Impact of Value Frameworks on the Magnitude of Clinical Benefit: Evaluating a Decade of Randomized Trials for Systemic Therapy in Solid Malignancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Abstraction
2.3. Data Synthesis and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
| Concept | Synonym |
|---|---|
| Drug Therapy | “drug therap*” [Keyword]; drug therapy [MeSH]; chemotherap* [Keyword]; immunotherap* [Keyword]; immunotherapy [MeSH]; “antineoplastic agent*” [Keyword]; antineoplastic agents [MeSH]; chemoradiotherap* [Keyword]; chemoradiotherapy [MeSH]; |
| Solid Tumour Cancers 1 | “solid tumour*” [Keyword]; “solid tumor*” “solid tumor* cancer” [Keyword]; “solid tumour* cancer” [Keyword]; “bladder cancer” [Keyword]; “bladder neoplasm*” [Keyword]; “bladder carcinoma” [Keyword]; “bladder tumor*” [Keyword]; “bladder tumour*” [Keyword]; urinary bladder neoplasms [MeSH]; “breast cancer” [Keyword]; “breast neoplasm*” [Keyword]; breast neoplasms [MeSH]; “breast carcinoma” [Keyword]; “breast tumor*” [Keyword]; “breast tumour*” [Keyword]; “cervical cancer” [Keyword]; “cervical neoplasm*” [Keyword]; “cervical carcinoma” [Keyword]; “cervical tumor*” [Keyword]; “cervical tumour*” [Keyword]; uterine cervical neoplasms [MeSH]; “colon cancer” [Keyword]; “colon neoplasm*” [Keyword]; “colon carcinoma” [Keyword]; “colon tumor*” [Keyword]; “colon tumour*” [Keyword]; colonic neoplasms [MeSH]; “colorectal cancer” [Keyword]; “colorectal neoplasm*” [Keyword]; colorectal neoplasms [MeSH]; “colorectal carcinoma” [Keyword]; “colorectal tumor*” [Keyword]; “colorectal tumour*” [Keyword]; “rectal cancer” [Keyword]; “rectal neoplasm*” [Keyword]; rectal neoplasms [MeSH]; “rectal carcinoma” [Keyword]; “rectal tumor*” [Keyword]; “rectal tumour*” [Keyword]; “endometrial cancer” [Keyword]; “endometrial neoplasm*” [Keyword]; “endometrial carcinoma” [Keyword]; “endometrial tumor*” [Keyword]; “endometrial tumour*” [Keyword]; endometrial neoplasms [MeSH]; “kidney cancer” [Keyword]; “kidney neoplasm*” [Keyword]; kidney neoplasms [MeSH]; “kidney carcinoma” [Keyword]; “kidney tumor*” [Keyword]; “kidney tumour*” [Keyword]; “lip cancer” [Keyword]; “lip neoplasm*” [Keyword]; lip neoplasms [MeSH]; “lip carcinoma” [Keyword]; “lip tumor*” [Keyword]; “lip tumour*” [Keyword]; “oral cancer” [Keyword]; “oral neoplasm*” [Keyword]; mouth neoplasms [MeSH]; “oral carcinoma” [Keyword]; “oral tumor*” [Keyword]; “oral tumour*” [Keyword]; “head and neck cancer” [Keyword]; “head and neck neoplasm*” [Keyword]; head and neck neoplasms [MESH]; “head and neck carcinoma” [Keyword]; “head and neck tumor*” [Keyword]; “head and neck tumour*” [Keyword]; “liver cancer” [Keyword]; “liver neoplasm*” [Keyword]; liver neoplasms [MeSH]; “liver carcinoma” [Keyword]; “liver tumour*” [Keyword]; “liver tumor*” [Keyword]; melanoma [Keyword, MeSH]; mesothelioma [Keyword, MeSH]; “non-small-cell lung cancer” [Keyword]; “non-small-cell lung neoplasm*” [Keyword]; “non-small-cell lung carcinoma” [Keyword]; carcinoma, non-small-cell lung [MeSH]; ”non-small-cell lung tumor*” [Keyword]; “non-small-cell lung tumour*” [Keyword]; “non-melanoma skin cancer” [Keyword]; “nonmelanoma skin cancer” [Keyword]; “nonmelanoma skin neoplasm*” [Keyword]; “non-melanoma skin neoplasm*” [Keyword]; “nonmelanoma carcinoma” [Keyword]; “non-melanoma skin cancer” [Keyword]; “nonmelanoma skin tumor*” [Keyword]; “nonmelanoma skin tumour*” [Keyword]; “non-melanoma skin tumour*” [Keyword]; “non-melanoma skin tumor*” [Keyword]; “ovarian cancer” [Keyword]; “ovarian neoplasm*” [Keyword]; ovarian neoplasms [MeSH]; “ovarian carcinoma” [Keyword]; “ovarian tumor*” [Keyword]; “ovarian tumour*” [Keyword]; “pancreatic cancer” [Keyword]; “pancreatic neoplasm*” [Keyword]; pancreatic neoplasms [MeSH]; “pancreatic carcinoma” [Keyword]; “pancreatic tumor*” [Keyword]; “pancreatic tumour*” [Keyword]; “prostate cancer” [Keyword]; “prostate neoplasm*” [Keyword]; prostatic neoplasms [MeSH]; “prostate carcinoma” [Keyword]; “prostate tumor*” [Keyword]; “prostate tumour*” [Keyword]; sarcoma [Keyword, MeSH]; “small cell lung cancer” [Keyword]; “small cell lung neoplasm*” [Keyword]; “small cell lung carcinoma” [Keyword, MeSH]; “small cell lung tumor*” [Keyword]; “small cell lung tumour*” [Keyword]; “thyroid cancer” [Keyword]; “thyroid neoplasm*” [Keyword]; thyroid neoplasms [MeSH]; “thyroid carcinoma” [Keyword]; “thyroid tumour*” [Keyword]; “thyroid tumor*” [Keyword]; |
| Language | English |
| Age | Open |
| Publication Date | 2010–present |
| Publication Type | Phase 2/Phase 3 trials |
| Organism | humans |
References
- Porter, M.E. What is value in health care? N. Engl. J. Med. 2010, 363, 2477–2481. [Google Scholar] [CrossRef]
- Prasad, V.; De Jesús, K.; Mailankody, S. The high price of anticancer drugs: Origins, implications, barriers, solutions. Nat. Rev. Clin. Oncol. 2017, 14, 381–390. [Google Scholar] [CrossRef]
- Saluja, R.; Arciero, V.S.; Cheng, S.; McDonald, E.; Wong, W.W.; Cheung, M.C.; Chan, K.K. Examining Trends in Cost and Clinical Benefit of Novel Anticancer Drugs Over Time. J. Oncol. Pract. 2018, 14, e280–e294. [Google Scholar] [CrossRef]
- Schnipper, L.E.; Davidson, N.E.; Wollins, D.S.; Tyne, C.; Blayney, D.W.; Blum, D.; Dicker, A.P.; Ganz, P.A.; Hoverman, J.R.; Langdon, R.M.; et al. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J. Clin. Oncol. 2015, 33, 2563–2577. [Google Scholar] [CrossRef]
- Schnipper, L.E.; Davidson, N.E.; Wollins, D.S.; Blayney, D.W.; Dicker, A.P.; Ganz, P.A.; Hoverman, J.R.; Langdon, R.; Lyman, G.H.; Meropol, N.J.; et al. Updating the American Society of Clinical Oncology Value Framework: Revisions and Reflections in Response to Comments Received. J. Clin. Oncol. 2016, 34, 2925–2934. [Google Scholar] [CrossRef] [PubMed]
- Cherny, N.I.; Sullivan, R.; Dafni, U.; Kerst, J.M.; Sobrero, A.; Zielinski, C.; de Vries, E.; Piccart, M.J. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann. Oncol. 2015, 26, 1547–1573. [Google Scholar] [CrossRef]
- Cherny, N.I.; Dafni, U.; Bogaerts, J.; Latino, N.J.; Pentheroudakis, G.; Douillard, J.-Y.; Tabernero, J.; Zielinski, C.; Piccart, M.J.; de Vries, E.G.E. ESMO-Magnitude of Clinical Benefit Scale version 1. Ann. Oncol. 2017, 28, 2340–2366. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807, Erratum in 2021, 21, 796–807. [Google Scholar] [CrossRef]
- Abrams, R.A.; Winter, K.A.; Safran, H.; Goodman, K.A.; Regine, W.F.; Berger, A.C.; Gillin, M.T.; Philip, P.A.; Lowy, A.M.; Wu, A.; et al. Results of the NRG Oncology/RTOG 0848 Adjuvant Chemotherapy Question—Erlotinib+Gemcitabine for Resected Cancer of the Pancreatic Head. Am. J. Clin. Oncol. 2020, 43, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Adkins, D.; Ley, J.; Neupane, P.; Worden, F.; Sacco, A.G.; Palka, K.; Grilley-Olson, E.J.; Maggiore, R.; Salama, N.; Trinkaus, K.; et al. Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: A multicentre, multigroup, phase 2 trial. Lancet Oncol. 2019, 20, 1295–1305. [Google Scholar] [CrossRef]
- Agarwal, N.; McQuarrie, K.; Bjartell, A.; Chowdhury, S.; de Santana Gomes, A.J.P.; Chung, B.H.; Özgüroglu, M.; Soto, J.; Merseburger, A.S.; Uemura, H.; et al. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): A randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019, 20, 1518–1530. [Google Scholar] [CrossRef]
- Aghajanian, C.; Goff, B.; Nycum, L.R.; Wang, Y.V.; Husain, A.; Blank, S.V. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 2015, 139, 10–16. [Google Scholar] [CrossRef]
- Ahlgren, G.M.; Flodgren, P.; Tammela, T.L.; Kellokumpu-Lehtinen, P.; Borre, M.; Angelsen, A.; Iversen, J.R.; Sverrisdottir, A.; Jonsson, E.; Sengelov, L. Docetaxel versus Surveillance after Radical Prostatectomy for High-Risk Prostate Cancer: Results from the Prospective Randomised, Open-Label Phase 3 Scandinavian Prostate Cancer Group 12 Trial. Eur. Urol. 2018, 73, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Alderson, D.; Cunningham, D.; Nankivell, M.; Blazeby, J.; Griffin, S.M.; Crellin, A.; Grabsch, I.H.; Langer, R.; Pritchard, S.; Okines, A.; et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): An open-label, randomised phase 3 trial. Lancet Oncol. 2017, 18, 1249–1260. [Google Scholar] [CrossRef]
- Amaria, R.N.; Reddy, S.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.-J.; Hanna, E.; et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Aparicio, T.; Bouché, O.; Taieb, J.; Maillard, E.; Kirscher, S.; Etienne, P.-L.; Faroux, R.; Akouz, F.K.; El Hajbi, F.; Locher, C.; et al. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: A randomized phase II trial—PRODIGE 20 study results. Ann. Oncol. 2017, 29, 133–138. [Google Scholar] [CrossRef]
- Aranda, E.; García-Alfonso, P.; Benavides, M.; Ruiz, A.S.; Guillén-Ponce, C.; Safont, M.; Alcaide, J.; Gómez, A.; López, R.; Manzano, J.; et al. First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: Phase II randomised MACRO2 TTD study. Eur. J. Cancer 2018, 101, 263–272. [Google Scholar] [CrossRef]
- Arend, R.C.; Davis, A.M.; Chimiczewski, P.; O’Malley, D.M.; Provencher, D.; Vergote, I.; Ghamande, S.; Birrer, M.J. EMR 20006-012: A phase II randomized double-blind placebo controlled trial comparing the combination of pimasertib (MEK inhibitor) with SAR245409 (PI3K inhibitor) to pimasertib alone in patients with previously treated unresectable borderline or low grade ovarian cancer. Gynecol. Oncol. 2019, 156, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Bauman, J.E.; Ohr, J.; Gooding, W.E.; Heron, D.E.; Duvvuri, U.; Kubicek, G.J.; Posluszny, D.M.; Vassilakopoulou, M.; Kim, S.; et al. Phase II randomized trial of radiation therapy, cetuximab, and pemetrexed with or without bevacizumab in patients with locally advanced head and neck cancer. Ann. Oncol. 2016, 27, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Lee, J.; Stevenson, J.; Sulecki, M.; Hugec, V.; Choong, N.; Saltzman, J.; Song, W.; Hansen, R.; Evans, T.; et al. Phase II randomized trial of carboplatin, paclitaxel, bevacizumab with or without cixutumumab (IMC-A12) in patients with advanced non-squamous, non-small-cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E3508). Ann. Oncol. 2017, 28, 3037–3043. [Google Scholar] [CrossRef]
- Argiris, A.; Li, S.; Savvides, P.; Ohr, J.P.; Gilbert, J.; Levine, M.A.; Chakravarti, A.; Haigentz, M., Jr.; Saba, N.F.; Ikpeazu, C.V.; et al. Phase III Randomized Trial of Chemotherapy with or without Bevacizumab in Patients with Recurrent or Metastatic Head and Neck Cancer. J. Clin. Oncol. 2019, 37, 3266–3274. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Ascierto, A.P.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.-J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dummer, R.; Gogas, H.J.; Flaherty, K.T.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; et al. Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600–mutant melanoma. Eur. J. Cancer 2020, 126, 33–44. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Ferrucci, P.F.; Fisher, R.; Del Vecchio, M.; Atkinson, V.; Schmidt, H.; Schachter, J.; Queirolo, P.; Long, G.V.; Di Giacomo, A.M.; et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat. Med. 2019, 25, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Awada, A.; Garcia, A.A.; Chan, S.; Jerusalem, G.H.M.; Coleman, E.R.; Huizing, M.T.; Mehdi, A.; O’Reilly, S.M.; Hamm, J.T.; Barrett-Lee, P.J.; et al. Two schedules of etirinotecan pegol (NKTR-102) in patients with previously treated metastatic breast cancer: A randomised phase 2 study. Lancet Oncol. 2013, 14, 1216–1225. [Google Scholar] [CrossRef]
- Baize, N.; Monnet, I.; Greillier, L.; Geier, M.; Lena, H.; Janicot, H.; Vergnenegre, A.; Crequit, J.; Lamy, R.; Auliac, J.-B.; et al. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1224–1233. [Google Scholar] [CrossRef]
- Barlesi, F.; Garon, E.B.; Kim, D.-W.; Felip, E.; Han, J.-Y.; Kim, J.-H.; Ahn, M.-J.; Fidler, M.J.; Gubens, M.A.; de Castro, G.; et al. Health-Related Quality of Life in KEYNOTE-010: A Phase II/III Study of Pembrolizumab versus Docetaxel in Patients with Previously Treated Advanced, Programmed Death Ligand 1–Expressing NSCLC. J. Thorac. Oncol. 2019, 14, 793–801. [Google Scholar] [CrossRef]
- Baselga, J.; Im, S.-A.; Iwata, H.; Cortes, J.; DE Laurentiis, M.; Jiang, Z.; Arteaga, C.L.; Jonat, W.; Clemons, M.; Ito, Y.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 904–916, Erratum in 2017, 20, e71–e72. [Google Scholar] [CrossRef]
