Immune-Related Pneumonitis Was Decreased by Addition of Chemotherapy with PD-1/L1 Inhibitors: Systematic Review and Network Meta-Analysis of Randomized Controlled Trials (RCTs)
Abstract
:1. Introduction
2. Methods and Materials
2.1. Search Methods and Study Selection
2.2. Data Extraction and Quality Assessment
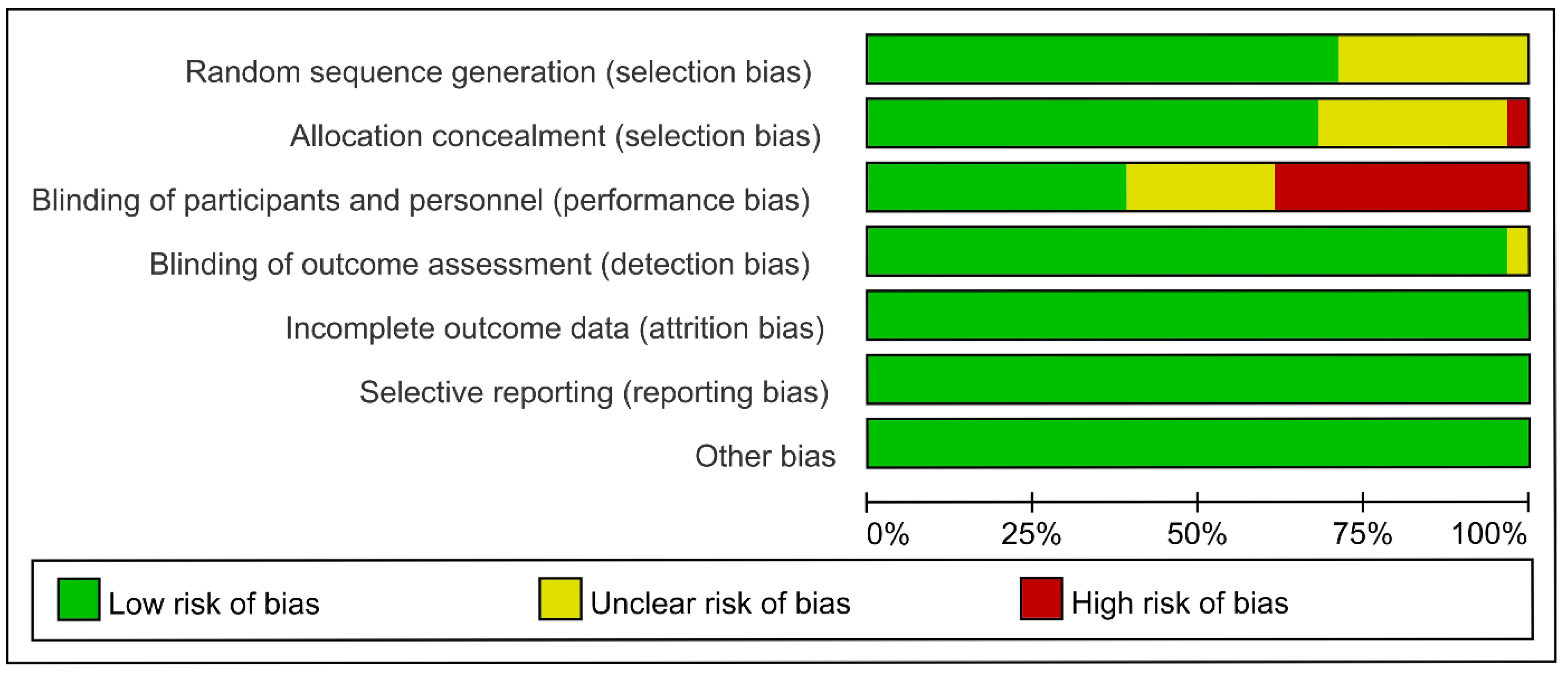
2.3. Risk of Bias Assessment
2.4. Outcome
2.5. Statistical Analysis
3. Results
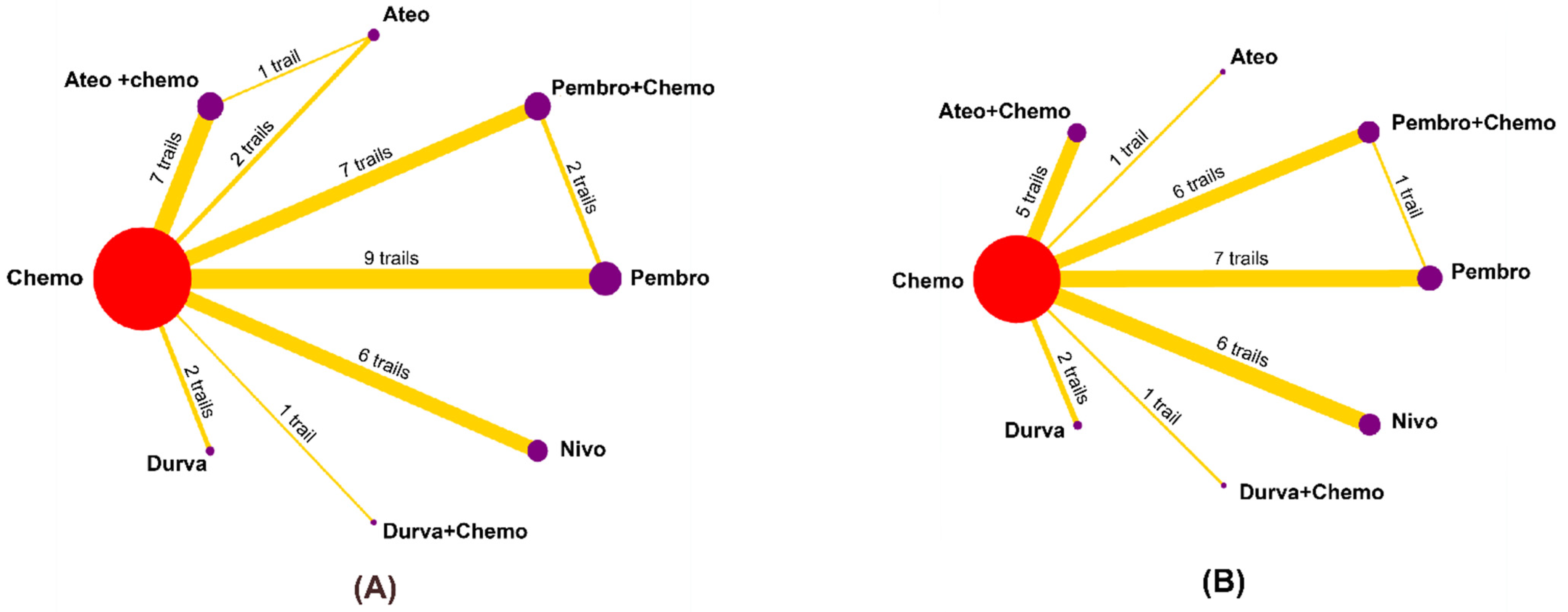
3.1. Eligible Studies and Characteristics
3.2. Risk of Bias within Studies
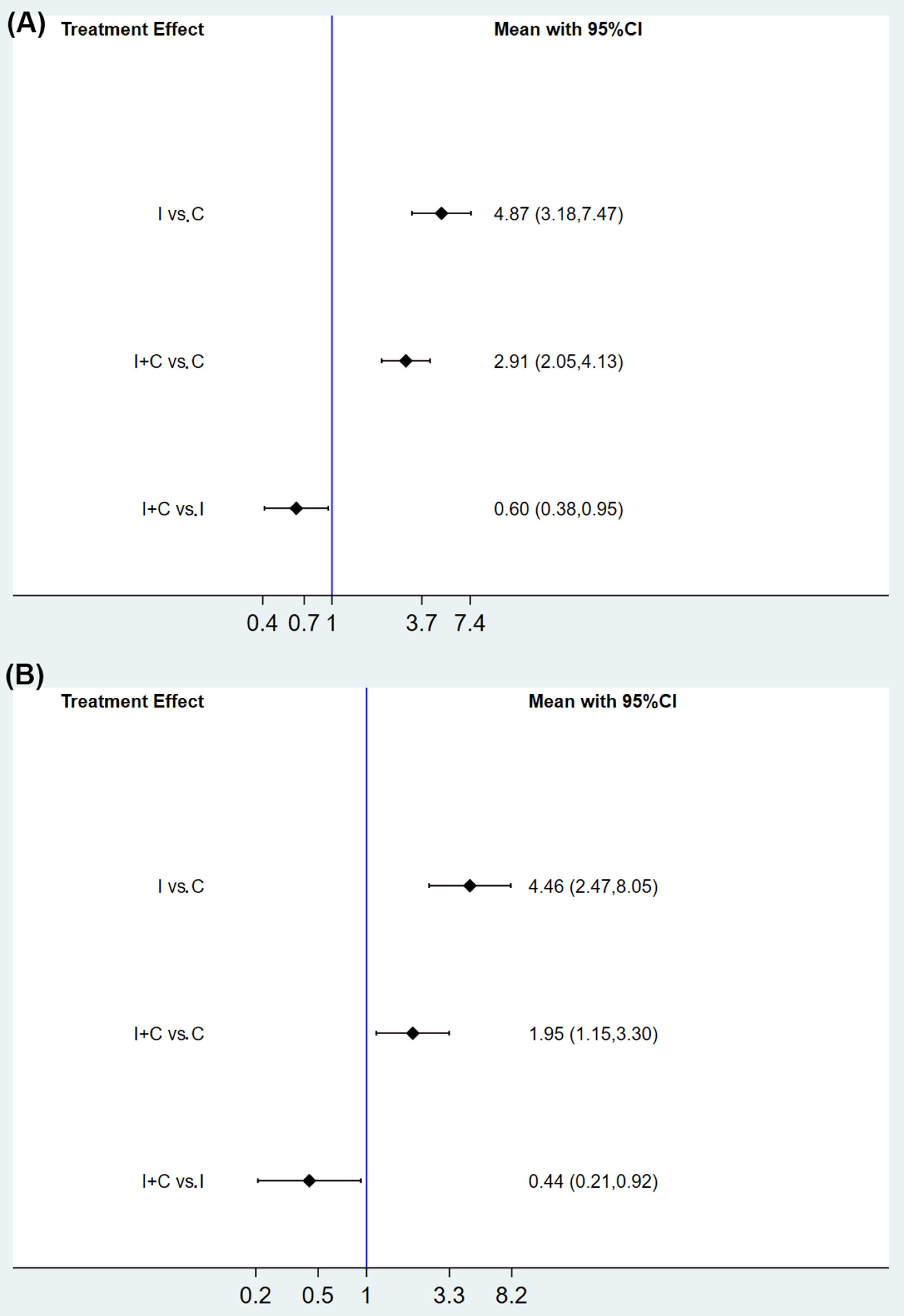
3.3. Primary Outcome
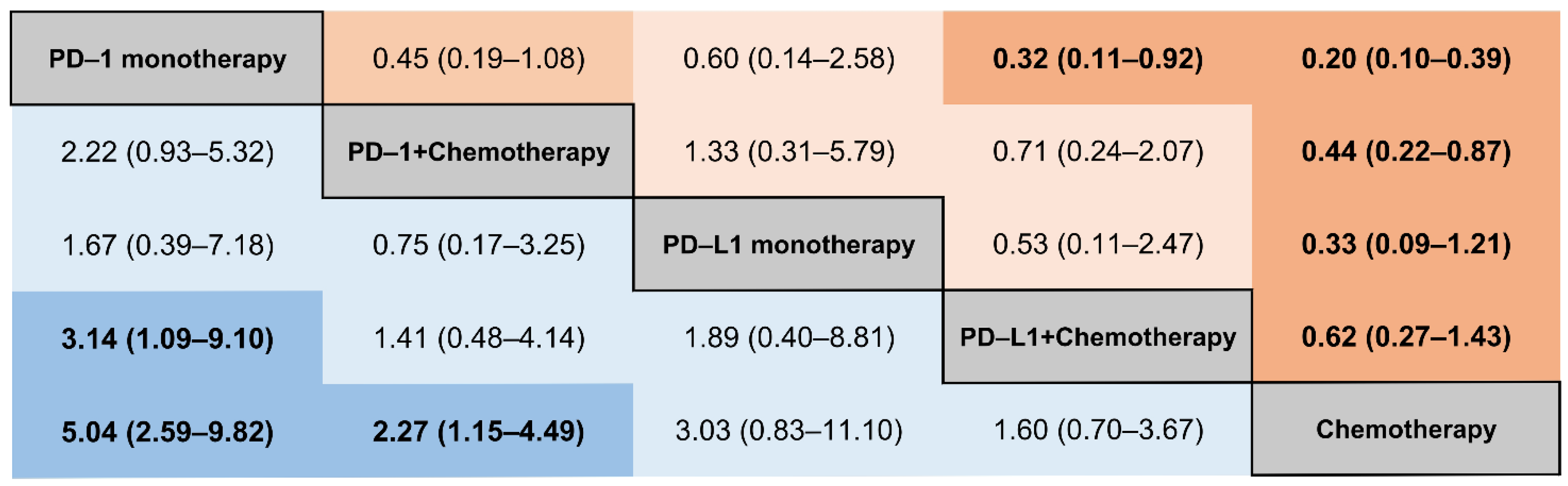
3.4. Subgroup Analysis of IRPs by the Different Types of ICIs
3.5. Subgroup Analysis of IRP by Caner Type
3.6. Heterogeneity, Inconsistency, and Publication Bias
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Shao, C.; Shi, Y.; Han, W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 2018, 11, 31. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodriguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.C.; et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J. Thorac. Oncol. 2020, 15, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Lu, S.; Cheng, Y.; Zhou, C.; Wang, J.; Mok, T.; Zhang, L.; Tu, H.Y.; Wu, L.; Feng, J.; et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J. Thorac. Oncol. 2019, 14, 867–875. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Humeau, J.; Buque, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef]
- Ervin, S.M.; Ramanan, S.V.; Bhatt, A.P. Relationship Between the Gut Microbiome and Systemic Chemotherapy. Dig. Dis. Sci. 2020, 65, 874–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschella, F.; Valentini, M.; Aricò, E.; Macchia, I.; Sestili, P.; D’Urso, M.; Alessandri, C.; Belardelli, F.; Proietti, E. Unraveling cancer chemoimmunotherapy mechanisms by gene and protein expression profiling of responses to cyclophosphamide. Cancer Res. 2011, 71, 3528–3539. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, L.; Arvin, A. Chemotherapy-induced immunosuppression. Environ. Health Perspect. 1982, 43, 21–25. [Google Scholar] [CrossRef]
