Identification of Tobacco-Related Cancer Diagnoses among Individuals with Psychiatric Disorders: A Population-Based Matched Cohort Study Using a Competing Risks Approach from British Columbia
Abstract
:1. Introduction
2. Methods
2.1. Study Population: Data Sources
2.2. Measurement of Outcomes
2.3. Statistical Methods
3. Results
3.1. Clinical Characteristics
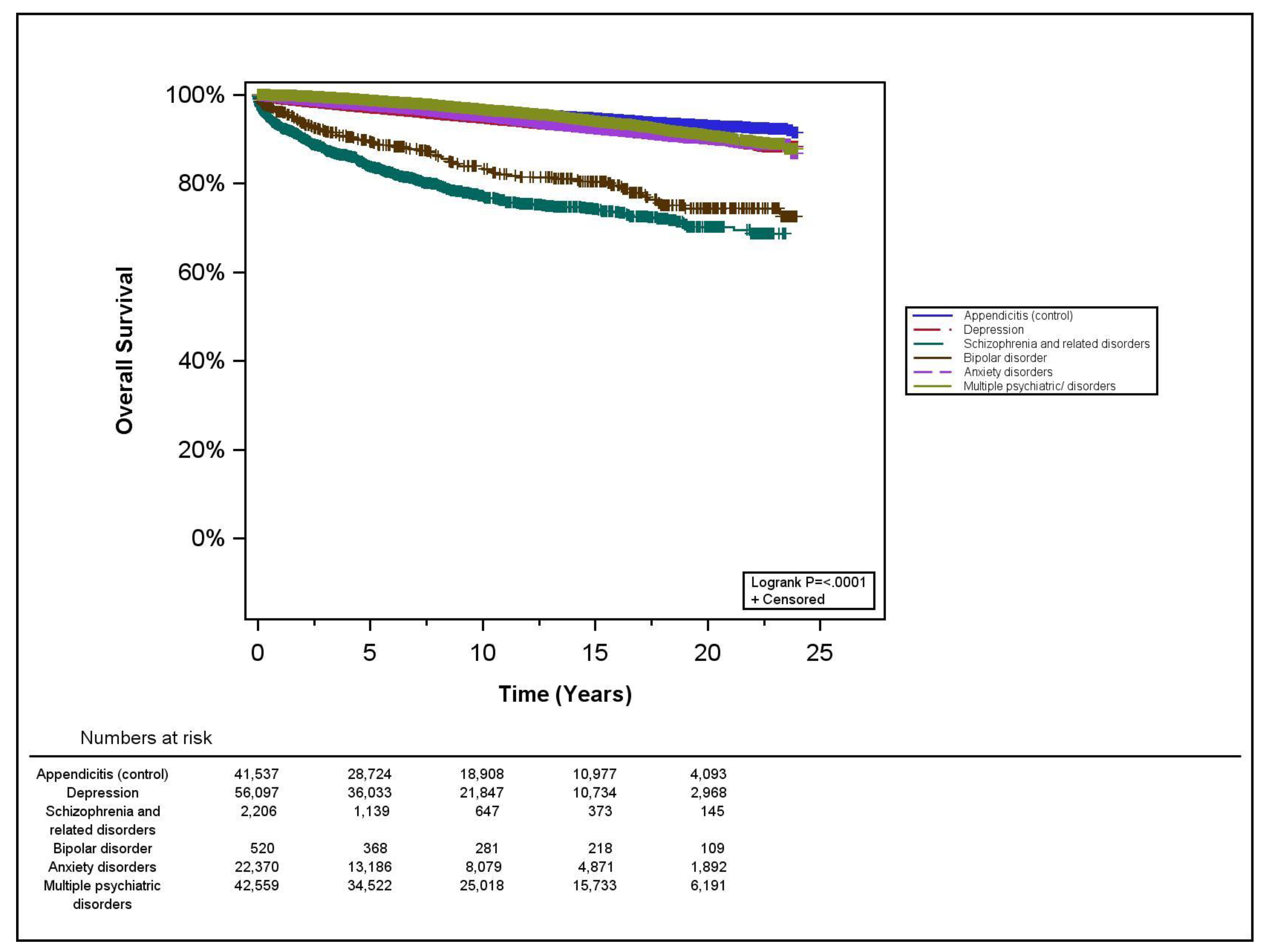
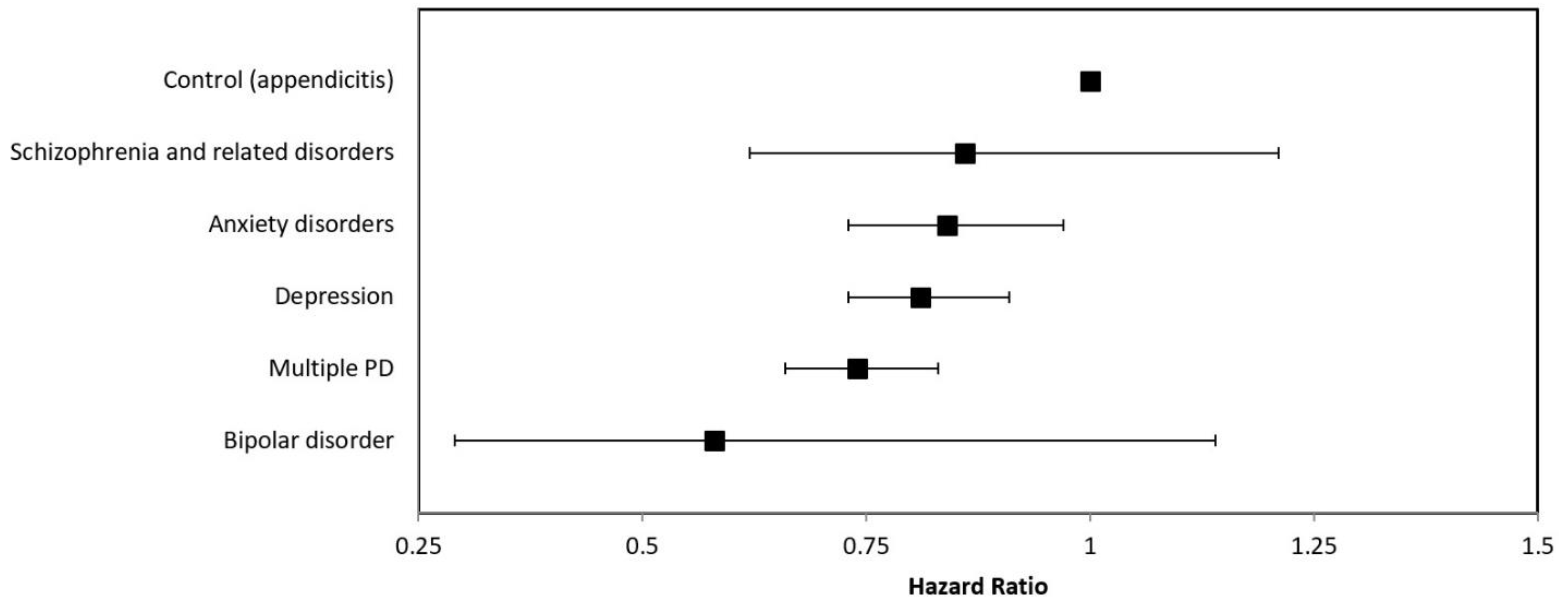
3.2. Survival Analyses
3.3. Tobacco-Related (TR) Cancer Analysis
3.4. Interpretation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lasser, K.; Boyd, J.W.; Woolhandler, S.; Himmelstein, D.; McCormick, D.; Bord, H. Smoking and mental illness: A population-based prevalence study. JAMA 2000, 284, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.H.; Mazure, C.M.; McKee, S.A. Smoking and mental illness in the U.S. population. Tob Control 2014, 23, 147. [Google Scholar] [CrossRef] [PubMed]
- Signs, V. Current cigarette smoking among adults aged ≥18 years with mental illness-united states, 2009–2011. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 81–87. [Google Scholar]
- Kalman, D.; Morissette, S.B.; George, T.P. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am. J. Addict. 2005, 14, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Hasin, D.S.; Chou, S.P.; Patricia, S.; Stinson, F.; Dawson, D. Nicotine dependence and psychiatric disorders in the united states: Results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry 2004, 61, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.L.; Wayne, G.F.; Kafali, E.N.; Liu, Z.; Shu, C.; Flores, M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA 2014, 311, 172–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leon, J.; Diaz, F.J. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005, 76, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiatry 2015, 72, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.C.; Veldhuizen, S.; Jeysingh, T.; Orlan, C.; Graham, C.; Kakouris, G.; Remington, G.; Gatley, J. Patterns of tobacco-related mortality among individuals diagnosed with schizophrenia, bipolar disorder, or depression. J. Psychiatr. Res. 2014, 48, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Population Data BC. Available online: http://www.popdata.bc.ca/data (accessed on 1 November 2021).