- Basu, P.; Mehta, A.; Jain, M.; Gupta, S.; Nagarkar, R.V.; John, S.; Petit, R. A Randomized Phase 2 Study of ADXS11-001 Listeria monocytogenes–Listeriolysin O Immunotherapy with or without Cisplatin in Treatment of Advanced Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 764–772. [Google Scholar] [CrossRef]
- Baumann, K.; Pfisterer, J.; Wimberger, P.; Burchardi, N.; Kurzeder, C.; du Bois, A.; Loibl, S.; Sehouli, J.; Huober, J.; Schmalfeldt, B.; et al. Intraperitoneal treatment with the trifunctional bispecific antibody Catumaxomab in patients with platinum-resistant epithelial ovarian cancer: A phase IIa study of the AGO Study Group. Gynecol. Oncol. 2011, 123, 27–32. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Ou, F.-S.; Ahn, D.H.; Boland, P.M.; Ciombor, K.K.; Heying, E.N.; Dockter, T.J.; Jacobs, N.L.; Pasche, B.C.; Cleary, J.M.; et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): A randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019, 20, 1070–1082. [Google Scholar] [CrossRef]
- Bergmann, L.; Grünwald, V.; Maute, L.; Grimm, M.-O.; Weikert, S.; Schleicher, J.; Klotz, T.; Greiner, J.; Flörcken, A.; Hartmann, A.; et al. A Randomized Phase IIa Trial with Temsirolimus versus Sunitinib in Advanced Non-Clear Cell Renal Cell Carcinoma: An Intergroup Study of the CESAR Central European Society for Anticancer Drug Research-EWIV and the Interdisciplinary Working Group on Renal Cell Cancer (IAGN) of the German Cancer Society. Oncol. Res. Treat. 2020, 43, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Biganzoli, L.; Cinieri, S.; Berardi, R.; Pedersini, R.; McCartney, A.; Minisini, A.M.; Caremoli, E.R.; Spazzapan, S.; Magnolfi, E.; Brunello, A.; et al. EFFECT: A randomized phase II study of efficacy and impact on function of two doses of nab-paclitaxel as first-line treatment in older women with advanced breast cancer. Breast Cancer Res. 2020, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Birtle, A.; Johnson, M.; Chester, J.; Jones, R.; Dolling, D.; Bryan, R.; Harris, C.; Winterbottom, A.; Blacker, A.; Catto, J.W.F.; et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 2020, 395, 1268–1277. [Google Scholar] [CrossRef]
- Bisogno, G.; De Salvo, G.L.; Bergeron, C.; Melcón, S.G.; Merks, J.H.; Kelsey, A.; Martelli, H.; Minard-Colin, V.; Orbach, D.; Glosli, H.; et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 1566–1575. [Google Scholar] [CrossRef]
- Blagden, S.P.; Cook, A.D.; Poole, C.; Howells, L.; McNeish, I.; Dean, A.; Kim, J.-W.; O’Donnell, D.M.; Hook, J.; James, E.C.; et al. Weekly platinum-based chemotherapy versus 3-weekly platinum-based chemotherapy for newly diagnosed ovarian cancer (ICON8): Quality-of-life results of a phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 969–977. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef]
- Bordoni, R.; Ciardiello, F.; von Pawel, J.; Cortinovis, D.; Karagiannis, T.; Ballinger, M.; Sandler, A.; Yu, W.; He, P.; Matheny, C.; et al. Patient-Reported Outcomes in OAK: A Phase III Study of Atezolizumab versus Docetaxel in Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, 441–449.e4. [Google Scholar] [CrossRef] [PubMed]
- Borg, C.; Mantion, G.; Boudghène, F.; Mornex, F.; Ghiringhelli, F.; Adenis, A.; Azria, D.; Balosso, J.; Ben Abdelghani, M.; Bachet, J.B.; et al. Efficacy and Safety of Two Neoadjuvant Strategies with Bevacizumab in MRI-Defined Locally Advanced T3 Resectable Rectal Cancer: Final Results of a Randomized, Noncomparative Phase 2 INOVA Study. Clin. Color. Cancer 2019, 18, 200–208.e1. [Google Scholar] [CrossRef]
- Boudewijns, S.; Bloemendal, M.; De Haas, N.; Westdorp, H.; Bol, K.F.; Schreibelt, G.; Aarntzen, E.H.J.G.; Lesterhuis, W.J.; Gorris, M.A.J.; Croockewit, A.; et al. Autologous monocyte-derived DC vaccination combined with cisplatin in stage III and IV melanoma patients: A prospective, randomized phase 2 trial. Cancer Immunol. Immunother. 2020, 69, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): A multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017, 18, 1600–1609. [Google Scholar] [CrossRef]
- Brandes, A.A.; Gil-Gil, M.; Saran, F.; Carpentier, A.F.; Nowak, A.K.; Mason, W.; Zagonel, V.; Dubois, F.; Finocchiaro, G.; Fountzilas, G.; et al. A Randomized Phase II Trial (TAMIGA) Evaluating the Efficacy and Safety of Continuous Bevacizumab through Multiple Lines of Treatment for Recurrent Glioblastoma. Oncologist 2018, 24, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.; Angioli, R.; Scambia, G.; Lorusso, D.; Terranova, C.; Panici, P.B.; Raspagliesi, F.; Scollo, P.; Plotti, F.; Ferrandina, G.; et al. Front-line chemo-immunotherapy with carboplatin-paclitaxel using oregovomab indirect immunization in advanced ovarian cancer: A randomized phase II study. Gynecol. Oncol. 2020, 156, 523–529. [Google Scholar] [CrossRef]
- Bridgewater, A.J.; Pugh, A.S.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; Griffiths, O.G.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef]
- Broom, R.J.; Hinder, V.; Sharples, K.; Proctor, J.; Duffey, S.; Pollard, S.; Fong, P.C.; Forgeson, G.; Harris, D.L.; Jameson, M.B.; et al. Everolimus and Zoledronic Acid in Patients with Renal Cell Carcinoma with Bone Metastases: A Randomized First-Line Phase II Trial. Clin. Genitourin. Cancer 2014, 13, 50–58. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Burris, H.A.; Lebrun, F.; Rugo, H.S.; Beck, J.T.; Piccart, M.; Neven, P.; Baselga, J.; Petrakova, K.; Hortobagyi, G.N.; Komorowski, A.; et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer 2013, 119, 1908–1915. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Campone, M.; Im, S.-A.; Iwata, H.; Clemons, M.; Ito, Y.; Awada, A.; Chia, S.; Jagiello-Gruszfeld, A.; Pistilli, B.; Tseng, L.-M.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: Overall survival results from BELLE-2. Eur. J. Cancer 2018, 103, 147–154. [Google Scholar] [CrossRef]
- Campone, M.; Lacroix-Triki, M.; Roca, L.; Spielmann, M.; Wildiers, H.; Cottu, P.; Kerbrat, P.; Levy, C.; Desmoulins, I.; Bachelot, T.; et al. UCBG 2-08: 5-year efficacy results from the UNICANCER-PACS08 randomised phase III trial of adjuvant treatment with FEC100 and then either docetaxel or ixabepilone in patients with early-stage, poor prognosis breast cancer. Eur. J. Cancer 2018, 103, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-M.; Yang, Q.; Guo, L.; Mai, H.-Q.; Mo, H.-Y.; Cao, K.-J.; Qian, C.-N.; Zhao, C.; Xiang, Y.-Q.; Zhang, X.-P.; et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur. J. Cancer 2017, 75, 14–23. [Google Scholar] [CrossRef]
- Cash, H.; Steiner, U.; Heidenreich, A.; Klotz, T.; Albers, P.; Melchior, S.; Martus, P.; Fuller, F.; Magheli, A.; Hinz, S.; et al. Intermittent vs continuous docetaxel therapy in patients with metastatic castration-resistant prostate cancer-a phase III study (PRINCE). BJU Int. 2018, 122, 774–782. [Google Scholar] [CrossRef]
- Chamie, K.; Donin, N.M.; Klöpfer, P.; Bevan, P.; Fall, B.; Wilhelm, O.; Störkel, S.; Said, J.; Gambla, M.; Hawkins, R.E.; et al. Adjuvant Weekly Girentuximab Following Nephrectomy for High-Risk Renal Cell Carcinoma. JAMA Oncol. 2017, 3, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Charton, E.; Bachet, J.; Hammel, P.; Desramé, J.; Chibaudel, B.; Cohen, R.; Debourdeau, P.; Dauba, J.; Lecomte, T.; Seitz, J.; et al. Impact on health-related quality of life deterioration-free survival of a first-line therapy combining nab-paclitaxel plus either gemcitabine or simplified leucovorin and fluorouracil for patients with metastatic pancreatic cancer: Results of the randomized phase II AFUGEM GERCOR clinical trial. Cancer Med. 2019, 8, 5079–5088. [Google Scholar] [CrossRef]
- Chawla, S.P.; Papai, Z.; Mukhametshina, G.; Sankhala, K.; Vasylyev, L.; Fedenko, A.; Khamly, K.; Ganjoo, K.; Nagarkar, R.; Wieland, S.; et al. First-Line Aldoxorubicin vs Doxorubicin in Metastatic or Locally Advanced Unresectable Soft-Tissue Sarcoma: A Phase 2b Randomized Clinical Trial. JAMA Oncol. 2015, 1, 1272. [Google Scholar] [CrossRef]
- Chekerov, R.; Hilpert, F.; Mahner, S.; El-Balat, A.; Harter, P.; De Gregorio, N.; Fridrich, C.; Markmann, S.; Potenberg, J.; Lorenz, R.; et al. Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 1247–1258. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Q.; Huang, M.; Wang, B.; Zhang, J.; Wang, T.; Ming, Y.; Zhou, X.; Jia, Q.; Huan, Y.; et al. A randomized Phase III trial of neoadjuvant recombinant human endostatin, docetaxel and epirubicin as first-line therapy for patients with breast cancer (CBCRT01). Int. J. Cancer 2017, 142, 2130–2138. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Q.; Li, D.; Zhang, J.; Wang, T.; Yu, M.; Zhou, X.; Huan, Y.; Wang, J.; Wang, L. Neoadjuvant rh-endostatin, docetaxel and epirubicin for breast cancer: Efficacy and safety in a prospective, randomized, phase II study. BMC Cancer 2013, 13, 248. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.-S.; Chen, X.-Z.; Hu, G.-Q.; Cheng, Z.-B.; Sun, Y.; Li, W.-X.; Chen, Y.-Y.; Xie, F.-Y.; Liang, S.-B.; et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012, 13, 163–171. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, J.; Zhu, Z.; Zhao, W.; Zhou, J.; Wu, C.; Tang, H.; Fan, M.; Li, L.; Lin, Q.; et al. Comparing Paclitaxel Plus Fluorouracil versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: A Randomized, Multicenter, Phase III Clinical Trial. J. Clin. Oncol. 2019, 37, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Protheroe, A.; Rodríguez-Antolín, A.; Facchini, G.; Suttman, H.; Matsubara, N.; Ye, Z.; Keam, B.; Damião, R.; Li, T.; et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): An international, randomised phase 3 trial. Lancet Oncol. 2018, 19, 194–206. [Google Scholar] [CrossRef]
- Chitapanarux, I.; Tharavichitkul, E.; Kamnerdsupaphon, P.; Pukanhapan, N.; Vongtama, R. Randomized phase III trial of concurrent chemoradiotherapy vs accelerated hyperfractionation radiotherapy in locally advanced head and neck cancer. J. Radiat. Res. 2013, 54, 1110–1117. [Google Scholar] [CrossRef]
- Ciruelos, E.; Apellániz-Ruiz, M.; Cantos, B.; Martinez-Jáñez, N.; Bueno-Muiño, C.; Echarri, M.; Enrech, S.; Guerra, J.; Manso, L.; Pascual, T.; et al. A Pilot, Phase II, Randomized, Open-Label Clinical Trial Comparing the Neurotoxicity of Three Dose Regimens of Nab-Paclitaxel to That of Solvent-Based Paclitaxel as the First-Line Treatment for Patients with Human Epidermal Growth Factor Receptor Type 2-Negative Metastatic Breast Cancer. Oncologist 2019, 24, e1024–e1033. [Google Scholar] [CrossRef]
- Clamp, A.R.; James, E.; McNeish, I.; Dean, A.; Kim, J.-W.; O’Donnell, D.M.; Hook, J.; Coyle, C.; Blagden, S.; Brenton, J.D.; et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): Primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 2019, 394, 2084–2095. [Google Scholar] [CrossRef]
- Clarke, N.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Fléchon, A.; Redfern, C.; et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 975–986. [Google Scholar] [CrossRef]
- Coens, C.; Suciu, S.; Sileni, V.C.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, A.P.; et al. Health-related quality of life with adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): Secondary outcomes of a multinational, randomised, double-blind, phase 3 trial. Lancet Oncol. 2017, 18, 393–403. [Google Scholar] [CrossRef]
- Cognetti, F.; Bagnato, A.; Colombo, N.; Savarese, A.; Scambia, G.; Sehouli, J.; Wimberger, P.; Sorio, R.; Harter, P.; Mari, E.; et al. A Phase II, randomized, double-blind study of zibotentan (ZD4054) in combination with carboplatin/paclitaxel versus placebo in combination with carboplatin/paclitaxel in patients with advanced ovarian cancer sensitive to platinum-based chemotherapy (AGO-OVAR 2.14). Gynecol. Oncol. 2013, 130, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; Karrison, T.G.; Kocherginsky, M.; Mueller, J.; Egan, R.; Huang, C.H.; Brockstein, B.E.; Agulnik, M.B.; Mittal, B.B.; Yunus, F.; et al. Phase III Randomized Trial of Induction Chemotherapy in Patients with N2 or N3 Locally Advanced Head and Neck Cancer. J. Clin. Oncol. 2014, 32, 2735–2743. [Google Scholar] [CrossRef]
- Cohen, S.J.; Zalupski, M.M.; Conkling, P.; Nugent, F.; Manuel, M.; Modiano, M.; Pascual, R.; Lee, F.C.; Wong, L.; Hersh, E. A Phase 2 Randomized, Double-Blind, Multicenter Trial of Imexon Plus Gemcitabine versus Gemcitabine Plus Placebo in Patients with Metastatic Chemotherapy-Naïve Pancreatic Adenocarcinoma. Am. J. Clin. Oncol. 2018, 41, 230–235. [Google Scholar] [CrossRef]