- Higgins, J.; Altman, D.; Gøtzsche, P.; Jüni, P.; Moher, D.; Oxman, A.; Savovic, J.; Schulz, K.; Weeks, L.; Sterne, J.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D.J.B. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Sjölander, A.; Vansteelandt, S. Frequentist versus Bayesian approaches to multiple testing. Eur. J. Epidemiol. 2019, 34, 809–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Satouchi, M.; Nosaki, K.; Takahashi, T.; Nakagawa, K.; Aoe, K.; Kurata, T.; Sekine, A.; Horiike, A.; Fukuhara, T.; Sugawara, S.; et al. First-line pembrolizumab vs chemotherapy in metastatic non-small-cell lung cancer: KEYNOTE-024 Japan subset. Cancer Sci. 2020, 111, 4480–4489. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Soto Parra, H.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J.; et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamamoto, Y.; Sassa, N.; Nishimura, K.; Fujimoto, K.; Fukasawa, S.; Yokoyama, M.; Enokida, H.; Takahashi, K.; Tanaka, Y.; et al. Pembrolizumab versus chemotherapy in recurrent, advanced urothelial cancer in Japanese patients: A subgroup analysis of the phase 3 KEYNOTE-045 trial. Int. J. Clin. Oncol. 2020, 25, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Liu, S.; Reck, M.; Mansfield, A.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Perez-Gracia, J.L.; Han, J.Y.; Arvis, C.D.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Long-Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death-Ligand 1—Positive, Advanced Non-Small-Cell Lung Cancer in the KEYNOTE-010 Study. J. Clin. Oncol. 