Prevalence of Lung Metastases among 19,321 Metastatic Colorectal Cancer Patients in Eight Countries of Europe and Asia
Abstract
1. Introduction
2. Methods
2.1. Database
2.2. Patient Selection and Study Outcome
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Population
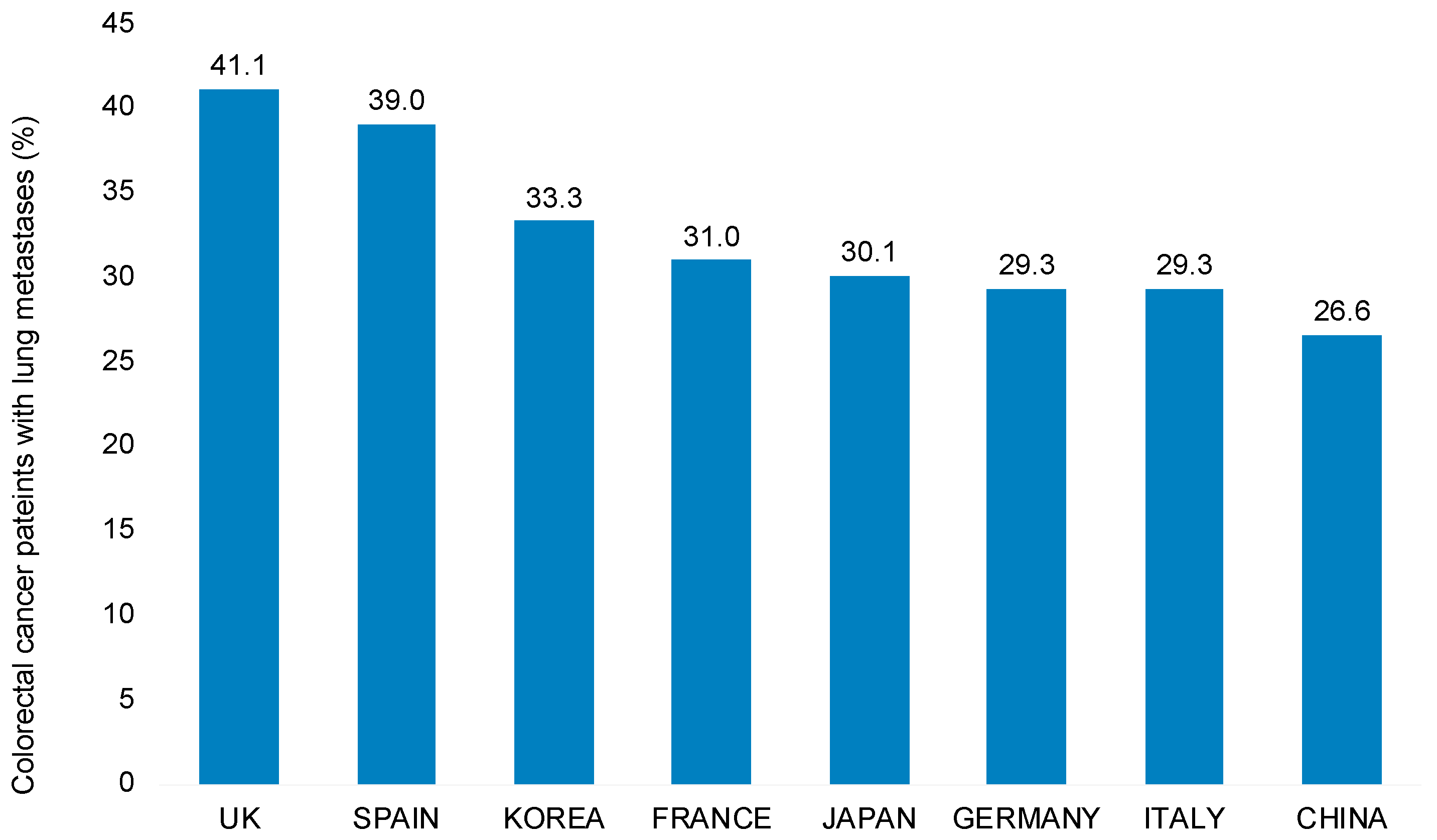
3.2. Prevalence of Lung Metastases among Patients with Metastatic Colorectal Cancer
3.3. Results of Multivariable Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; Van De Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.P.; Bos, A.C.; Lemmens, V.E.; Tanis, P.; Hugen, N.; Nagtegaal, I.; De Wilt, J.H.; Verhoeven, R. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.J.; Lansdorp-Vogelaar, I.; Seeff, L.C.; et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.K.; Lopes Gde, L., Jr.; Sim, R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. J. Gastrointest. Surg. 2009, 13, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Parnaby, C.N.; Bailey, W.; Balasingam, A.; Beckert, L.; Eglinton, T.; Fife, J.; Frizelle, F.A.; Jeffery, M.; Watson, A.J.M. Pulmonary staging in colorectal cancer: A review. Color. Dis. 2012, 14, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Liu, Y.; Zhang, C.; Li, H.; Lai, B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med. 2019, 9, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Pelletier, E.; Barber, B.; Bhosle, M.; Wang, S.; Klingman, D.; Gao, S. Major Surgery in Patients with Metastatic Colorectal Cancer in Western Europe. J. Gastrointest. Cancer 2012, 43, 456–461. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marchetti, P.; Maass, N.; Gligorov, J.; Berger, K.; MacDougall, F.; Montonen, J.; Lewis, J. Patient database analysis of fulvestrant 500 mg in the treatment of metastatic breast cancer: A European perspective. Breast 2017, 32, 247–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chambers, P.; Man, K.; Lui, V.W.; Mpima, S.; Nasuti, P.; Forster, M.D.; Wong, I.C. Understanding Molecular Testing Uptake Across Tumor Types in Eight Countries: Results From a Multinational Cross-Sectional Survey. JCO Oncol. Pract. 2020, 16, e770–e778. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Grundmann, E.; Torhorst, J.; Hartveit, F.; Moberg, I.; Eder, M.; Fenoglio-Preiser, C.M.; Napier, J.; Horne, C.H.W.; Lopez, M.J.; et al. Haematogenous metastastic patterns in colonic carcinoma: An analysis of 1541 necropsies. J. Pathol. 1986, 150, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Hu, J.; Yang, D.; Cosgrove, D.P.; Xu, R. Pattern of distant metastases in colorectal cancer: A SEER based study. Oncotarget 2015, 6, 38658–38666. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, M.; Tsuruta, M.; Hasegawa, H.; Okabayashi, K.; Toyoda, N.; Iwama, N.; Morita, S.; Kitagawa, Y. Smoking is a risk factor for pulmonary metastasis in colorectal cancer. Color. Dis. 2017, 19, O322–O328. [Google Scholar] [CrossRef] [PubMed]
- Van de Schans, S.; Janssen-Heijnen, M.; Biesma, B.; Smeenk, F.; van de Poll-Franse, L.; Seynaeve, C.; Coebergh, J. COPD in cancer patients: Higher prevalence in the elderly, a different treatment strategy in case of primary tumours above the diaphragm, and a worse overall survival in the elderly patient. Eur. J. Cancer 2007, 43, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Li, M.-C.; Yu, Y.-H.; Lin, C.-M.; Wu, S.-Y. Chronic Obstructive Pulmonary Disease and Its Acute Exacerbation before Colon Adenocarcinoma Treatment Are Associated with Higher Mortality: A Propensity Score-Matched, Nationwide, Population-Based Cohort Study. Cancers 2021, 13, 4728. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Lampert, T.; von der Lippe, E.; Muters, S. Prevalence of smoking in the adult population of Germany: Results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Parascandola, M.; Xiao, L. Tobacco and the lung cancer epidemic in China. Transl. Lung Cancer Res. 2019, 8, S21–S30. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Zheng, X.-Y.; Chung, K.F.; Zhong, N.-S. Impact of air pollution on the burden of chronic respiratory diseases in China: Time for urgent action. Lancet 2016, 388, 1939–1951. [Google Scholar] [CrossRef]
| Variable | N (%) |
|---|---|
| N | 19,321 |
| Cancer type | |
| Colon | 15,086 (78.1) |
| Rectum | 4235 (21.9) |
| Age (mean, SD) | 65.2 (11.3) |
| Males (%) | 11,863 (61.4) |
| Facility | |
| Hospital | 13,903 (72.0) |
| Office-based oncologists | 1930 (10.0) |
| Unknown | 3488 (18.0) |