- Coleman, R.; Finkelstein, D.M.; Barrios, C.; Martin, M.; Iwata, H.; Hegg, R.; Glaspy, J.; Periañez, A.M.; Tonkin, K.; Deleu, I.; et al. Adjuvant denosumab in early breast cancer (D-CARE): An international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 21, 60–72. [Google Scholar] [CrossRef]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.-G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and paclitaxel–carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef]
- Coleman, R.L.; Moon, J.; Sood, A.K.; Hu, W.; Delmore, J.E.; Bonebrake, A.J.; Anderson, G.L.; Chambers, S.K.; Markman, M. Randomised phase II study of docetaxel plus vandetanib versus docetaxel followed by vandetanib in patients with persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: SWOG S0904. Eur. J. Cancer 2014, 50, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, I.J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Colombo, N.; Zaccarelli, E.; Baldoni, A.; Frezzini, S.; Scambia, G.; Palluzzi, E.; Tognon, G.; Lissoni, A.A.; Rubino, D.; Ferrero, A.; et al. Multicenter, randomised, open-label, non-comparative phase 2 trial on the efficacy and safety of the combination of bevacizumab and trabectedin with or without carboplatin in women with partially platinum-sensitive recurrent ovarian cancer. Br. J. Cancer 2019, 121, 744–750. [Google Scholar] [CrossRef]
- Corn, P.G.; Heath, I.E.; Zurita, A.; Ramesh, N.; Xiao, L.; Sei, E.; Li-Ning-Tapia, E.; Tu, S.-M.; Subudhi, S.K.; Wang, J.; et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: A randomised, open-label, phase 1–2 trial. Lancet Oncol. 2019, 20, 1432–1443. [Google Scholar] [CrossRef]
- Cortés, J.; Dieras, V.; Ro, J.; Barriere, J.; Bachelot, T.; Hurvitz, S.; Le Rhun, E.; Espié, M.; Kim, S.-B.; Schneeweiss, A.; et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): A randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Cote, G.M.; Choy, E.; Chen, T.; Marino-Enriquez, A.; Morgan, J.; Merriam, P.; Thornton, K.; Wagner, A.J.; Nathenson, M.J.; Demetri, G.; et al. A phase II multi-strata study of lurbinectedin as a single agent or in combination with conventional chemotherapy in metastatic and/or unresectable sarcomas. Eur. J. Cancer 2020, 126, 21–32. [Google Scholar] [CrossRef]
- Cremolini, C.; Antoniotti, C.; Rossini, D.; Lonardi, S.; Loupakis, F.; Pietrantonio, F.; Bordonaro, R.; Latiano, T.P.; Tamburini, E.; Santini, D.; et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 497–507. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Hamid, O.A.; Tarhini, A.; Schadendorf, D.; Chmielowski, B.; Collichio, F.A.; Pavlick, A.C.; Lewis, K.D.; Weil, S.C.; Heyburn, J.; et al. A phase 2 study of ontuxizumab, a monoclonal antibody targeting endosialin, in metastatic melanoma. Investig. New Drugs 2017, 36, 103–113. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Da Costa, S.C.S.; Bonadio, R.C.; Gabrielli, F.C.G.; Aranha, A.S.; Dias Genta, M.L.N.; Miranda, V.C.; De Freitas, D.; Filho, E.A.; Ferreira, P.A.; Machado, K.K.; et al. Neoadjuvant Chemotherapy with Cisplatin and Gemcitabine Followed by Chemoradiation versus Chemoradiation for Locally Advanced Cervical Cancer: A Randomized Phase II Trial. J. Clin. Oncol. 2019, 37, 3124–3131. [Google Scholar] [CrossRef]
- Daud, A.; Kluger, H.M.; Kurzrock, R.; Schimmoller, F.; Weitzman, A.L.; Samuel, T.A.; Moussa, A.H.; Gordon, M.S.; Shapiro, G.I. Phase II randomised discontinuation trial of the MET/VEGF receptor inhibitor cabozantinib in metastatic melanoma. Br. J. Cancer 2017, 116, 432–440. [Google Scholar] [CrossRef]
- Davis, L.E.; Bolejack, V.; Ryan, C.W.; Ganjoo, K.N.; Loggers, E.T.; Chawla, S.; Agulnik, M.; Livingston, M.B.; Reed, D.; Keedy, V.; et al. Randomized Double-Blind Phase II Study of Regorafenib in Patients with Metastatic Osteosarcoma. J. Clin. Oncol. 2019, 37, 1424–1431. [Google Scholar] [CrossRef]
- De Placido, S.; Gallo, C.; De Laurentiis, M.; Bisagni, G.; Arpino, G.; Sarobba, M.G.; Riccardi, F.; Russo, A.; Del Mastro, L.; Cogoni, A.A.; et al. Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 474–485. [Google Scholar] [CrossRef]
- Decker, T.; Marschner, N.; Muendlein, A.; Welt, A.; Hagen, V.; Rauh, J.; Schröder, H.; Jaehnig, P.; Potthoff, K.; Lerchenmüller, C. VicTORia: A randomised phase II study to compare vinorelbine in combination with the mTOR inhibitor everolimus versus vinorelbine monotherapy for second-line chemotherapy in advanced HER2-negative breast cancer. Breast Cancer Res. Treat. 2019, 176, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.M.; Hitt, R.; Sebastian, P.; Carracedo, C.; Lokanatha, D.; Bourhis, J.; Temam, S.; Cupissol, D.; De Raucourt, D.; Maroudias, N.; et al. Effects of lapatinib monotherapy: Results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neck. Br. J. Cancer 2011, 105, 618–627. [Google Scholar] [CrossRef]
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lønning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.-A.; Kalev, D.; Egle, D.; et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 87–100, Erratum in 2018, 19, E137. [Google Scholar] [CrossRef]
- Diéras, V.; Bonnefoi, H.; Alba, E.; Awada, A.; Coudert, B.; Pivot, X.; Gligorov, J.; Jager, A.; Zambelli, S.; Lindeman, G.J.; et al. Iniparib administered weekly or twice-weekly in combination with gemcitabine/carboplatin in patients with metastatic triple-negative breast cancer: A phase II randomized open-label study with pharmacokinetics. Breast Cancer Res. Treat. 2019, 177, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Han, H.S.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.-P.; Puhalla, S.L.; Bondarenko, I.; Campone, M.; Jakobsen, E.H.; et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1269–1282. [Google Scholar] [CrossRef]
- Dittrich, C.; Pápai-Székely, Z.; Viñolas, N.; Sederholm, C.; Hartmann, J.T.; Behringer, D.; Kazeem, G.; Desaiah, D.; Leschinger, M.I.; Von Pawel, J. A randomised phase II study of pemetrexed versus pemetrexed+erlotinib as second-line treatment for locally advanced or metastatic non-squamous non-small cell lung cancer. Eur. J. Cancer 2014, 50, 1571–1580. [Google Scholar] [CrossRef]
- Dong, L.; Han, Z.-F.; Feng, Z.-H.; Jia, Z.-Y. Comparison of pemetrexed and docetaxel as salvage chemotherapy for the treatment for nonsmall-cell lung cancer after the failure of epidermal growth factor receptor-tyrosine kinase inhibitors. J. Int. Med. Res. 2014, 42, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Dorff, T.B.; Quinn, D.I.; Pinski, J.K.; Goldkorn, A.; Sadeghi, S.; Tsao-Wei, D.; Groshen, S.; Kuhn, P.; Gross, M.E. Randomized Phase II Trial of Abiraterone Alone or with Dasatinib in Men with Metastatic Castration-resistant Prostate Cancer (mCRPC). Clin. Genitourin. Cancer 2019, 17, 241–247.e1. [Google Scholar] [CrossRef]
- Dreno, B.; Thompson, J.; Smithers, B.M.; Santinami, M.; Jouary, T.; Gutzmer, R.; Levchenko, E.; Rutkowski, P.; Grob, J.-J.; Korovin, S.; et al. MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 916–929. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Vallier, A.-L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Harnett, A.N.; Ah-See, M.-L.; Simcock, R.; Rea, D.; et al. 6 vs. 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019, 393, 2599–2612. [Google Scholar] [CrossRef]
- Eberst, G.; Anota, A.; Scherpereel, A.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Léna, H.; et al. Health-Related Quality of Life Impact from Adding Bevacizumab to Cisplatin-Pemetrexed in Malignant Pleural Mesothelioma in the MAPS IFCT-GFPC-0701 Phase III Trial. Clin. Cancer Res. 2019, 25, 5759–5765. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Sileni, V.C.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Sileni, V.C.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: Long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur. J. Cancer 2019, 119, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eisen, T.; Trefzer, U.; Hamilton, A.; Hersey, P.; Millward, M.; Knight, R.D.; Jungnelius, J.U.; Glaspy, J. Results of a multicenter, randomized, double-blind phase 2/3 study of lenalidomide in the treatment of pretreated relapsed or refractory metastatic malignant melanoma. Cancer 2009, 116, 146–154. [Google Scholar] [CrossRef]
- Eisenberger, M.; Hardy-Bessard, A.-C.; Kim, C.S.; Géczi, L.; Ford, D.; Mourey, L.; Carles, J.; Parente, P.; Font, A.; Kacso, G.; et al. Phase III Study Comparing a Reduced Dose of Cabazitaxel (20 mg/m2) and the Currently Approved Dose (25 mg/m2) in Postdocetaxel Patients with Metastatic Castration-Resistant Prostate Cancer—PROSELICA. J. Clin. Oncol. 2017, 35, 3198–3206. [Google Scholar] [CrossRef]
- Emens, A.L.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Costantini, B.; Margariti, P.A.; Alletti, S.G.; Cosentino, F.; et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer 2016, 59, 22–33. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E., Jr.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.-F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy with or without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and with IHC 1+ or 2+. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Feng, Y.-R.; Zhu, Y.; Liu, L.-Y.; Wang, W.-H.; Wang, S.-L.; Song, Y.-W.; Wang, X.; Tang, Y.; Liu, Y.-P.; Ren, H.; et al. Interim analysis of postoperative chemoradiotherapy with capecitabine and oxaliplatin versus capecitabine alone for pathological stage II and III rectal cancer: A randomized multicenter phase III trial. Oncotarget 2016, 7, 25576–25584. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, O.W.; Raab, H.-R.; Lordick, F.; Hartmann, J.T.; Lang, H.; Frilling, A.; Stoehlmacher, J.; Weitz, J.; et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010, 11, 38–47. [Google Scholar] [CrossRef]
- Forster, M.D.; Dillon, M.T.; Kocsis, J.; Remenár, É.; Pajkos, G.; Rolland, F.; Greenberg, J.; Harrington, K.J. Patritumab or placebo, with cetuximab plus platinum therapy in recurrent or metastatic squamous cell carcinoma of the head and neck: A randomised phase II study. Eur. J. Cancer 2019, 123, 36–47. [Google Scholar] [CrossRef]
- Fountzilas, G.; Ciuleanu, T.E.; Bobos, M.; Kalogera-Fountzila, A.; Eleftheraki, A.; Karayannopoulou, G.; Zaramboukas, T.; Nikolaou, A.; Markou, K.; Resiga, L.; et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: A randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann. Oncol. 2012, 23, 427–435. [Google Scholar] [CrossRef]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef]
- Friedlander, M.; Gebski, V.; Gibbs, E.; Davies, L.; Bloomfield, R.; Hilpert, F.; Wenzel, L.B.; Eek, D.; Rodrigues, M.; Clamp, A.; et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): A placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018, 19, 1126–1134. [Google Scholar] [CrossRef]
- Fruscio, R.; Garbi, A.; Parma, G.; Lissoni, A.A.; Garavaglia, D.; Bonazzi, C.M.; Dell’Anna, T.; Mangioni, C.; Milani, R.; Colombo, N. Randomized Phase III Clinical Trial Evaluating Weekly Cisplatin for Advanced Epithelial Ovarian Cancer. J. Natl. Cancer Inst. 2011, 103, 347–351. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ushijima, K.; Nagao, S.; Takei, Y.; Shimada, M.; Takano, M.; Yoshino, K.; Kawano, Y.; Hirashima, Y.; Nagase, S.; et al. A phase II randomized controlled study of pegylated liposomal doxorubicin and carboplatin vs. gemcitabine and carboplatin for platinum-sensitive recurrent ovarian cancer (GOTIC003/intergroup study). Int. J. Clin. Oncol. 2019, 24, 1284–1291. [Google Scholar] [CrossRef]
- Fukuda, M.; Kitazaki, T.; Ogawara, D.; Ichiki, M.; Mukae, H.; Maruyama, R.; Nakagaki, N.; Shimada, M.; Ikeda, T.; Kishimoto, J.; et al. Randomized phase II study of pemetrexed or pemetrexed plus bevacizumab for elderly patients with previously untreated non-squamous non-small cell lung cancer: Results of the Lung Oncology Group in Kyushu (LOGIK1201). Lung Cancer 2019, 132, 1–8. [Google Scholar] [CrossRef]