2020, 38, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Gadgeel, S.; Esteban, E.; Felip, E.; Speranza, G.; Domine, M.; Hochmair, M.J.; Powell, S.; Cheng, S.Y.; Bischoff, H.G.; et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Hou, X.; Zhang, Y.; Zhou, T.; Liu, J.; Lin, Z.; Fang, W.; Yang, Y.; Ma, Y.; et al. Immune-related pneumonitis associated with immune checkpoint inhibitors in lung cancer: A network meta-analysis. J. Immunother. Cancer 2020, 8, e001170. [Google Scholar] [CrossRef]
- Wang, M.; Liang, H.; Wang, W.; Zhao, S.; Cai, X.; Zhao, Y.; Li, C.; Cheng, B.; Xiong, S.; Li, J.; et al. Immune-related adverse events of a PD-L1 inhibitor plus chemotherapy versus a PD-L1 inhibitor alone in first-line treatment for advanced non-small cell lung cancer: A meta-analysis of randomized control trials. Cancer 2021, 127, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Waxman, D.J. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018, 419, 210–221. [Google Scholar] [CrossRef]
- Borg, C.; Ray-Coquard, I.; Philip, I.; Clapisson, G.; Bendriss-Vermare, N.; Menetrier-Caux, C.; Sebban, C.; Biron, P.; Blay, J.Y. CD4 lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients with cancer. Cancer 2004, 101, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Mackall, C.; Fleisher, T.; Brown, M.; Andrich, M.; Chen, C.; Feuerstein, I.; Magrath, I.; Wexler, L.; Dimitrov, D.; Gress, R.J.B. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 1997, 89, 3700–3707. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Naidoo, J.; Zhong, Q.; Xiong, Y.; Mammen, J.; de Flores, M.V.; Cappelli, L.; Balaji, A.; Palmer, T.; Forde, P.M.; et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J. Clin. Investig. 2019, 129, 4305–4315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpathiou, G.; Mihailidis, V.; Nakou, E.; Anevlavis, S.; Tzouvelekis, A.; Kouliatsis, G.; Ntolios, P.; Bouros, D.; Kotsianidis, I.; Froudarakis, M.E. Chemotherapy-induced changes in bronchoalveolar lavage fluid CD4 + and CD8 + cells of the opposite lung to the cancer. Sci. Rep. 2020, 10, 19927. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M. Clinical Pharmacology of Corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; Committee, E.G. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Brahmer, J.; Lacchetti, C.; Schneider, B.; Atkins, M.; Brassil, K.; Caterino, J.; Chau, I.; Ernstoff, M.; Gardner, J.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Schneider, B.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.; Costa, L.; Davies, M.; Dunnington, D.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J. NCCN 2019, 17, 255–289. [Google Scholar] [CrossRef] [Green Version]
- Khunger, M.; Rakshit, S.; Pasupuleti, V.; Hernandez, A.; Mazzone, P.; Stevenson, J.; Pennell, N.; Velcheti, V.J.C. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Orig. Res. Lung Cancer 2017, 152, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Yu, S.; Zhu, B.; Bedoret, D.; Bu, X.; Francisco, L.; Hua, P.; Duke-Cohan, J.; Umetsu, D.; Sharpe, A.; et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J. Exp. Med. 2014, 211, 943–959. [Google Scholar] [CrossRef]
- Tabchi, S.; Messier, C.; Blais, N. Immune-mediated respiratory adverse events of checkpoint inhibitors. Curr. Opin. Oncol. 2016, 28, 269–277. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Chen, W.W.; Siu, L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, E.; Wu, J.; Li, T.; Hou, Y.; Wang, D.; Gao, Z.J.F. Risk of Pneumonitis and Pneumonia Associated With Immune Checkpoint Inhibitors for Solid Tumors: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, K.; Psoter, K.; Voong, K.; Shankar, B.; Forde, P.; Ettinger, D.; Marrone, K.; Kelly, R.; Hann, C.; Levy, B.; et al. Impact of Checkpoint Inhibitor Pneumonitis on Survival in NSCLC Patients Receiving Immune Checkpoint Immunotherapy. J. Thorac. Oncol. 2019, 14, 494–502. [Google Scholar] [CrossRef] [Green Version]