- Ministry of Health. Medical Services Plan Data. Population Data BC. Data Extract. BC Ministry of Health (2017). Available online: http://www.popdata.bc.ca/data (accessed on 1 November 2021).
- Ministry of Health. Discharge Abstracts Database Data: Population Data BC. Data Extract. BC Ministry of Health (2017). Available online: http://www.popdata.bc.ca/data (accessed on 1 November 2021).
- Ministry of Health. PharmaNet/PharmaCare Data. Population Data BC. Data Extract. Data Stewardship Committee (2017) & Ministry of Health (2017). Available online: https://www.popdata.bc.ca/data_access/publishing_research_materials (accessed on 1 November 2021).
- Callaghan, R.C.; Cunningham, J.K.; Sykes, J.; Kish, S. Increased risk of parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012, 120, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Poikolainen, K.; Saarinen, M.; Eskola, J. Acute appendicitis not associated with social class among children. Int. J. Epidemiol. 1985, 14, 333–334. [Google Scholar] [CrossRef] [PubMed]
- BC Cancer Registry Data: Population Data BC. Data Extract. BC Cancer (2017). Available online: http://www.popdata.bc.ca/data (accessed on 1 November 2021).
- The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014.
- Smoking-attributable mortality, years of potential life lost, and productivity losses—United states, 2000–2004. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 1226–1228.
- Davis, L.E.; Bogner, E.; Coburn, N.G.; Natalie, G.; Hanna, T.; Kurdyak, P.; Groome, P.; Mahar, A. Stage at diagnosis and survival in patients with cancer and a pre-existing mental illness: A meta-analysis. J. Epidemiol. Community Health 2020, 74, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Clifton, A.; Burgess, C.; Clement, S.; Ohlsen, R.; Ramluggun, P.; Sturt, J.; Walters, P.; Barley, E. Influences on uptake of cancer screening in mental health service users: A qualitative study. BMC Health Serv. Res. 2016, 16, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgson, R.; Wildgust, H.J.; Bushe, C.J. Cancer and schizophrenia: Is there a paradox? J. Psychopharmacol. 2010, 24, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.M.; Lau, M.; Jönsson, C.; Zhang, C.; Riso Berglind, L.-L.; Jonasson, J.M.; Strander, B. Participation in a swedish cervical cancer screening program among women with psychiatric diagnoses: A population-based cohort study. BMC Public Health 2019, 19, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, B.A.; Rivara, F.P.; Lii, S.M.; Weiss, N.S. Environmental factors and the risk for childhood pedestrian-motor vehicle collision occurrence. Am. J. Epidemiol. 1990, 132, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Hsiao, F.; Pfeiffer, S.; Hwang, Y.-T.; Lee, H.-C. An increased risk of stroke among young schizophrenia patients. Schizophr. Res. 2008, 101, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tsai, S.; Lee, H. No higher risk of myocardial infarction among bipolar patients in a 6-year follow-up of acute mood episodes. Psychosom Med. 2008, 70, 73–76. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Control | A | B | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (Appendicitis) n = 41,537 | All PD n = 123,752 | p-Value | Depression n = 56,097 | Schizophrenia n = 2206 | Bipolar n = 520 | Anxiety n = 22,370 | Multiple PD n = 42,559 | p-Value | |
| Median age at diagnosis in years (range) | 29 (13–85) | 30 (13–85) | 0.29 | 30 (13–85) | 33 (13–85) | 29 (13–85) | 31 (13–85) | 28 (13–85) | <0.001 |
| Age, n (%) | |||||||||