- Fukuoka, M.; Wu, Y.-L.; Thongprasert, S.; Sunpaweravong, P.; Leong, S.-S.; Sriuranpong, V.; Chao, T.-Y.; Nakagawa, K.; Chu, D.-T.; Saijo, N.; et al. Biomarker Analyses and Final Overall Survival Results from a Phase III, Randomized, Open-Label, First-Line Study of Gefitinib versus Carboplatin/Paclitaxel in Clinically Selected Patients with Advanced Non–Small-Cell Lung Cancer in Asia (IPASS). J. Clin. Oncol. 2011, 29, 2866–2874. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Gamboa-Vignolle, C.; Ferrari-Carballo, T.; Arrieta, Ó.; Mohar, A. Whole-brain irradiation with concomitant daily fixed-dose Temozolomide for brain metastases treatment: A randomised phase II trial. Radiother. Oncol. 2012, 102, 187–191. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.; Esteban, E.; Felip, E.; Speranza, G.; Domine, M.; Hochmair, M.J.; Powell, S.; Cheng, S.Y.-S.; Bischoff, H.G.; et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Garcia Garcia, Y.; de Juan Ferre, A.; Mendiola, C.; Barretina-Ginesta, M.P.; Gaba Garcia, L.; Santaballa Bertran, A.; Bover Barcelo, I.; Gil-Martin, M.; Manzano, A.; Rubio Perez, M.J.; et al. Efficacy and safety results from GEICO 1205, a randomized phase II trial of neoadjuvant chemotherapy with or without bevacizumab for advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer 2019, 29, 1050–1056. [Google Scholar] [CrossRef]
- Garon, E.B.; Siegfried, J.M.; Stabile, L.P.; Young, P.A.; Marquez-Garban, D.C.; Park, D.J.; Patel, R.; Hu, E.H.; Sadeghi, S.; Parikh, R.J.; et al. Randomized phase II study of fulvestrant and erlotinib compared with erlotinib alone in patients with advanced or metastatic non-small cell lung cancer. Lung Cancer 2018, 123, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Sun, Z.; Hasler-Strub, U.; Colleoni, M.; Kennedy, M.; Von Moos, R.; Cortés, J.; Vidal, M.; Hennessy, B.; Walshe, J.; et al. A randomized phase II study evaluating different maintenance schedules of nab-paclitaxel in the first-line treatment of metastatic breast cancer: Final results of the IBCSG 42-12/BIG 2-12 SNAP trial. Ann. Oncol. 2017, 29, 661–668. [Google Scholar] [CrossRef]
- Gerber, D.E.; Socinski, M.A.; Neal, J.W.; Wakelee, H.A.; Shirai, K.; Sequist, L.V.; Rosovsky, R.P.; Lilenbaum, R.C.; Bastos, B.R.; Huang, C.; et al. Randomized phase 2 study of tivantinib plus erlotinib versus single-agent chemotherapy in previously treated KRAS mutant advanced non-small cell lung cancer. Lung Cancer 2018, 117, 44–49. [Google Scholar] [CrossRef]
- Gezelius, E.; Bendahl, P.; de Oliveira, K.G.; Ek, L.; Bergman, B.; Sundberg, J.; Strandberg, K.; Krämer, R.; Belting, M. Low-molecular-weight heparin adherence and effects on survival within a randomised phase III lung cancer trial (RASTEN). Eur. J. Cancer 2019, 118, 82–90. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Vincent, J.; Bengrine, L.; Borg, C.; Jouve, J.L.; Loffroy, R.; Guiu, B.; Blanc, J.; Bertaut, A. Hepatic arterial chemotherapy with raltitrexed and oxaliplatin versus standard chemotherapy in unresectable liver metastases from colorectal cancer after conventional chemotherapy failure (HEARTO): A randomized phase-II study. J. Cancer Res. Clin. Oncol. 2019, 145, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Sanborn, R.E.; Waqar, S.N.; Martinez-Marti, A.; Ponce, S.; Zhen, H.; Kennealey, G.; Erickson-Viitanen, S.; Schaefer, E. A Placebo-Controlled Phase II Study of Ruxolitinib in Combination with Pemetrexed and Cisplatin for First-Line Treatment of Patients with Advanced Nonsquamous Non–Small-Cell Lung Cancer and Systemic Inflammation. Clin. Lung Cancer 2018, 19, e567–e574. [Google Scholar] [CrossRef]
- Gilbert, J.; Lee, J.W.; Argiris, A.; Haigentz, M.; Feldman, L.E.; Jang, M.; Arun, P.; Van Waes, C.; Forastiere, A.A. Phase II 2-arm trial of the proteasome inhibitor, PS-341 (bortezomib) in combination with irinotecan or PS-341 alone followed by the addition of irinotecan at time of progression in patients with locally recurrent or metastatic squamous cell carcinoma of. Head Neck 2012, 35, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.-M.; Bereder, J.-M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.-J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP–PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef]
- Gordon, A.N.; Teneriello, M.; Janicek, M.F.; Hines, J.; Lim, P.C.; Chen, M.D.; Vaccarello, L.; Homesley, H.D.; McMeekin, S.; Burkholder, T.L.; et al. Phase III trial of induction gemcitabine or paclitaxel plus carboplatin followed by paclitaxel consolidation in ovarian cancer. Gynecol. Oncol. 2011, 123, 479–485. [Google Scholar] [CrossRef]
- Gore, M.; Hackshaw, A.; Brady, W.E.; Penson, R.T.; Zaino, R.; McCluggage, W.G.; Ganesan, R.; Wilkinson, N.; Perren, T.; Montes, A.; et al. An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: And experience of conducting a clinical trial in a rare gynecological tumor. Gynecol. Oncol. 2019, 153, 541–548. [Google Scholar] [CrossRef]
- Jin, F.; Gou, X.-X.; Wu, W.-L.; Long, J.-H.; Li, Y.-Y.; Gong, X.-Y.; Chen, G.-Y.; Chen, X.-X.; Liu, L.-N. Induction chronomodulated chemotherapy plus radiotherapy for nasopharyngeal carcinoma: A Phase II prospective randomized study. J. Cancer Res. Ther. 2018, 14, 1613. [Google Scholar] [CrossRef]
- Grávalos, C.; Carrato, A.; Tobeña, M.; Rodriguez-Garrote, M.; Soler, G.; Vieitez, J.M.; Robles, L.; Valladares-Ayerbes, M.; Polo, E.; Limón, M.L.; et al. A Randomized Phase II Study of Axitinib as Maintenance Therapy after First-line Treatment for Metastatic Colorectal Cancer. Clin. Color. Cancer 2018, 17, e323–e329. [Google Scholar] [CrossRef]
- Gregoire, V.; Hamoir, M.; Chen, C.; Kane, M.; Kawecki, A.; Julka, P.K.; Wang, H.-M.; Prasad, S.; D’Cruz, A.K.; Radosevic-Jelic, L.; et al. Gefitinib plus cisplatin and radiotherapy in previously untreated head and neck squamous cell carcinoma: A phase II, randomized, double-blind, placebo-controlled study. Radiother. Oncol. 2011, 100, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, V.; Gaafar, R.M.; Favaretto, A.; Grossi, F.; Jassem, J.; Polychronis, A.; Bidoli, P.; Tiseo, M.; Shah, R.; Taylor, P.; et al. NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2018, 19, 799–811. [Google Scholar] [CrossRef]
- Greimel, E.; Kristensen, G.B.; van der Burg, M.E.; Coronado, P.; Rustin, G.; del Rio, A.S.; Reed, N.S.; Nordal, R.R.; Coens, C.; Vergote, I. Quality of life of advanced ovarian cancer patients in the randomized phase III study comparing primary debulking surgery versus neo-adjuvant chemotherapy. Gynecol. Oncol. 2013, 131, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Groen, H.J.M.; van der Heijden, E.; Klinkenberg, T.J.; Biesma, B.; Aerts, J.; Verhagen, A.; Kloosterziel, C.; Pieterman, R.; Borne, B.V.D.; Smit, H.J.M.; et al. Randomised phase 3 study of adjuvant chemotherapy with or without nadroparin in patients with completely resected non-small-cell lung cancer: The NVALT-8 study. Br. J. Cancer 2019, 121, 372–377. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, S.; Wang, H.-Y.; Guo, Y.; Xiao, W.-W.; Chen, C.-Y.; Zhao, C.; Lu, T.-X.; Han, F. Long-term outcomes of a phase II randomized controlled trial comparing intensity-modulated radiotherapy with or without weekly cisplatin for the treatment of locally recurrent nasopharyngeal carcinoma. Chin. J. Cancer 2016, 35, 20. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, Y.; Kong, E.; Rao, L.; Chen, J.; Wu, Q.; Zhang, T.; Liu, N.; Li, M.; Sun, L. Apatinib combined with chemotherapy or concurrent chemo-brachytherapy in patients with recurrent or advanced cervical cancer. Medicine 2020, 99, e19372. [Google Scholar] [CrossRef]
- Guo, Y.; Ahn, M.-J.; Chan, A.; Wang, C.-H.; Kang, J.-H.; Kim, S.-B.; Bello, M.; Arora, R.; Zhang, Q.; He, X.; et al. Afatinib versus methotrexate as second-line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 3): An open-label, randomised phase III trial. Ann. Oncol. 2019, 30, 1831–1839. [Google Scholar] [CrossRef]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Ohashi, Y.; Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; et al. Health-related quality of life of adjuvant chemotherapy with S-1 versus gemcitabine for resected pancreatic cancer: Results from a randomised phase III trial (JASPAC 01). Eur. J. Cancer 2018, 93, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Reeves, J.A.; Mace, J.R.; Crane, E.J.; Hamid, O.; Stille, J.R.; Flynt, A.; Roberson, S.; Polzer, J.; Arrowsmith, E.R. A Randomized, Open-Label Phase 2 Study of the CXCR4 Inhibitor LY2510924 in Combination with Sunitinib versus Sunitinib Alone in Patients with Metastatic Renal Cell Carcinoma (RCC). Target. Oncol. 2016, 11, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Thompson, D.S.; Bismayer, J.A.; Gian, V.G.; Merritt, W.M.; Whorf, R.C.; Finney, L.H.; Dudley, B.S. Paclitaxel/carboplatin with or without sorafenib in the first-line treatment of patients with stage III/IV epithelial ovarian cancer: A randomized phase II study of the Sarah Cannon Research Institute. Cancer Med. 2014, 4, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Foster, N.R.; Kim, G.P.; Meyers, J.P.; Smyrk, T.C.; McCullough, A.E.; Ames, M.M.; Jaffe, J.P.; Alberts, S.R. A Phase II Randomized Trial of Panitumumab, Erlotinib, and Gemcitabine versus Erlotinib and Gemcitabine in Patients with Untreated, Metastatic Pancreatic Adenocarcinoma: North Central Cancer Treatment Group Trial N064B (Alliance). Oncologist 2019, 24, 589.e160. [Google Scholar] [CrossRef]
- Hamidou, Z.; Chibaudel, B.; Hebbar, M.; De Larauze, M.H.; Andre, T.; Louvet, C.; Brusquant, D.; Garcia-Larnicol, M.-L.; De Gramont, A.; Bonnetain, F. Time to Definitive Health-Related Quality of Life Score Deterioration in Patients with Resectable Metastatic Colorectal Cancer Treated with FOLFOX4 versus Sequential Dose-Dense FOLFOX7 followed by FOLFIRI: The MIROX Randomized Phase III Trial. PLoS ONE 2016, 11, e0157067. [Google Scholar] [CrossRef]
- Hammel, P.; Fabienne, P.; Mineur, L.; Metges, J.-P.; Andre, T.; De La Fouchardiere, C.; Louvet, C.; El Hajbi, F.; Faroux, R.; Guimbaud, R.; et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase IIb trial. Eur. J. Cancer 2020, 124, 91–101. [Google Scholar] [CrossRef]
- Han, B.; Li, K.; Zhao, Y.; Li, B.; Cheng, Y.; Zhou, J.; Lu, Y.; Shi, Y.; Wang, Z.; Jiang, L.; et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: A multicentre, randomised phase II trial (ALTER0302). Br. J. Cancer 2018, 118, 654–661. [Google Scholar] [CrossRef]
- Han, H.S.; Diéras, V.; Robson, M.; Palácová, M.; Marcom, P.K.; Jager, A.; Bondarenko, I.; Citrin, D.; Campone, M.; Telli, M.L.; et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: Randomized phase II study. Ann. Oncol. 2018, 29, 154–161. [Google Scholar] [CrossRef]
- Harari, P.M.; Harris, J.; Kies, M.S.; Myers, J.N.; Jordan, R.C.; Gillison, M.L.; Foote, R.L.; Machtay, M.; Rotman, M.; Khuntia, D.; et al. Postoperative Chemoradiotherapy and Cetuximab for High-Risk Squamous Cell Carcinoma of the Head and Neck: Radiation Therapy Oncology Group RTOG-0234. J. Clin. Oncol. 2014, 32, 2486–2495. [Google Scholar] [CrossRef]
- Harrington, K.; Temam, S.; Mehanna, H.; D’Cruz, A.; Jain, M.; D’Onofrio, I.; Manikhas, G.; Horvath, Z.; Sun, Y.; Dietzsch, S.; et al. Postoperative Adjuvant Lapatinib and Concurrent Chemoradiotherapy Followed by Maintenance Lapatinib Monotherapy in High-Risk Patients with Resected Squamous Cell Carcinoma of the Head and Neck: A Phase III, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Oncol. 2015, 33, 4202–4209. [Google Scholar] [CrossRef]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Kopp, H.-G.; Gruenwald, V.; Piperno-Neumann, S.; Kunitz, A.; Hofheinz, R.; Mueller, L.; Geissler, M.; Horger, M.; Fix, P.; et al. Randomised phase II trial of trofosfamide vs. doxorubicin in elderly patients with untreated metastatic soft-tissue sarcoma. Eur. J. Cancer 2020, 124, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.; Heilbrun, L.; Mannuel, H.; Liu, G.; Lara, P.; Monk, J.P.; Flaig, T.; Zurita, A.; Mack, P.; Vaishampayan, U.; et al. Phase II, Multicenter, Randomized Trial of Docetaxel plus Prednisone with or without Cediranib in Men with Chemotherapy-Naive Metastatic Castrate-Resistant Prostate Cancer. Oncology 2019, 24, 1149. [Google Scholar] [CrossRef] [PubMed]
- Hellerstedt, B.A.; Vogelzang, N.J.; Kluger, H.M.; Yasenchak, C.A.; Aftab, D.T.; Ramies, D.A.; Gordon, M.S.; Lara, P. Results of a Phase II Placebo-controlled Randomized Discontinuation Trial of Cabozantinib in Patients with Non–small-cell Lung Carcinoma. Clin. Lung Cancer 2018, 20, 74–81.e1. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef]