| Study Name | Year | Phase | Blind | History | Patients, No. | Median Age | Treatment | Median Follow-Up Time | Endpoint | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment | Control | Experiment | Control | Experiment | Control | Primary | Second | ||||||
| CASPIAN | 2021 | 3 | Double | SCLC | 268 | 269 | 62 (58–68) | 63 (57–68) | Durvalumab + (platinum + etoposide) | Platinum + etoposide | 25.1 (22.3–27.9) | OS | PFS, ORR |
| KEYNOTE-062 | 2020 | 3 | Partially | G/GEJ | 257 | 256 | 62 (22–83) | 61 (20–83) | Pembrolizumab + (cisplatin + fluorouracil/capecitabine q3w) | Pembrolizumab | 29.4 (22.0–41.3) | OS, PFS | ORR, DOR, safety, |
| IMpassion130 | 2020 | 3 | Double | TNBC | 451 | 451 | 55 (46–64) | 56 (47–65) | Atezolizumab + nab-paclitaxel | Placebo + nab-paclitaxel | 18.5 (9.6–22.8) | PFS, OS | ORR, DOR, QOF, |
| KEYNOTE-522 | 2020 | 3 | Double | TNBC | 784 | 390 | 49 (22–80) | 48 (24–79) | Pembrolizumab + (paclitaxel + carboplatin) | Paclitaxel + carboplatin | 15.0 (2.7–25.0) | pCR; EFS | pCR; safety |
| KEYNOTE-024 | 2019 | 3 | Open | NSCLC | 154 | 151 | 64 (33–90) | 66 (38–85) | Pembrolizumab (200 mg, q3w, up to 2 years) | Platinum-based (4–6 cycles) | 25.2 (20.4–33.7) | PFS | OS, ORR, DOR |
| KEYNOTE-604 | 2020 | 3 | Double | SCLC | 228 | 225 | 64 (24–81) | 65 (37–83) | Pembrolizumab + (platinum + etoposide) | Platinum + etoposide | 26.1 (16.1–30.6) | PFS, OS | ORR, DOR, safety |
| MYSTIC | 2020 | 3 | Open | NSCLC | 369 | 352 | 65 (28–84) | 64 (30–85) | Durvalumab (20 mg/kg, q4w) | Platinum-based | 30.2 (0.3–37.2) | OS, PFS | ORR, DOR, safety |
| KEYNOTE-407 | 2020 | 3 | Double | NSCLC | 278 | 281 | 65 (29–87) | 65 (36–88) | Pembrolizumab + (carboplatin + paclitaxel/nab-paclitaxel) | Placebo + (carboplatin + paclitaxel/nab-paclitaxel) | 14.3 (0.1–31.3) | OS, PFS | ORR, DOR, safety |