| 13–25 | 17,105 (41%) | 50,600 (41%) | 0.02 | 22,251 (40%) | 815 (37%) | 228 (44%) | 8262 (37%) | 19,044 (45%) | <0.001 |
| 26–35 | 8523 (21%) | 25,493 (21%) | 0.95 | 11,326 (20%) | 343 (16%) | 89 (17%) | 4680 (21%) | 9055 (21%) | 0.57 |
| 36–45 | 6216 (15%) | 18,644 (15%) | 0.92 | 8682 (16%) | 221 (10%) | 57 (11%) | 3429 (15%) | 6255 (15%) | 0.24 |
| 46–55 | 4303 (10%) | 12,885 (10%) | 0.92 | 6304 (11%) | 179 (8%) | 43 (8%) | 2488 (11%) | 3871 (9%) | 0.01 |
| 56–65 | 2766 (7%) | 8257 (7%) | 0.58 | 3961 (7%) | 160 (7%) | 40 (8%) | 1874 (8%) | 2222 (5%) | <0.001 |
| 66–75 | 1689 (4%) | 5075 (4%) | 0.63 | 2287 (4%) | 215 (10%) | 31 (6%) | 1141 (5%) | 1401 (3%) | 0.001 |
| 76–85 | 935 (2%) | 2798 (2%) | 0.98 | 1286 (2%) | 273 (12%) | 32 (6%) | 496 (2%) | 711 (2%) | 0.06 |
| Male, n (%) | 25,457 (61%) | 75,596 (61%) | 0.47 | 34,699 (62%) | 1704 (77%) | 351 (68%) | 14,114 (63%) | 24,728 (58%) | <0.001 |
| Mean years of follow-up (to cancer) | 9.9 | 9.8 | 0.06 | 8.7 | 7.4 | 11.6 | 8.5 | 12.0 | <0.001 |
| Developed TR cancer during study period, n (%) | 730 (2%) | 1689 (1%) | <0.001 | 721 (1%) | 55 (3%) | 11 (2%) | 312 (1%) | 590 (1%) | <0.001 |
| Mean years of follow-up (to death) | 10.9 | 10.8 | 0.003 | 9.7 | 8.2 | 12.5 | 9.5 | 13.0 | <0.001 |
| Died during study period, n (%) | 1577 (4%) | 6329 (5%) | <0.001 | 2696 (5%) | 416 (19%) | 96 (19%) | 993 (4%) | 2128 (5%) | <0.001 |
| Total person-years of follow-up | 409 251 | 1 210 346 | - | 487 950 | 16 285 | 6044 | 189 820 | 510 247 | - |
| Cancer-Specific Survival | Overall Survival | ||
|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p-Value | |
| Control (appendicitis) | 1 | reference | |
| Depression | 1.41 | 1.33–1.50 | <0.001 |
| Schizophrenia and related disorders | 6.47 | 5.81–7.21 | <0.001 |
| Bipolar disorder | 4.25 | 3.46–5.22 | <0.001 |
| Anxiety disorders | 1.33 | 1.23–1.44 | <0.001 |
| Multiple PD | 1.11 | 1.04–1.18 | <0.001 |
| Hazard Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Control (appendicitis) | 1 | reference | |
| Depression | 0.81 | 0.73–0.91 | <0.01 |
| Schizophrenia and related disorders | 0.86 | 0.62–1.21 | 0.40 |
| Bipolar disorder | 0.58 | 0.29–1.14 | 0.12 |
| Anxiety disorders | 0.84 | 0.73–0.97 | 0.02 |
| Multiple PD | 0.74 | 0.66–0.83 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, R.; McLay, M.; Hamm, J.; Callaghan, R.C. Identification of Tobacco-Related Cancer Diagnoses among Individuals with Psychiatric Disorders: A Population-Based Matched Cohort Study Using a Competing Risks Approach from British Columbia. Curr. Oncol. 2021, 28, 4953-4960. https://doi.org/10.3390/curroncol28060415
Olson R, McLay M, Hamm J, Callaghan RC. Identification of Tobacco-Related Cancer Diagnoses among Individuals with Psychiatric Disorders: A Population-Based Matched Cohort Study Using a Competing Risks Approach from British Columbia. Current Oncology. 2021; 28(6):4953-4960. https://doi.org/10.3390/curroncol28060415
Chicago/Turabian StyleOlson, Robert, Mary McLay, Jeremy Hamm, and Russell C. Callaghan. 2021. "Identification of Tobacco-Related Cancer Diagnoses among Individuals with Psychiatric Disorders: A Population-Based Matched Cohort Study Using a Competing Risks Approach from British Columbia" Current Oncology 28, no. 6: 4953-4960. https://doi.org/10.3390/curroncol28060415
APA StyleOlson, R., McLay, M., Hamm, J., & Callaghan, R. C. (2021). Identification of Tobacco-Related Cancer Diagnoses among Individuals with Psychiatric Disorders: A Population-Based Matched Cohort Study Using a Competing Risks Approach from British Columbia. Current Oncology, 28(6), 4953-4960. https://doi.org/10.3390/curroncol28060415