- Holmsten, K.; Jensen, N.V.; Mouritsen, L.S.; Jonsson, E.; Mellnert, C.; Agerbaek, M.; Nilsson, C.; Moe, M.; Carus, A.; Öfverholm, E.; et al. Vinflunine/gemcitabine versus carboplatin/gemcitabine as first-line treatment in cisplatin-ineligible patients with advanced urothelial carcinoma: A randomised phase II trial (VINGEM). Eur. J. Cancer 2019, 127, 173–182. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, O.O.C.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Hu, Z.I.; Bendell, J.C.; Bullock, A.; LoConte, N.K.; Hatoum, H.; Ritch, P.; Hool, H.; Leach, J.W.; Sanchez, J.; Sohal, D.P.S.; et al. A randomized phase II trial of nab-paclitaxel and gemcitabine with tarextumab or placebo in patients with untreated metastatic pancreatic cancer. Cancer Med. 2019, 8, 5148–5157. [Google Scholar] [CrossRef]
- Huang, P.-W.; Lin, C.-Y.; Hsieh, C.-H.; Hsu, C.-L.; Fan, K.-H.; Huang, S.-F.; Liao, C.-T.; Ng, S.-K.; Yen, T.-C.; Chang, J.T.-C.; et al. A phase II randomized trial comparing neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in advanced squamous cell carcinoma of the pharynx or larynx. Biomed. J. 2018, 41, 129–136. [Google Scholar] [CrossRef]
- Huober, J.; Holmes, E.; Baselga, J.; de Azambuja, E.; Untch, M.; Fumagalli, D.; Sarp, S.; Lang, I.; Smith, I.; Boyle, F.; et al. Survival outcomes of the NeoALTTO study (BIG 1–06): Updated results of a randomised multicenter phase III neoadjuvant clinical trial in patients with HER2-positive primary breast cancer. Eur. J. Cancer 2019, 118, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Andre, F.; Jiang, Z.; Shao, Z.; Mano, M.S.; Neciosup, S.P.; Tseng, L.-M.; Zhang, Q.; Shen, K.; Liu, D.; et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015, 16, 816–829. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Jung, K.H.; Huang, C.-S.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; Campone, M.; Boileau, J.-F.; et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Three-Year Outcomes from the Phase III KRISTINE Study. J. Clin. Oncol. 2019, 37, 2206–2216. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Tan, B.R.; Reeves, J.A.; Xiong, H.; Somer, B.; Lenz, H.; Hochster, H.S.; Scappaticci, F.; Palma, J.F.; Price, R.; et al. Phase II Randomized Trial of Sequential or Concurrent FOLFOXIRI-Bevacizumab versus FOLFOX-Bevacizumab for Metastatic Colorectal Cancer (STEAM). Oncology 2019, 24, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; De Phung, B.S.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Kawashima, H.; Ohno, E.; Matsubara, H.; Sasaki, Y.; Achiwa, K.; Kanamori, A.; Sumi, H.; Hirai, T.; Nonogaki, K.; et al. Randomized Phase II Study of Consecutive-Day versus Alternate-Day Treatment with S-1 as Second-Line Chemotherapy in Advanced Pancreatic Cancer. Oncology 2018, 96, 1–7. [Google Scholar] [CrossRef]
- Jameson, M.B.; Gormly, K.; Espinoza, D.; Hague, W.; Asghari, G.; Jeffery, G.M.; Price, T.J.; Karapetis, C.; Arendse, M.; Armstrong, J.; et al. SPAR—A randomised, placebo-controlled phase II trial of simvastatin in addition to standard chemotherapy and radiation in preoperative treatment for rectal cancer: An AGITG clinical trial. BMC Cancer 2019, 19, 1229. [Google Scholar] [CrossRef]
- Janni, W.; Alba, E.; Bachelot, T.; Diab, S.; Gil-Gil, M.; Beck, T.J.; Ryvo, L.; López, R.L.; Tsai, M.; Esteva, F.; et al. First-line ribociclib plus letrozole in postmenopausal women with HR+, HER2− advanced breast cancer: Tumor response and pain reduction in the phase 3 MONALEESA-2 trial. Breast Cancer Res. Treat. 2018, 169, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, W.; Hu, X.; Zhang, Q.; Sun, T.; Cui, S.; Wang, S.; Ouyang, Q.; Yin, Y.; Geng, C.; et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 806–815. [Google Scholar] [CrossRef]
- Jimeno, A.; Posner, M.R.; Wirth, L.J.; Saba, N.F.; Cohen, R.B.; Popa, E.C.; Argiris, A.; Grossmann, K.F.; Sukari, A.; Wilson, D.; et al. A phase 2 study of dalantercept, an activin receptor-like kinase-1 ligand trap, in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Cancer 2016, 122, 3641–3649. [Google Scholar] [CrossRef]
- Jimeno, A.; Shirai, K.; Choi, M.; Laskin, J.; Kochenderfer, M.; Spira, A.; Cline-Burkhardt, V.; Winquist, E.; Hausman, D.; Walker, L.; et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann. Oncol. 2014, 26, 556–561. [Google Scholar] [CrossRef]
- Jones, R.H.; Casbard, A.; Carucci, M.; Cox, C.; Butler, R.; Alchami, F.; Madden, T.-A.; Bale, C.; Bezecny, P.; Joffe, J.; et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020, 21, 345–357. [Google Scholar] [CrossRef]
- Jones, R.L.; Chawla, S.P.; Attia, S.; Schöffski, P.; Gelderblom, H.; Chmielowski, B.; Le Cesne, A.; Van Tine, B.A.; Trent, J.C.; Patel, S.; et al. A phase 1 and randomized controlled phase 2 trial of the safety and efficacy of the combination of gemcitabine and docetaxel with ontuxizumab (MORAb-004) in metastatic soft-tissue sarcomas. Cancer 2019, 125, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Ratain, M.J.; O’Dwyer, P.J.; Siu, L.L.; Jassem, J.; Medioni, J.; DeJonge, M.; Rudin, C.; Sawyer, M.; Khayat, D.; et al. Phase II randomised discontinuation trial of brivanib in patients with advanced solid tumours. Eur. J. Cancer 2019, 120, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Jonker, D.J.; Tang, P.A.; Kennecke, H.; Welch, S.A.; Cripps, M.C.; Asmis, T.; Chalchal, H.; Tomiak, A.; Lim, H.; Ko, Y.-J.; et al. A Randomized Phase II Study of FOLFOX6/Bevacizumab with or without Pelareorep in Patients with Metastatic Colorectal Cancer: IND.210, a Canadian Cancer Trials Group Trial. Clin. Color. Cancer 2018, 17, 231–239.e7. [Google Scholar] [CrossRef] [PubMed]
- Judson, I.; Morden, J.P.; Kilburn, L.; Leahy, M.; Benson, C.; Bhadri, V.; Campbell-Hewson, Q.; Cubedo, R.; Dangoor, A.; Fox, L.; et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): A double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1023–1034. [Google Scholar] [CrossRef]
- Kaira, K.; Imai, H.; Souma, R.; Sakurai, R.; Miura, Y.; Sunaga, N.; Kasahara, N.; Tsukagoshi, Y.; Koga, Y.; Kitahara, S.; et al. An Exploratory Randomized Phase II Trial Comparing CDDP Plus S-1 with Bevacizumab and CDDP Plus Pemetrexed with Bevacizumab Against Patients with Advanced Non-squamous Non-small Cell Lung Cancer. Anticancer Res. 2019, 39, 2483–2491. [Google Scholar] [CrossRef]
- Karasic, T.B.; O’Hara, M.H.; Loaiza-Bonilla, A.; Reiss, K.A.; Teitelbaum, U.R.; Borazanci, E.; De Jesus-Acosta, A.; Redlinger, C.; Burrell, J.A.; Laheru, D.A.; et al. Effect of Gemcitabine and nab-Paclitaxel with or without Hydroxychloroquine on Patients with Advanced Pancreatic Cancer. JAMA Oncol. 2019, 5, 993–998. [Google Scholar] [CrossRef]
- Karoui, M.; Rullier, A.; Piessen, G.; Legoux, J.L.; Barbier, E.; De Chaisemartin, C.; Lecaille, C.; Bouche, O.; Ammarguellat, H.; Brunetti, F.; et al. Perioperative FOLFOX 4 versus FOLFOX 4 Plus Cetuximab versus Immediate Surgery for High-Risk Stage II and III Colon Cancers. Ann. Surg. 2020, 271, 637–645. [Google Scholar] [CrossRef]
- Kashefi, S.; Omranipour, R.; Mahmoodzadeh, H.; Ahmadi, H.; Alikhassi, A.; Hosseini, M.; Cuzzocrea, S.; Rehm, B.H.A.; Matsuo, H.; Mirshafiey, A. A randomized, controlled, phaseIIclinical trial of β-D-mannuronic acid (M2000) in pre-surgical breast cancer patients at early stage (T1-T2). Clin. Exp. Pharmacol. Physiol. 2019, 46, 527–532. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.-Y.; Chin, K.; Kadowaki, S.; Ahn, M.-J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Kaye, S.B.; Lubinski, J.; Matulonis, U.; Ang, J.E.; Gourley, C.; Karlan, B.Y.; Amnon, A.; Bell-McGuinn, K.M.; Chen, L.-M.; Friedlander, M.; et al. Phase II, Open-Label, Randomized, Multicenter Study Comparing the Efficacy and Safety of Olaparib, a Poly (ADP-Ribose) Polymerase Inhibitor, and Pegylated Liposomal Doxorubicin in Patients with BRCA1 or BRCA2 Mutations and Recurrent Ovarian Cancer. J. Clin. Oncol. 2012, 30, 372–379. [Google Scholar] [CrossRef]
- Kaye, S.B.; Poole, C.J.; Dańska-Bidzińska, A.; Gianni, L.; Del Conte, G.; Gorbunova, V.; Novikova, E.; Strauss, A.; Moczko, M.; McNally, V.A.; et al. A randomized phase II study evaluating the combination of carboplatin-based chemotherapy with pertuzumab versus carboplatin-based therapy alone in patients with relapsed, platinum-sensitive ovarian cancer. Ann. Oncol. 2013, 24, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.D.; Tan, W.S.; Porta, N.; Mostafid, H.; Huddart, R.; Protheroe, A.; Bogle, R.; Blazeby, J.; Palmer, A.; Cresswell, J.; et al. BOXIT—A Randomised Phase III Placebo-controlled Trial Evaluating the Addition of Celecoxib to Standard Treatment of Transitional Cell Carcinoma of the Bladder (CRUK/07/004). Eur. Urol. 2018, 75, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.G.; Mor, G.; Husband, A.; O’Malley, D.M.; Baker, L.; Azodi, M.; Schwartz, P.E.; Rutherford, T.J. Phase II Evaluation of Phenoxodiol in Combination with Cisplatin or Paclitaxel in Women with Platinum/Taxane-Refractory/Resistant Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancers. Int. J. Gynecol. Cancer 2011, 21, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.; Shepherd, F.; Krivoshik, A.; Jie, F.; Horn, L. A phase III, randomized, open-label study of ASP8273 versus erlotinib or gefitinib in patients with advanced stage IIIB/IV non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1127–1133. [Google Scholar] [CrossRef]
- Kerr, R.S.; Love, S.; Segelov, E.; Johnstone, E.; Falcon, B.; Hewett, P.; Weaver, A.; Church, D.; Scudder, C.; Pearson, S.; et al. Adjuvant capecitabine plus bevacizumab versus capecitabine alone in patients with colorectal cancer (QUASAR 2): An open-label, randomised phase 3 trial. Lancet Oncol. 2016, 17, 1543–1557. [Google Scholar] [CrossRef]
- Khalaf, D.J.; Annala, M.; Taavitsainen, S.; Finch, D.L.; Oja, C.; Vergidis, J.; Zulfiqar, M.; Sunderland, K.; Azad, A.A.; Kollmannsberger, C.K.; et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: A multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019, 20, 1730–1739. [Google Scholar] [CrossRef]
- Khalaf, D.J.; Sunderland, K.; Eigl, B.; Kollmannsberger, C.K.; Ivanov, N.; Finch, D.L.; Oja, C.; Vergidis, J.; Zulfiqar, M.; Gleave, M.; et al. Health-related Quality of Life for Abiraterone Plus Prednisone versus Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer: Results from a Phase II Randomized Trial. Eur. Urol. 2018, 75, 940–947. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kim, H.-G.; Kim, J.-H.; Park, K.; Kim, H.-K.; Jang, J.S.; Kim, B.-S.; Kang, J.-H.; Lee, K.H.; Kim, S.-W.; et al. Randomized Phase III Trial of Irinotecan Plus Cisplatin versus Etoposide Plus Cisplatin in Chemotherapy-Naïve Korean Patients with Extensive-Disease Small Cell Lung Cancer. Cancer Res. Treat. 2019, 51, 119–127. [Google Scholar] [CrossRef]
- Kim, H.-A.; Lee, J.W.; Nam, S.J.; Park, B.-W.; Im, S.-A.; Lee, E.S.; Jung, Y.S.; Yoon, J.H.; Kang, S.S.; Lee, S.-J.; et al. Adding Ovarian Suppression to Tamoxifen for Premenopausal Breast Cancer: A Randomized Phase III Trial. J. Clin. Oncol. 2020, 38, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Jang, J.S.; Sun, J.-M.; Ahn, M.-J.; Kim, D.-W.; Jung, I.; Lee, K.H.; Kim, J.-H.; Lee, D.H.; Kim, S.-W.; et al. A randomized, phase II study of gefitinib alone versus nimotuzumab plus gefitinib after platinum-based chemotherapy in advanced non-small cell lung cancer (KCSG LU12-01). Oncotarget 2016, 8, 15943–15951. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Im, S.-A.; Jung, K.H.; Ro, J.; Sohn, J.; Kim, J.H.; Park, Y.H.; Kim, T.-Y.; Kim, S.-B.; Lee, K.S.; et al. Fulvestrant plus goserelin versus anastrozole plus goserelin versus goserelin alone for hormone receptor-positive, HER2-negative tamoxifen-pretreated premenopausal women with recurrent or metastatic breast cancer (KCSG BR10-04): A multicentre, open-label, three-arm, randomised phase II trial (FLAG study). Eur. J. Cancer 2018, 103, 127–136. [Google Scholar] [CrossRef]
- Kim, S.Y.; Joo, J.; Kim, T.W.; Hong, Y.S.; Kim, J.E.; Hwang, I.G.; Kim, B.G.; Lee, K.-W.; Kim, J.-W.; Oh, H.-S.; et al. A Randomized Phase 2 Trial of Consolidation Chemotherapy after Preoperative Chemoradiation Therapy versus Chemoradiation Therapy Alone for Locally Advanced Rectal Cancer: KCSG CO 14-03. Int. J. Radiat. Oncol. 2018, 101, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Klinghammer, K.; Gauler, T.; Dietz, A.; Grünwald, V.; Stöhlmacher, J.; Knipping, S.; Schroeder, M.; Guntinas-Lichius, O.; Frickhofen, N.; Lindeman, H.-W.; et al. Cetuximab, fluorouracil and cisplatin with or without docetaxel for patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CeFCiD): An open-label phase II randomised trial (AIO/IAG-KHT trial 1108). Eur. J. Cancer 2019, 122, 53–60. [Google Scholar] [CrossRef]