| KEYNOTE-045 | 2019 | 3 | Open | UC | 270 | 272 | 67 (29–88) | 65 (26–84) | Pembrolizumab (200 mg, q3w) | Paclitaxel, docetaxel, or vinflunine | 26 | OS, PFS | ORR, DOR, safety |
| IMpassion031 | 2020 | 3 | Double | TNBC | 165 | 168 | 51 (22–76) | 51 (26–78) | Atezolizumab plus (nab-paclitaxel + doxorubicin + cyclophosphamide) | Nab-paclitaxel + doxorubicin + cyclophosphamide | 20.6 (8.7–24.9) | pCR | EFS, OS, safety, tolerability |
| IMpower133 | 2020 | I/III | Double | SCLC | 201 | 202 | 64 (28–90) | 64 (26–87) | Atezolizumab plus (carboplatin + etoposide) | Carboplatin + etoposide | 23.1 (0–29.5) | OS, PFS | ORR, DOR, safety |
| IMpower110 | 2020 | 3 | Open | NSCLC | 277 | 277 | 64 (30–81) | 65 (30–87) | Atezolizumab | Platinum-based | 13.4 (0–35) | OS | PFS, DOR, safety |
| KEYNOTE-010 | 2020 | II/III | Open | NSCLC | 690 | 343 | 63 (56–69) | 62 (56–69) | Pembrolizumab | Docetaxel | 42.6 (35.2–53.2) | OS, PFS | ORR, DOR, safety |
| KEYNOTE-189 | 2020 | 3 | Double | NSCLC | 410 | 206 | 65 (34–84) | 63 (34–84) | Pembrolizumab plus (pemetrexed + platinum) | Pemetrexed + platinum | 23.1 (18.6–30.9) | OS, PFS | ORR, DOR, safety |
| IMvigor130 | 2020 | 3 | Double | UC | 451 | 362 | 69 (62–75) | 67 (62–74) | Atezolizumab plus platinum-based chemotherapy | Atezolizumab | 11.8 (6.1–17.2) | OS, safety | ORR, DOR, PFS |
| IMpower130 | 2019 | 3 | Open | NSCLC | 483 | 240 | 64 (18–86) | 65 (38–85) | Atezolizumab plus (carboplatin + nab-paclitaxel) | Carboplatin + nab-paclitaxel | 18.5 (15.2–23.6) | PFS, OS | ORR, DOR |
| KEYNOTE-042 | 2019 | 3 | Open | NSCLC | 637 | 637 | 63 (57–69) | 63 (57–69) | Pembrolizumab | Platinum-based | 12.8 (6.0–20.0). | OS | ORR, DOR, PFS |
| KEYNOTE-061 | 2018 | 3 | Partially | G/GEJ | 296 | 296 | 62 (54–70) | 60 (53–68) | Pembrolizumab | Paclitaxel | 7.9 (3.4–14.6) | OS, PFS | ORR, DOR, safety |
