
| 1998–2007 | 13,608 (34.3%) | 29 (36.3%) | 95 (37.4%) | 28 (38.9%) | ||
| 2008–2018 | 13,070 (33.0%) | 29 (36.3%) | 95 (37.4%) | 27 (37.5%) | ||
| size | ||||||
| >15 cm | 3749 (9.5%) | 26 (32.5%) | <0.001 | 65 (25.6%) | 23 (31.9%) | 0.809 |
| 10–15 cm | 5224 (13.2%) | 18 (22.5%) | 51 (20.1%) | 15 (20.8%) | ||
| 5–10 cm | 7225 (18.2%) | 10 (12.5%) | 42 (16.5%) | 10 (13.9%) | ||
| 0–5 cm | 5946 (15.0%) | 4 (5.0%) | 20 (7.9%) | 4 (5.6%) | ||
| No/Micro | 165 (0.4%) | 0 (0%) | ||||
| Unknown | 17,353 (43.8%) | 22 (27.5%) | 76 (29.9%) | 20 (27.8%) | ||
| FIGO stage | ||||||
| I | 11,647 (29.4%) | 27 (33.8%) | 0.475 | 98 (38.6%) | 23 (31.9%) | 0.764 |
| II | 3892 (9.8%) | 10 (12.5%) | 27 (10.6%) | 9 (12.5%) | ||
| III | 15,088 (38.0%) | 24 (30.0%) | 77 (30.3%) | 23 (31.9%) | ||
| IV | 9035 (22.8%) | 19 (23.8%) | 52 (20.5%) | 17 (23.6%) | ||
| Radiation | ||||||
| No | 38,759 (97.7%) | 77 (96.3%) | 0.612 | 249 (98.0%) | 70 (97.2%) | 1 |
| Yes | 903 (2.3%) | 3 (3.8%) | 5 (2.0%) | 2 (2.8%) | ||
| Chemotherapy | ||||||
| No/Unknown | 11,819 (29.8%) | 18 (22.5%) | 0.192 | 62 (24.4%) | 17 (23.6%) | 1 |
| Yes | 27,843 (70.2%) | 62 (77.5%) | 192 (75.6%) | 55 (76.4%) | ||
| Surgery type | ||||||
| DEB/EXE | 3982 (10.0%) | 5 (6.3%) | 0.463 | 16 (6.3%) | 4 (5.6%) | 0.956 |
| Non-DEB | 9002 (22.7%) | 17 (21.3%) | 48 (18.9%) | 13 (18.1%) | ||
| Unknown | 26,678 (67.3%) | 58 (72.5%) | 190 (74.8%) | 55 (76.4%) | ||
| LND | ||||||
| No | 20,746 (52.3%) | 39 (48.8%) | 0.6 | 117 (46.1%) | 35 (48.6%) | 0.804 |
| Yes | 18,916 (47.7%) | 41 (51.3%) | 137 (53.9%) | 37 (51.4%) | ||
| Characteristics | Non-SCCO | SCCO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | Crude HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | ||
| Marital status | |||||||||
| Married and other | 1 | 1 | |||||||
| Single | 0.39 (0.24–0.62) | <0.001 | 0.89 (0.49–1.63) | 0.7095 | 1.17 (0.68–2.03) | 0.566 | |||
| Unknown | 1.49 (0.54–4.08) | 0.439 | 0.97 (0.33–2.9) | 0.9629 | 6.08 (0.78–47.63) | 0.086 | |||
| Race | |||||||||
| Black | 1 | 1 | |||||||
| Other | 0.49 (0.23–1.05) | 0.066 | 0.67 (0.19–2.34) | 0.536 | |||||
| White | 0.62 (0.35–1.07) | 0.087 | 1.12 (0.44–2.84) | 0.809 | |||||
| Malignancy | |||||||||
| ≥2 | |||||||||
| 1 | 0.74 (0.45–1.24) | 0.251 | 2.6 (1.03–6.59) | 0.044 | 2.73 (1.03–7.2) | 0.0427 | |||
| Grade | |||||||||
| III | 1 | 1 | |||||||
| IV | 1.14 (0.72–1.79) | 0.572 | 1.31 (0.78–2.22) | 0.3062 | 1.03 (1.03–6.59) | 0.926 | |||
| Unknown | 0.55 (0.33–0.91) | 0.02 | 0.93 (0.52–1.64) | 0.7908 | 1.03 (0.52–2.03) | 0.941 | |||
| Laterality | |||||||||
| Bilateral | 1 | 1 | |||||||
| Unilateral | 0.25 (0.16–0.37) | <0.001 | 0.42 (0.27–0.67) | <0.001 | 0.33 (0.17–0.63) | 0.001 | 0.43 (0.19–0.95) | 0.0365 | |
| Age | |||||||||
| <40 | 1 | 1 | |||||||