- Kondo, M.; Morimoto, M.; Kobayashi, S.; Ohkawa, S.; Hidaka, H.; Nakazawa, T.; Aikata, H.; Hatanaka, T.; Takizawa, D.; Matsunaga, K.; et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer 2019, 19, 954. [Google Scholar] [CrossRef]
- Kong, X.-Y.; Lu, J.-X.; Yu, X.-W.; Zhang, J.; Xu, Q.-L.; Zhang, R.-J.; Mi, J.-L.; Liao, S.-F.; Fan, J.-F.; Qin, X.-L.; et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as a first-line concurrent chemotherapy regimen in nasopharyngeal carcinoma: A prospective, multi-institution, randomized controlled phase II study. Cancer Chemother. Pharmacol. 2019, 84, 155–161. [Google Scholar] [CrossRef]
- Konstantinopoulos, A.P.; Cheng, S.-C.; Hendrickson, A.E.W.; Penson, R.T.; Schumer, S.T.; Doyle, L.A.; Lee, E.K.; Kohn, E.C.; Duska, L.R.; Crispens, A.M.; et al. Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 957–968. [Google Scholar] [CrossRef]
- Koshkin, V.S.; Mir, M.C.; Barata, P.; Gul, A.; Gupta, R.; Stephenson, A.J.; Kaouk, J.; Berglund, R.; Magi-Galluzzi, C.; Klein, E.A.; et al. Randomized phase II trial of neoadjuvant everolimus in patients with high-risk localized prostate cancer. Investig. New Drugs 2019, 37, 559–566. [Google Scholar] [CrossRef]
- Kristeleit, R.; Davidenko, I.; Shirinkin, V.; El-Khouly, F.; Bondarenko, I.; Goodheart, M.J.; Gorbunova, V.; Penning, C.A.; Shi, J.G.; Liu, X.; et al. A randomised, open-label, phase 2 study of the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as therapy for biochemically recurrent (CA-125 relapse)–only epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer. Gynecol. Oncol. 2017, 146, 484–490. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Mohanti, B.K.; Thakar, A.; Shukla, N.K.; Thulkar, S.P.; Sikka, K.; Bhasker, S.; Singh, C.A.; Vishnubhatla, S. A phase 2 randomized study to compare short course palliative radiotherapy with short course concurrent palliative chemotherapy plus radiotherapy in advanced and unresectable head and neck cancer. Radiother. Oncol. 2015, 117, 145–151. [Google Scholar] [CrossRef]
- Aoyama, T.; Kusano, M.; Okabayashi, K.; Hirata, K.; Tsuji, Y.; Nakamori, S.; Asahara, T.; Ohashi, Y.; Yoshikawa, T.; Sakamoto, J.; et al. A randomized phase III study of hepatic arterial infusion chemotherapy with 5-fluorouracil and subsequent systemic chemotherapy versus systemic chemotherapy alone for colorectal cancer patients with curatively resected liver metastases (Japanese Foundation for Multidisciplinary Treatment of Cancer 32). J. Cancer Res. Ther. 2018, 14, 761–S766. [Google Scholar] [CrossRef]
- Kuwayama, T.; Nakamura, S.; Hayashi, N.; Takano, T.; Tsugawa, K.; Sato, T.; Kitani, A.; Okuyama, H.; Yamauchi, H. Randomized Multicenter Phase II Trial of Neoadjuvant Therapy Comparing Weekly Nab-paclitaxel Followed by FEC with Docetaxel Followed by FEC in HER2− Early-stage Breast Cancer. Clin. Breast Cancer 2018, 18, 474–480. [Google Scholar] [CrossRef]
- Kwakman, J.J.; Van Werkhoven, E.; Simkens, L.H.; Van Rooijen, J.M.; Van De Wouw, Y.A.; Tije, A.J.T.; Creemers, G.-J.M.; Hendriks, M.P.; Los, M.; Van Alphen, R.J.; et al. Updated Survival Analysis of the Randomized Phase III Trial of S-1 versus Capecitabine in the First-Line Treatment of Metastatic Colorectal Cancer by the Dutch Colorectal Cancer Group. Clin. Color. Cancer 2019, 18, e229–e230. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, A.V.; Patnaik, A.; Powell, S.F.; Gentzler, R.; Martins, R.G.; Stevenson, J.P.; Jalal, I.S.; et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, A.J.; Embleton-Thirsk, A.; Raja, F.; Perren, T.; Jayson, G.; Rustin, G.J.S.; Kaye, S.B.; Hirte, H.; Eisenhauer, E.; Vaughan, M.; et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 387, 1066–1074. [Google Scholar] [CrossRef]
- Ledermann, A.J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: An updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016, 17, 1579–1589. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sun, J.-M.; Oh, D.R.; Lim, S.H.; Goo, J.; Lee, S.-H.; Kim, S.-B.; Park, K.U.; Kim, H.-K.; Hong, D.S.; et al. Comparison of weekly versus triweekly cisplatin delivered concurrently with radiation therapy in patients with locally advanced nasopharyngeal cancer: A multicenter randomized phase II trial (KCSG-HN10-02). Radiother. Oncol. 2015, 118, 244–250. [Google Scholar] [CrossRef]
- Lee, O.; Sullivan, M.E.; Xu, Y.; Rodgers, C.; Muzzio, M.; Helenowski, I.; Shidfar, A.; Zeng, Z.; Singhal, H.; Jovanovic, B.; et al. Selective Progesterone Receptor Modulators in Early-Stage Breast Cancer: A Randomized, Placebo-Controlled Phase II Window-of-Opportunity Trial Using Telapristone Acetate. Clin. Cancer Res. 2019, 26, 25–34. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.; Kim, M.; Lee, J.; Park, Y.H.; Im, Y.-H.; Park, S.H. Capecitabine in combination with either cisplatin or weekly paclitaxel as a first-line treatment for metastatic esophageal squamous cell carcinoma: A randomized phase II study. BMC Cancer 2015, 15, 693. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, Y.-M.; Cho, C.H.; Kim, Y.T.; Kim, S.M.; Hur, S.Y.; Kim, J.-H.; Kim, B.-G.; Kim, S.-C.; Ryu, H.-S.; et al. An Open-Label, Randomized, Parallel, Phase II Trial to Evaluate the Efficacy and Safety of a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel as First-Line Treatment for Ovarian Cancer: A Korean Gynecologic Oncology Group Study (KGOG-3021). Cancer Res. Treat. 2018, 50, 195–203. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.-Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Argiles, G.; Yoshino, T.; Lonardi, S.; Falcone, A.; Limón, M.L.; Sobrero, A.; Hastedt, C.; Peil, B.; Voss, F.; et al. Health-related Quality of Life in the Phase III LUME-Colon 1 Study: Comparison and Interpretation of Results from EORTC QLQ-C30 Analyses. Clin. Color. Cancer 2019, 18, 269–279.e5. [Google Scholar] [CrossRef]
- Li, J.; Xu, R.; Qin, S.; Liu, T.; Pan, H.; Xu, J.; Bi, F.; Lim, R.; Zhang, S.; Ba, Y.; et al. Aflibercept plus FOLFIRI in Asian patients with pretreated metastatic colorectal cancer: A randomized Phase III study. Future Oncol. 2018, 14, 2031–2044. [Google Scholar] [CrossRef]
- Li, L.; Jiang, L.; Wang, Y.; Zhao, Y.; Zhang, X.-J.; Wu, G.; Zhou, X.; Sun, J.; Bai, J.; Ren, B.; et al. Combination of Metformin and Gefitinib as First-Line Therapy for Nondiabetic Advanced NSCLC Patients with EGFR Mutations: A Randomized, Double-Blind Phase II Trial. Clin. Cancer Res. 2019, 25, 6967–6975. [Google Scholar] [CrossRef]
- Liu, J.F.; Barry, W.T.; Birrer, M.; Lee, J.-M.; Buckanovich, R.J.; Fleming, G.F.; Rimel, B.; Buss, M.K.; Nattam, S.; Hurteau, J.; et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: A randomised phase 2 study. Lancet Oncol. 2014, 15, 1207–1214. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Ruiz, A.; Antón, A.; Barnadas, A.; Antolín, S.; Alés-Martínez, J.E.; Alvarez, I.; Andres, R.; Lao, J.; Carrasco, E.; et al. Exemestane versus anastrozole as front-line endocrine therapy in postmenopausal patients with hormone receptor-positive, advanced breast cancer. Cancer 2011, 118, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Lluch, A.; Barrios, C.H.; Torrecillas, L.; Ruiz-Borrego, M.; Bines, J.; Segalla, J.; Guerrero-Zotano, Á.; García-Sáenz, J.A.; Torres, R.; de la Haba, J.; et al. Phase III Trial of Adjuvant Capecitabine after Standard Neo-/Adjuvant Chemotherapy in Patients with Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01). J. Clin. Oncol. 2020, 38, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.-U.; Grischke, E.-M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.-J.; Kim, T.M.; et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage IIIBRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Lorusso, D.; Ferrandina, G.; Colombo, N.; Pignata, S.; Pietragalla, A.; Sonetto, C.; Pisano, C.; Lapresa, M.; Savarese, A.; Tagliaferri, P.; et al. Carboplatin-paclitaxel compared to Carboplatin-Paclitaxel-Bevacizumab in advanced or recurrent endometrial cancer: MITO END-2—A randomized phase II trial. Gynecol. Oncol. 2019, 155, 406–412. [Google Scholar] [CrossRef]
- Lorusso, D.; Hilpert, F.; Martín, A.G.; Rau, J.; Ottevanger, P.; Greimel, E.; Lück, H.-J.; Selle, F.; Colombo, N.; Kroep, J.; et al. Patient-reported outcomes and final overall survival results from the randomized phase 3 PENELOPE trial evaluating pertuzumab in low tumor human epidermal growth factor receptor 3 (HER3) mRNA-expressing platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer 2019, 29, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Olson, D.J.; Allred, J.B.; Strand, C.A.; Bao, R.; Zha, Y.; Carll, T.; Labadie, B.W.; Bastos, B.R.; Butler, M.O.; et al. Randomized Phase II Trial and Tumor Mutational Spectrum Analysis from Cabozantinib versus Chemotherapy in Metastatic Uveal Melanoma (Alliance A091201). Clin. Cancer Res. 2020, 26, 804–811. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, L.; Yang, B.; Feng, Y.; Xiong, Y.; Zhang, S.; Li, X.; Qian, C.; Dong, W.; Dai, N. A randomized phase 2 trial of apatinib vs observation as maintenance treatment following first-line induction chemotherapy in extensive-stage small cell lung cancer. Investig. New Drugs 2019, 38, 148–159. [Google Scholar] [CrossRef]
- Ma, D.-Y.; Tan, B.-X.; Liu, M.; Li, X.-F.; Zhou, Y.-Q.; Lu, Y. Concurrent three-dimensional conformal radiotherapy and chemotherapy for postoperative recurrence of mediastinal lymph node metastases in patients with esophageal squamous cell carcinoma: A phase 2 single-institution study. Radiat. Oncol. 2014, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Ouyang, Q.; Li, W.; Jiang, Z.; Tong, Z.; Liu, Y.; Li, H.; Yu, S.; Feng, J.; Wang, S.; et al. Pyrotinib or Lapatinib Combined with Capecitabine in HER2–Positive Metastatic Breast Cancer with Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase II Study. J. Clin. Oncol. 2019, 37, 2610–2619. [Google Scholar] [CrossRef]
- Machiels, J.-P.H.; Haddad, I.R.; Fayette, J.; Licitra, L.; Tahara, M.; Vermorken, J.B.; Clement, P.M.; Gauler, T.; Cupissol, D.; Grau, J.J.; et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015, 16, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.-P.; Subramanian, S.; Ruzsa, A.; Repassy, G.; Lifirenko, I.; Flygare, A.; Sørensen, P.; Nielsen, T.; Lisby, S.; Clement, P.M. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: An open-label, randomised phase 3 trial. Lancet Oncol. 2011, 12, 333–343. [Google Scholar] [CrossRef]
- Machiels, J.-P.; Van Maanen, A.; Vandenbulcke, J.-M.; Filleul, B.; Seront, E.; Henry, S.; D’Hondt, L.; Lonchay, C.; Holbrechts, S.; Boegner, P.; et al. Randomized Phase II Study of Cabazitaxel versus Methotrexate in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Previously Treated with Platinum-Based Therapy. Oncology 2016, 21, 1416.e17. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Lewis, K.; Demidov, L.; Mandalà, M.; Bondarenko, I.; Ascierto, A.P.; Herbert, C.; Mackiewicz, A.; Rutkowski, P.; Guminski, A.; et al. Adjuvant vemurafenib in resected, BRAF V600 mutation-positive melanoma (BRIM8): A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 510–520. [Google Scholar] [CrossRef]
- Maio, M.; Scherpereel, A.; Calabrò, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; Fennell, A.D.; Kowalski, D.; Tsao, A.S.; et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): A multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef]
- Mannel, R.S.; Brady, M.F.; Kohn, E.C.; Hanjani, P.; Hiura, M.; Lee, R.; DeGeest, K.; Cohn, D.; Monk, B.J.; Michael, H. A randomized phase III trial of IV carboplatin and paclitaxel × 3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2011, 122, 89–94. [Google Scholar] [CrossRef]
- Marec-Berard, P.; Dalban, C.; Gaspar, N.; Brugieres, L.; Gentet, J.-C.; Lervat, C.; Corradini, N.; Castex, M.-P.; Schmitt, C.; Pacquement, H.; et al. A multicentric randomized phase II clinical trial evaluating high-dose thiotepa as adjuvant treatment to standard chemotherapy in patients with resectable relapsed osteosarcoma. Eur. J. Cancer 2019, 125, 58–68. [Google Scholar] [CrossRef]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; Berg, H.V.D.; Brennan, B.; et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 21, 162–174. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sill, M.W.; Makker, V.; Mutch, D.G.; Carlson, J.W.; Darus, C.J.; Mannel, R.S.; Bender, D.P.; Crane, E.K.; Aghajanian, C. A randomized phase II study of cabozantinib versus weekly paclitaxel in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2019, 152, 548–553. [Google Scholar] [CrossRef]