| KEYNOTE-048 | 2019 | 3 | Open | HNSCC | 281 | 301 | 61 (55–68) | 62 (56–68) | Pembrolizumab plus (platinum + 5-fluorouracil) | Pembrolizumab | 11.5 (5.1–20.8) | OS, PFS | ORR, DOR, safety |
| IMpower132 | 2020 | 3 | Open | NSCLC | 292 | 286 | 64 (31–85) | 63 (33–83) | Atezolizumab plus (carboplatin/cisplatin + pemetrexed) | Carboplatin/cisplatin + pemetrexed | 14.8 (11.7–25.5) | OS, PFS | ORR, DOR, safety |
| IMpower131 | 2018 | 3 | Open | NSCLC | 343 | 340 | 65 (23–83) | 65 (38–86) | Atezolizumab + Carboplatin + Nab-Paclitaxel | Carboplatin + Nab-Paclitaxel | NR | PFS, OS | ORR, DOR, safety |
| KEYNOTE-355 | 2020 | 3 | Double | TNBC | 566 | 281 | 53 (44–63) | 53 (43–63) | Pembrolizumab + Chemotherapy (nab-paclitaxel/paclitaxel/gemcitabine + carboplatin) | Nab-paclitaxel/paclitaxel/gemcitabine + carboplatin | 25.9 (22.8–29.9) | Safety; PFS | ORR; DOR |
| KEYNOTE-119 | 2021 | 3 | Open | TNBC | 312 | 310 | 50 (43–59) | 53 (44–61) | Pembrolizumab | Capecitabine, eribulin, gemcitabine, vinorelbine | 31.4 (27·8–34·4) | OS | PFS ORR DOR safety |
| DANUBE | 2020 | 3 | Open | UC | 346 | 344 | 67 (60–73) | 68 (60–73) | Durvalumab | Gemcitabine + cisplatin/carboplatin | 41.2 (37.9–43.2) | OS | ORR DOR |
| KEYNOTE-181 | 2021 | 3 | Open | ES | 314 | 314 | 63 (23-84) | 62 (24–84) | Pembrolizumab | Paclitaxel/docetaxel/irinotecan | 7.1 (0.5–31.3) | OS | PFS ORR |
| CheckMate 017 | 2015 | 3 | Open | NSCLC | 135 | 137 | 62 (39–85) | 64 (42–84) | Nivolumab | Docetaxel | NR | OS, ORR | PFS, Efficacy |
| CheckMate 057 | 2015 | 3 | Open | NSCLC | 292 | 290 | 61 (37–84) | 64 (21–85) | Nivolumab | Docetaxel | 12.2 (9.7–15.1) | OS | Efficacy PFS, ORR Efficacy |
| CheckMate 078 | 2015 | 3 | Open | NSCLC | 338 | 166 | 60 (27–78) | 60 (38–78) | Nivolumab | Docetaxel | 8.8 (0.2–21.1) | OS | PFS, ORR |
| CheckMate 026 | 2017 | 3 | Open | NSCLC | 271 | 270 | 63 (32–89) | 65 (29–87) | Nivolumab | Platinum doublet chemotherapy | 13.5 | PFS | OS |