| Co-Diagnosis of COPD | 1732 (9.0) |
| Country | |
| Germany | 2988 (15.5) |
| France | 2594 (13.4) |
| Italy | 3486 (18.0) |
| Spain | 2203 (11.4) |
| UK | 2224 (11.5) |
| Japan | 1713 (8.9) |
| Korea | 1074 (5.6) |
| China | 3039 (15.7) |
| Variable | OR (95%CI) | p Value |
|---|---|---|
| Age | ||
| Age ≤ 50 | Reference | |
| Age 51–60 | 1.04 (0.92–1.17) | 0.571 |
| Age 61–70 | 1.09 (0.97–1.21) | 0.157 |
| Age > 70 | 1.09 (0.97–1.22) | 0.154 |
| Sex | ||
| Male sex | 0.98 (0.92–1.04) | 0.444 |
| Female sex | Reference | |
| Cancer type | ||
| Colon cancer | Reference | |
| Rectum cancer | 1.52 (1.42–1.64) | <0.001 |
| COPD | 1.22 (1.09–1.36) | <0.001 |
| Country | ||
| Germany | 1.47 (1.26–1.71) | <0.001 |
| France | 1.29 (1.15–1.46) | <0.001 |
| Italy | 1.86 (1.52–2.27) | <0.001 |
| Spain | 1.85 (1.64–2.09) | <0.001 |
| UK | 2.02 (1.80–2.28) | <0.001 |
| Japan | 1.20 (1.05–1.37) | 0.008 |
| Korea | 1.28 (1.10–1.50) | 0.002 |
| China | Reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jördens, M.S.; Labuhn, S.; Luedde, T.; Hoyer, L.; Kostev, K.; Loosen, S.H.; Roderburg, C. Prevalence of Lung Metastases among 19,321 Metastatic Colorectal Cancer Patients in Eight Countries of Europe and Asia. Curr. Oncol. 2021, 28, 5035-5040. https://doi.org/10.3390/curroncol28060423
Jördens MS, Labuhn S, Luedde T, Hoyer L, Kostev K, Loosen SH, Roderburg C. Prevalence of Lung Metastases among 19,321 Metastatic Colorectal Cancer Patients in Eight Countries of Europe and Asia. Current Oncology. 2021; 28(6):5035-5040. https://doi.org/10.3390/curroncol28060423
Chicago/Turabian StyleJördens, Markus S., Simon Labuhn, Tom Luedde, Laura Hoyer, Karel Kostev, Sven H. Loosen, and Christoph Roderburg. 2021. "Prevalence of Lung Metastases among 19,321 Metastatic Colorectal Cancer Patients in Eight Countries of Europe and Asia" Current Oncology 28, no. 6: 5035-5040. https://doi.org/10.3390/curroncol28060423
APA StyleJördens, M. S., Labuhn, S., Luedde, T., Hoyer, L., Kostev, K., Loosen, S. H., & Roderburg, C. (2021). Prevalence of Lung Metastases among 19,321 Metastatic Colorectal Cancer Patients in Eight Countries of Europe and Asia. Current Oncology, 28(6), 5035-5040. https://doi.org/10.3390/curroncol28060423