- Mavratzas, A.; Baek, S.; Gerber, B.; Schmidt, M.; Moebus, V.; Foerster, F.; Grischke, E.; Fasching, P.; Strumberg, D.; Solomayer, E.; et al. Sorafenib in combination with docetaxel as first-line therapy for HER2-negative metastatic breast cancer: Final results of the randomized, double-blind, placebo-controlled phase II MADONNA study. Breast 2019, 45, 22–28. [Google Scholar] [CrossRef]
- Mayer, I.A.; Prat, A.; Egle, D.; Blau, S.; Fidalgo, J.A.P.; Gnant, M.; Fasching, P.A.; Colleoni, M.; Wolff, A.C.; Winer, E.P.; et al. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB). Clin. Cancer Res. 2019, 25, 2975–2987. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef] [PubMed]
- McGuire, W.P.; Penson, R.T.; Gore, M.; Herraez, A.C.; Peterson, P.; Shahir, A.; Ilaria, R., Jr. Randomized phase II study of the PDGFRα antibody olaratumab plus liposomal doxorubicin versus liposomal doxorubicin alone in patients with platinum-refractory or platinum-resistant advanced ovarian cancer. BMC Cancer 2018, 18, 1292. [Google Scholar] [CrossRef]
- Meier, W.; du Bois, A.; Rau, J.; Gropp-Meier, M.; Baumann, K.; Huober, J.; Wollschlaeger, K.; Kreienberg, R.; Canzler, U.; Schmalfeldt, B.; et al. Randomized phase II trial of carboplatin and paclitaxel with or without lonafarnib in first-line treatment of epithelial ovarian cancer stage IIB–IV. Gynecol. Oncol. 2012, 126, 236–240. [Google Scholar] [CrossRef]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.-B.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef]
- Melosky, B.; Bradbury, P.; Tu, D.; Florescu, M.; Reiman, A.; Nicholas, G.; Basappa, N.; Rothenstein, J.; Goffin, J.R.; Laurie, S.A.; et al. Selumetinib in patients receiving standard pemetrexed and platinum-based chemotherapy for advanced or metastatic KRAS wildtype or unknown non-squamous non-small cell lung cancer: A randomized, multicenter, phase II study. Canadian Cancer Trials Group (CCTG) IND. Lung Cancer 2019, 133, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Merle, P.; Blanc, J.-F.; Phelip, J.-M.; Pelletier, G.; Bronowicki, J.-P.; Touchefeu, Y.; Pageaux, G.; Gerolami, R.; Habersetzer, F.; Nguyen-Khac, E.; et al. Doxorubicin-loaded nanoparticles for patients with advanced hepatocellular carcinoma after sorafenib treatment failure (RELIVE): A phase 3 randomised controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 454–465. [Google Scholar] [CrossRef]
- Mir, O.; Brodowicz, T.; Italiano, A.; Wallet, J.; Blay, J.-Y.; Bertucci, F.; Chevreau, C.; Piperno-Neumann, S.; Bompas, E.; Salas, S.; et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1732–1742. [Google Scholar] [CrossRef]
- Mirza, M.R.; Lundqvist, E.A.; Birrer, M.J.; Christensen, R.D.; Nyvang, G.-B.; Malander, S.; Anttila, M.; Werner, T.L.; Lund, B.; Lindahl, G.; et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): A randomised, phase 2, superiority trial. Lancet Oncol. 2019, 20, 1409–1419. [Google Scholar] [CrossRef]
- Mita, M.M.; Joy, A.A.; Mita, A.; Sankhala, K.; Jou, Y.-M.; Zhang, D.; Statkevich, P.; Zhu, Y.; Yao, S.-L.; Small, K.; et al. Randomized Phase II Trial of the Cyclin-Dependent Kinase Inhibitor Dinaciclib (MK-7965) versus Capecitabine in Patients with Advanced Breast Cancer. Clin. Breast Cancer 2014, 14, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Hiller, J.P.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Modest, D.P.; Martens, U.M.; Riera-Knorrenschild, J.; Greeve, J.; Florschütz, A.; Wessendorf, S.; Ettrich, T.; Kanzler, S.; Nörenberg, D.; Ricke, J.; et al. FOLFOXIRI Plus Panitumumab As First-Line Treatment of RAS Wild-Type Metastatic Colorectal Cancer: The Randomized, Open-Label, Phase II VOLFI Study (AIO KRK0109). J. Clin. Oncol. 2019, 37, 3401–3411. [Google Scholar] [CrossRef]
- Mohamma-Dianpanah, M.; Shafizad, A.; Khademi, B.; Ansari, M.; Mosalaei, A.; Razmjou-Ghalaei, S.; Ashouri-Taziani, Y.; Ahmadloo, N.; Omidvari, S.; Mosleh-Shirazi, M.A. Efficacy and safety of concurrent chemoradiation with weekly cisplatin ± low-dose celecoxib in locally advanced undifferentiated nasopharyngeal carcinoma: A phase II-III clinical trial. J. Cancer Res. Ther. 2011, 7, 442–447. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Monk, B.J.; Brady, M.F.; Aghajanian, C.; Lankes, H.A.; Rizack, T.; Leach, J.; Fowler, J.M.; Higgins, R.; Hanjani, P.; Morgan, M.; et al. A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: A Gynecologic Oncology Group partners study. Ann. Oncol. 2017, 28, 996–1004. [Google Scholar] [CrossRef]
- Monk, B.J.; Herzog, T.J.; Wang, G.; Triantos, S.; Maul, S.; Knoblauch, R.; McGowan, T.; Shalaby, W.S.; Coleman, R.L. A phase 3 randomized, open-label, multicenter trial for safety and efficacy of combined trabectedin and pegylated liposomal doxorubicin therapy for recurrent ovarian cancer. Gynecol. Oncol. 2020, 156, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Poveda, A.; Vergote, I.; Raspagliesi, F.; Fujiwara, K.; Bae, D.-S.; Oaknin, A.; Ray-Coquard, I.; Provencher, D.M.; Karlan, B.Y.; et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): A randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014, 15, 799–808. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Morris, M.J.; Loriot, Y.; Sweeney, C.J.; Fizazi, K.; Ryan, C.J.; Shevrin, D.H.; Antonarakis, E.S.; Pandit-Taskar, N.; Deandreis, D.; Jacene, H.A.; et al. Radium-223 in combination with docetaxel in patients with castration-resistant prostate cancer and bone metastases: A phase 1 dose escalation/randomised phase 2a trial. Eur. J. Cancer 2019, 114, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Mostafid, A.H.; Porta, N.; Cresswell, J.; Griffiths, T.R.; Kelly, J.D.; Penegar, S.R.; Davenport, K.; McGrath, J.S.; Campain, N.; Cooke, P.; et al. CALIBER: A phase II randomized feasibility trial of chemoablation with mitomycin-C vs surgical management in low-risk non-muscle-invasive bladder cancer. BJU Int. 2020, 125, 817–826. [Google Scholar] [CrossRef]
- Motzer, R.J.; Haas, N.B.; Donskov, F.; Gross-Goupil, M.; Varlamov, S.; Kopyltsov, E.; Lee, J.L.; Melichar, B.; Rini, B.I.; Choueiri, T.K.; et al. Randomized Phase III Trial of Adjuvant Pazopanib versus Placebo after Nephrectomy in Patients with Localized or Locally Advanced Renal Cell Carcinoma. J. Clin. Oncol. 2017, 35, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Rini, I.B.; McDermott, D.F.; Frontera, O.A.; Hammers, H.J.; Carducci, A.M.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Munemoto, Y.; Nakamura, M.; Takahashi, M.; Kotaka, M.; Kuroda, H.; Kato, T.; Minagawa, N.; Noura, S.; Fukunaga, M.; Kuramochi, H.; et al. SAPPHIRE: A randomised phase II study of planned discontinuation or continuous treatment of oxaliplatin after six cycles of modified FOLFOX6 plus panitumumab in patients with colorectal cancer. Eur. J. Cancer 2019, 119, 158–167. [Google Scholar] [CrossRef]
- Nakagawa, K.; Garon, E.B.; Seto, T.; Nishio, M.; Aix, S.P.; Paz-Ares, L.; Chiu, C.-H.; Park, K.; Novello, S.; Nadal, E.; et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1655–1669. [Google Scholar] [CrossRef]
- Nakayama, T.; Sagara, Y.; Takashima, T.; Matsunami, N.; Masuda, N.; Miyoshi, Y.; Taguchi, T.; Aono, T.; Ito, T.; Kagimura, T.; et al. Randomized phase II study of anastrozole plus tegafur-uracil as neoadjuvant therapy for ER-positive breast cancer in postmenopausal Japanese women (Neo-ACET BC). Cancer Chemother. Pharmacol. 2018, 81, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Narui, K.; Ishikawa, T.; Shimizu, D.; Yamada, A.; Tanabe, M.; Sasaki, T.; Oba, M.S.; Morita, S.; Nawata, S.; Kida, K.; et al. Anthracycline could be essential for triple-negative breast cancer: A randomised phase II study by the Kanagawa Breast Oncology Group (KBOG). Breast 2019, 47, 1–9. [Google Scholar] [CrossRef]
- Noguchi, M.; Arai, G.; Egawa, S.; Ohyama, C.; Naito, S.; Matsumoto, K.; Uemura, H.; Nakagawa, M.; Nasu, Y.; Eto, M.; et al. Mixed 20-peptide cancer vaccine in combination with docetaxel and dexamethasone for castration-resistant prostate cancer: A randomized phase II trial. Cancer Immunol. Immunother. 2020, 69, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Scagliotti, G.; De Castro, G., Jr.; Kiyik, M.; Kowalyszyn, R.; Deppermann, K.-M.; Arriola, E.; Bosquee, L.; Novosiadly, R.; Nguyen, T.S.; et al. An Open-Label, Multicenter, Randomized, Phase II Study of Cisplatin and Pemetrexed with or without Cixutumumab (IMC-A12) as a First-Line Therapy in Patients with Advanced Nonsquamous Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 383–389. [Google Scholar] [CrossRef]
- O’Neil, B.H.; Scott, A.J.; Ma, W.W.; Cohen, S.J.; Aisner, D.L.; Menter, A.R.; Tejani, M.A.; Cho, J.K.; Granfortuna, J.; Coveler, L.; et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Ann. Oncol. 2015, 26, 1923–1929. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Barone, D.; Mahalingam, D.; Bekaii-Saab, T.; Shao, S.H.; Wolf, J.; Rosano, M.; Krause, S.; Richards, D.A.; Yu, K.H.; et al. Randomised phase II trial of gemcitabine and nab-paclitaxel with necuparanib or placebo in untreated metastatic pancreas ductal adenocarcinoma. Eur. J. Cancer 2020, 132, 112–121. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; DeMichele, A.; Ma, C.X.; Richards, P.; Yardley, D.A.; Wright, G.S.; Kalinsky, K.; Steis, R.; Diab, S.; Kennealey, G.; et al. A randomized, double-blind, phase 2 study of ruxolitinib or placebo in combination with capecitabine in patients with advanced HER2-negative breast cancer and elevated C-reactive protein, a marker of systemic inflammation. Breast Cancer Res. Treat. 2018, 170, 547–557. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.; Petrakova, K.; Sonke, G.; Conte, P.; Arteaga, C.L.; Cameron, D.A.; Hart, L.L.; Villanueva, C.; Jakobsen, E.; Beck, J.T.; et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2− advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res. Treat. 2017, 168, 127–134. [Google Scholar] [CrossRef]
- Oh, I.-J.; Kim, K.-S.; Park, C.-K.; Kim, Y.-C.; Lee, K.-H.; Jeong, J.-H.; Kim, S.-Y.; Lee, J.E.; Shin, K.-C.; Jang, T.-W.; et al. Belotecan/cisplatin versus etoposide/cisplatin in previously untreated patients with extensive-stage small cell lung carcinoma: A multi-center randomized phase III trial. BMC Cancer 2016, 16, 690. [Google Scholar] [CrossRef]
- Okamoto, T.; Yano, T.; Shimokawa, M.; Takeo, S.; Yamazaki, K.; Sugio, K.; Takenoyama, M.; Nagashima, A.; Tsukamoto, S.; Hamatake, M.; et al. A phase II randomized trial of adjuvant chemotherapy with S-1 versus S-1 plus cisplatin for completely resected pathological stage II/IIIA non-small cell lung cancer. Lung Cancer 2018, 124, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Oki, E.; Emi, Y.; Yamanaka, T.; Uetake, H.; Muro, K.; Takahashi, T.; Nagasaka, T.; Hatano, E.; Ojima, H.; Manaka, D.; et al. Randomised phase II trial of mFOLFOX6 plus bevacizumab versus mFOLFOX6 plus cetuximab as first-line treatment for colorectal liver metastasis (ATOM trial). Br. J. Cancer 2019, 121, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Okuno, K.; Aoyama, T.; Oba, K.; Yokoyama, N.; Matsuhashi, N.; Kunieda, K.; Nishimura, Y.; Akamatsu, H.; Kobatake, T.; Morita, S.; et al. Randomized phase III trial comparing surgery alone to UFT + PSK for stage II rectal cancer (JFMC38 trial). Cancer Chemother. Pharmacol. 2017, 81, 65–71. [Google Scholar] [CrossRef]
- Oliveira, M.; Saura, C.; Nuciforo, P.; Calvo, I.; Andersen, J.; Passos-Coelho, J.L.; Gil Gil, M.; Bermejo, B.; Patt, D.A.; Ciruelos, E.; et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann. Oncol. 2019, 30, 1289–1297. [Google Scholar] [CrossRef]
- Onda, T.; Satoh, T.; Ogawa, G.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Mizutani, T.; Takehara, K.; Okamoto, A.; Ushijima, K.; et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur. J. Cancer 2020, 130, 114–125. [Google Scholar] [CrossRef]
- Ouyang, X.; Shi, M.; Jie, F.; Bai, Y.; Shen, P.; Yu, Z.; Wang, X.; Huang, C.; Tao, M.; Wang, Z.; et al. Phase III study of dulanermin (recombinant human tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand) combined with vinorelbine and cisplatin in patients with advanced non-small-cell lung cancer. Investig. New Drugs 2017, 36, 315–322. [Google Scholar] [CrossRef]