| CheckMate 066 | 2015 | 3 | Double | MM | 210 | 208 | 64 (18–86) | 66 (26–87) | Nivolumab | Dacarbazine | 16.7 | OS | PFS |
| CheckMate 037 | 2015 | 3 | Open | MM | 272 | 133 | 59 (23–88) | 62 (29–85) | Nivolumab | Dacarbazine or paclitaxel + carboplatin | 5.3 (3.3–6.5) | ORR | PFS, OS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.-X.; Sun, Y.; Liu, R.-Z.; Zhang, M.-Y.; Zhao, J.; Wang, Y.-Q.; Zhou, Y.-W.; Cheng, K.; Chen, Y.; Zhu, C.-R.; et al. Immune-Related Pneumonitis Was Decreased by Addition of Chemotherapy with PD-1/L1 Inhibitors: Systematic Review and Network Meta-Analysis of Randomized Controlled Trials (RCTs). Curr. Oncol. 2022, 29, 267-282. https://doi.org/10.3390/curroncol29010025
Long Y-X, Sun Y, Liu R-Z, Zhang M-Y, Zhao J, Wang Y-Q, Zhou Y-W, Cheng K, Chen Y, Zhu C-R, et al. Immune-Related Pneumonitis Was Decreased by Addition of Chemotherapy with PD-1/L1 Inhibitors: Systematic Review and Network Meta-Analysis of Randomized Controlled Trials (RCTs). Current Oncology. 2022; 29(1):267-282. https://doi.org/10.3390/curroncol29010025
Chicago/Turabian StyleLong, Yi-Xiu, Yue Sun, Rui-Zhi Liu, Ming-Yi Zhang, Jing Zhao, Yu-Qing Wang, Yu-Wen Zhou, Ke Cheng, Ye Chen, Cai-Rong Zhu, and et al. 2022. "Immune-Related Pneumonitis Was Decreased by Addition of Chemotherapy with PD-1/L1 Inhibitors: Systematic Review and Network Meta-Analysis of Randomized Controlled Trials (RCTs)" Current Oncology 29, no. 1: 267-282. https://doi.org/10.3390/curroncol29010025
APA StyleLong, Y.-X., Sun, Y., Liu, R.-Z., Zhang, M.-Y., Zhao, J., Wang, Y.-Q., Zhou, Y.-W., Cheng, K., Chen, Y., Zhu, C.-R., & Liu, J.-Y. (2022). Immune-Related Pneumonitis Was Decreased by Addition of Chemotherapy with PD-1/L1 Inhibitors: Systematic Review and Network Meta-Analysis of Randomized Controlled Trials (RCTs). Current Oncology, 29(1), 267-282. https://doi.org/10.3390/curroncol29010025