| 40–59 | 3.33 (1.98–5.62) | <0.001 | 1.62 (0.77–3.42) | 0.2025 | 0.54 (0.17–0.63) | 0.078 | |||
| ≥60 | 7.6 (4.33–13.33) | <0.001 | 3.67 (1.7–7.93) | <0.001 | 1.06 (0.47–2.38) | 0.892 | |||
| Year of diagnosis | |||||||||
| 1988–1997 | 1 | 1 | |||||||
| 1998–2007 | 0.8 (0.5–1.26) | 0.337 | 0.33 (0.16–0.65) | 0.001 | 0.37 (0.17–0.82) | 0.0149 | |||
| 2008–2018 | 0.68 (0.4–1.13) | 0.139 | 0.53 (0.27–1.05) | 0.069 | 0.91 (0.39–2.16) | 0.8356 | |||
| Size | |||||||||
| 0–5 cm | 1 | 1 | |||||||
| 5–10 cm | 0.51 (0.25–1.02) | 0.058 | 0.63 (0.3–1.31) | 0.2202 | 2.46 (0.51–11.96) | 0.263 | |||
| 10–15 cm | 0.36 (0.18–0.74) | 0.005 | 0.55 (0.26–1.2) | 0.136 | 2.98 (0.66–13.5) | 0.156 | |||
| >15 cm | 0.34 (0.17–0.68) | 0.002 | 0.82 (0.38–1.81) | 0.6302 | 1.56 (0.35–6.9) | 0.559 | |||
| Unknown | 0.53 (0.28–1) | 0.049 | 0.55 (0.28–1.07) | 0.0786 | 3.01 (0.69–13.22) | 0.144 | |||
| FIGO stage | |||||||||
| I | 1 | ||||||||
| II | 2.61 (1.13–6.04) | 0.025 | 2.11 (0.88–5.04) | 0.0924 | 1.86 (0.75–4.66) | 0.183 | 1.65 (0.65–4.19) | 0.2922 | |
| III | 4.63 (2.5–8.55) | <0.001 | 3.4 (1.77–6.53) | <0.001 | 1.94 (0.96–3.91) | 0.065 | 1.55 (0.73–3.28) | 0.2561 | |
| IV | 11.23 (6.05–20.84) | <0.001 | 7.19 (3.52–14.7) | <0.001 | 2.2 (0.96–3.91) | 0.038 | 1.18 (0.46–2.99) | 0.7327 | |
| Radiation | |||||||||
| No | 1 | ||||||||
| Yes | 0.68 (0.09–4.88) | 0.7 | 0.65 (0.09–4.74) | 0.675 | |||||
| Chemotherapy | |||||||||
| No/Unknown | 1 | 1 | |||||||
| Yes | 1.05 (0.67–1.65) | 0.837 | 0.54 (0.3–0.96) | 0.037 | 0.54 (0.28–1.04) | 0.0639 | |||
| Surgery | |||||||||
| DEB/EXE | 1 | 1 | |||||||
| Non-DEB | 0.47 (0.23–0.96) | 0.039 | 2.53 (1.19–5.38) | 0.0162 | 0.99 (0.32–3.08) | 0.983 | |||
| Unknown | 0.44 (0.24–0.81) | 0.008 | 1.7 (0.81–3.6) | 0.1627 | 0.4 (0.32–3.08) | 0.088 | |||
| LND | |||||||||
| No | 1 | 1 | |||||||
| Yes | 0.67 (0.45–0.99) | 0.045 | 0.82 (0.53–1.28) | 0.3903 | 0.43 (0.32–3.08) | 0.003 | 0.5 (0.25–0.99) | 0.0459 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ning, Y.; Du, Y.; Kang, Y. Lymphadenectomy Benefits Small Cell Carcinoma of Ovary: A Population-Based Analysis. Curr. Oncol. 2022, 29, 7802-7815. https://doi.org/10.3390/curroncol29100617
Wang J, Ning Y, Du Y, Kang Y. Lymphadenectomy Benefits Small Cell Carcinoma of Ovary: A Population-Based Analysis. Current Oncology. 2022; 29(10):7802-7815. https://doi.org/10.3390/curroncol29100617
Chicago/Turabian StyleWang, Jing, Yan Ning, Yan Du, and Yu Kang. 2022. "Lymphadenectomy Benefits Small Cell Carcinoma of Ovary: A Population-Based Analysis" Current Oncology 29, no. 10: 7802-7815. https://doi.org/10.3390/curroncol29100617
APA StyleWang, J., Ning, Y., Du, Y., & Kang, Y. (2022). Lymphadenectomy Benefits Small Cell Carcinoma of Ovary: A Population-Based Analysis. Current Oncology, 29(10), 7802-7815. https://doi.org/10.3390/curroncol29100617