- Oza, A.M.; Cibula, D.; Benzaquen, A.O.; Poole, C.; Mathijssen, R.H.J.; Sonke, G.; Colombo, N.; Špaček, J.; Vuylsteke, P.; Hirte, H.; et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: A randomised phase 2 trial. Lancet Oncol. 2014, 16, 87–97. [Google Scholar] [CrossRef]
- Oza, A.M.; Matulonis, A.U.; Malander, S.; Hudgens, S.; Sehouli, J.; del Campo, J.M.; Berton-Rigaud, D.; Banerjee, S.; Scambia, G.; Berek, J.S.; et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): Results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2018, 19, 1117–1125. [Google Scholar] [CrossRef]
- Pal, S.K.; McDermott, D.F.; Atkins, M.B.; Escudier, B.; Rini, B.I.; Motzer, R.J.; Fong, L.; Joseph, R.W.; Oudard, S.; Ravaud, A.; et al. Patient-reported outcomes in a phase 2 study comparing atezolizumab alone or with bevacizumab vs sunitinib in previously untreated metastatic renal cell carcinoma. BJU Int. 2020, 126, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, A.; Matikas, A.; Bengtsson, N.O.; Malmström, P.; Hedayati, E.; Steger, G.; Untch, M.; Hübbert, L.; Johansson, H.; Hellström, M.; et al. Efficacy and safety of tailored and dose-dense adjuvant chemotherapy and trastuzumab for resected HER2-positive breast cancer: Results from the phase 3 PANTHER trial. Cancer 2019, 126, 1175–1182. [Google Scholar] [CrossRef]
- Park, I.H.; Im, S.-A.; Jung, K.H.; Sohn, J.; Park, Y.H.; Lee, K.S.; Sim, S.H.; Park, K.-H.; Kim, J.H.; Nam, B.H.; et al. Randomized Open Label Phase III Trial of Irinotecan Plus Capecitabine versus Capecitabine Monotherapy in Patients with Metastatic Breast Cancer Previously Treated with Anthracycline and Taxane: PROCEED Trial (KCSG BR 11-01). Cancer Res. Treat. 2019, 51, 43–52. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, T.-Y.; Kim, G.M.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; Kim, H.-J.; et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1750–1759. [Google Scholar] [CrossRef]
- Patel, S.; Von Mehren, M.; Reed, D.R.; Kaiser, P.; Charlson, J.; Ryan, C.W.; Rushing, D.; Livingston, M.; Singh, A.; Seth, R.; et al. Overall survival and histology-specific subgroup analyses from a phase 3, randomized controlled study of trabectedin or dacarbazine in patients with advanced liposarcoma or leiomyosarcoma. Cancer 2019, 125, 2610–2620. [Google Scholar] [CrossRef]
- Patel, T.A.; Ensor, J.E.; Creamer, S.L.; Boone, T.; Rodriguez, A.A.; Niravath, P.A.; Darcourt, J.G.; Meisel, J.L.; Li, X.; Zhao, J.; et al. A randomized, controlled phase II trial of neoadjuvant ado-trastuzumab emtansine, lapatinib, and nab-paclitaxel versus trastuzumab, pertuzumab, and paclitaxel in HER2-positive breast cancer (TEAL study). Breast Cancer Res. 2019, 21, 100. [Google Scholar] [CrossRef]
- Patil, V.; Noronha, V.; Dhumal, S.B.; Joshi, A.; Menon, N.; Bhattacharjee, A.; Kulkarni, S.; Ankathi, S.K.; Mahajan, A.; Sable, N.; et al. Low-cost oral metronomic chemotherapy versus intravenous cisplatin in patients with recurrent, metastatic, inoperable head and neck carcinoma: An open-label, parallel-group, non-inferiority, randomised, phase 3 trial. Lancet Glob. Health 2020, 8, e1213–e1222. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Şenler, F.Ç.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, S.; Masini, L.; Turco, E.; Triggiani, L.; Krengli, M.; Meduri, B.; Pirtoli, L.; Borghetti, P.; Pegurri, L.; Riva, N.; et al. Hypofractionated radiation therapy versus chemotherapy with temozolomide in patients affected by RPA class V and VI glioblastoma: A randomized phase II trial. J. Neuro-Oncol. 2019, 143, 447–455. [Google Scholar] [CrossRef]
- Penel, N.; Mir, O.; Wallet, J.; Ray-Coquard, I.; Le Cesne, A.; Italiano, A.; Salas, S.; Delcambre, C.; Bompas, E.; Bertucci, F.; et al. A double-blind placebo-controlled randomized phase II trial assessing the activity and safety of regorafenib in non-adipocytic sarcoma patients previously treated with both chemotherapy and pazopanib. Eur. J. Cancer 2020, 126, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Barrios, C.; Eiermann, W.; Toi, M.; Im, Y.-H.; Conte, P.; Martin, M.; Pienkowski, T.; Pivot, X.; Burris, H.A.; et al. Trastuzumab Emtansine with or without Pertuzumab versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2–Positive, Advanced Breast Cancer: Primary Results from the Phase III MARIANNE Study. J. Clin. Oncol. 2017, 35, 141–148, Erratum in 2017, 35, 2342. [Google Scholar] [CrossRef]
- Pérol, M.; Pavlakis, N.; Levchenko, E.; Platania, M.; Oliveira, J.; Novello, S.; Chiari, R.; Moran, T.; Mitry, E.; Nüesch, E.; et al. Patient-reported outcomes from the randomized phase III ALEX study of alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer. Lung Cancer 2019, 138, 79–87. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Perrone, F.; De Laurentiis, M.; De Placido, S.; Orditura, M.; Cinieri, S.; Riccardi, F.; Ribecco, A.S.; Putzu, C.; Del Mastro, L.; Rossi, E.; et al. Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur. J. Cancer 2019, 118, 178–186. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Yilmaz, M.; Möller, S.; Zitnjak, D.; Krogh, M.; Petersen, L.N.; Poulsen, L.; Winther, S.B.; Thomsen, K.G.; Qvortrup, C. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: An investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 412–420. [Google Scholar] [CrossRef]
- Pfisterer, J.; Shannon, C.M.; Baumann, K.; Rau, J.; Harter, P.; Joly, F.; Sehouli, J.; Canzler, U.; Schmalfeldt, B.; Dean, A.P.; et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 699–709. [Google Scholar] [CrossRef]
- Pignata, S.; Scambia, G.; Lorusso, D.; De Giorgi, U.; Nicoletto, M.O.; Lauria, R.; Mosconi, A.M.; Sacco, C.; Omarini, C.; Tagliaferri, P.; et al. The MITO CERV-2 trial: A randomized phase II study of cetuximab plus carboplatin and paclitaxel, in advanced or recurrent cervical cancer. Gynecol. Oncol. 2019, 153, 535–540. [Google Scholar] [CrossRef]
- Pili, R.; Häggman, M.; Stadler, W.M.; Gingrich, J.; Assikis, V.J.; Björk, A.; Nordle, O.; Forsberg, G.; Carducci, M.A.; Armstrong, A.J. Phase II Randomized, Double-Blind, Placebo-Controlled Study of Tasquinimod in Men with Minimally Symptomatic Metastatic Castrate-Resistant Prostate Cancer. J. Clin. Oncol. 2011, 29, 4022–4028. [Google Scholar] [CrossRef]
- Pillai, R.N.; Fennell, D.A.; Kovcin, V.; Ciuleanu, T.E.; Ramlau, R.; Kowalski, D.; Schenker, M.; Yalcin, I.; Teofilovici, F.; Vukovic, V.M.; et al. Randomized Phase III Study of Ganetespib, a Heat Shock Protein 90 Inhibitor, with Docetaxel versus Docetaxel in Advanced Non–Small-Cell Lung Cancer (GALAXY-2). J. Clin. Oncol. 2020, 38, 613–622. [Google Scholar] [CrossRef]
- Pimentel, I.; Lohmann, A.E.; Ennis, M.; Dowling, R.J.O.; Cescon, D.; Elser, C.; Potvin, K.R.; Haq, R.; Hamm, C.; Chang, M.C.; et al. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast 2019, 48, 17–23. [Google Scholar] [CrossRef]
- Pivot, X.; Bondarenko, I.; Nowecki, Z.; Dvorkin, M.; Trishkina, E.; Ahn, J.-H.; Im, S.-A.; Sarosiek, T.; Chatterjee, S.; Wojtukiewicz, M.; et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: Final safety, immunogenicity and survival results. Eur. J. Cancer 2018, 93, 19–27. [Google Scholar] [CrossRef]
- Pivot, X.; Pegram, M.; Cortes, J.; Lüftner, D.; Lyman, G.H.; Curigliano, G.; Bondarenko, I.; Yoon, Y.C.; Kim, Y.; Kim, C. Three-year follow-up from a phase 3 study of SB3 (a trastuzumab biosimilar) versus reference trastuzumab in the neoadjuvant setting for human epidermal growth factor receptor 2–positive breast cancer. Eur. J. Cancer 2019, 120, 1–9. [Google Scholar] [CrossRef]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.-Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espié, M.; Fumoleau, P.; Serin, D.; et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): Final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 2019, 393, 2591–2598. [Google Scholar] [CrossRef]
- Platania, M.; Pasini, F.; Porcu, L.; Boeri, M.; Verderame, F.; Modena, Y.; Del Conte, A.; Nichetti, F.; Garassino, M.C.; Martinetti, A.; et al. Oral maintenance metronomic vinorelbine versus best supportive care in advanced non-small-cell lung cancer after platinum-based chemotherapy: The MA.NI.LA. multicenter, randomized, controlled, phase II trial. Lung Cancer 2019, 132, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Lackner, M.R.; Oudard, S.; Escudier, B.; Ralph, C.; Brown, J.E.; Hawkins, R.E.; Castellano, D.; Rini, B.I.; Staehler, M.D.; et al. Randomized Open-Label Phase II Trial of Apitolisib (GDC-0980), a Novel Inhibitor of the PI3K/Mammalian Target of Rapamycin Pathway, versus Everolimus in Patients with Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2016, 34, 1660–1668. [Google Scholar] [CrossRef]
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Munoz, M.; Pare, L.; Farré, B.G.; Fernández, P.L.; Galván, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2019, 21, 33–43. [Google Scholar] [CrossRef]
- Provencher, D.; Gallagher, C.; Parulekar, W.; Ledermann, J.; Armstrong, D.; Brundage, M.; Gourley, C.; Romero, I.; Gonzalez-Martin, A.; Feeney, M.; et al. OV21/PETROC: A randomized Gynecologic Cancer Intergroup phase II study of intraperitoneal versus intravenous chemotherapy following neoadjuvant chemotherapy and optimal debulking surgery in epithelial ovarian cancer. Ann. Oncol. 2017, 29, 431–438. [Google Scholar] [CrossRef]
- Pujol, J.-L.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Uwer, L.; Hureaux, J.; Guisier, F.; Carmier, D.; Madelaine, J.; Otto, J.; et al. A Randomized Non-Comparative Phase II Study of Anti-Programmed Cell Death-Ligand 1 Atezolizumab or Chemotherapy as Second-Line Therapy in Patients with Small Cell Lung Cancer: Results from the IFCT-1603 Trial. J. Thorac. Oncol. 2019, 14, 903–913. [Google Scholar] [CrossRef]
- Qin, S.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 571–580. [Google Scholar] [CrossRef]
- Quoix, E.; Lena, H.; Losonczy, G.; Forget, F.; Chouaid, C.; Papai, Z.; Gervais, R.; Ottensmeier, C.; Szczesna, A.; Kazarnowicz, A.; et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): Results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2015, 17, 212–223. [Google Scholar] [CrossRef]
- Quon, H.; Leong, T.; Haselow, R.; Leipzig, B.; Cooper, J.; Forastiere, A. Phase III Study of Radiation Therapy with or without Cis-Platinum in Patients with Unresectable Squamous or Undifferentiated Carcinoma of the Head and Neck: An Intergroup Trial of the Eastern Cooperative Oncology Group (E2382). Int. J. Radiat. Oncol. 2011, 81, 719–725. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Thomas, G.W.; Khorana, A.A.; Shah, S.; Zhou, C.; Wong, S.; Cole, G., Jr.; James, D.; Gabrail, N.Y. A Phase 2 Study of PCI-27483, a Factor VIIa Inhibitor in Combination with Gemcitabine for Advanced Pancreatic Cancer. Oncology 2019, 96, 217–222. [Google Scholar] [CrossRef]
- Randall, M.E.; Filiaci, V.; McMeekin, D.S.; Von Gruenigen, V.; Huang, H.; Yashar, C.M.; Mannel, R.S.; Kim, J.-W.; Salani, R.; DiSilvestro, P.A.; et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early-Stage Endometrial Cancer. J. Clin. Oncol. 2019, 37, 1810–1818. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cibula, D.; Mirza, M.R.; Reuss, A.; Ricci, C.; Colombo, N.; Koch, H.; Goffin, F.; González-Martin, A.; Ottevanger, P.B.; et al. Final results from GCIG/ENGOT/AGO-OVAR 12, a randomised placebo-controlled phase III trial of nintedanib combined with chemotherapy for newly diagnosed advanced ovarian cancer. Int. J. Cancer 2019, 146, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Brahmer, J.; Bennett, B.; Taylor, F.; Penrod, J.R.; DeRosa, M.; Dastani, H.; Spigel, D.R.; Gralla, R.J. Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur. J. Cancer 2018, 102, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Horn, L.; Novello, S.; Barlesi, F.; Albert, I.; Juhász, E.; Kowalski, D.; Robinet, G.; Cadranel, J.; Bidoli, P.; et al. Phase II Study of Roniciclib in Combination with Cisplatin/Etoposide or Carboplatin/Etoposide as First-Line Therapy in Patients with Extensive-Disease Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 701–711. [Google Scholar] [CrossRef]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.-Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014, 15, 143–155. [Google Scholar] [CrossRef]
- Reck, M.; Schenker, M.; Lee, K.H.; Provencio, M.; Nishio, M.; Lesniewski-Kmak, K.; Sangha, R.; Ahmed, S.; Raimbourg, J.; Feeney, K.; et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non–small-cell lung cancer with high tumour mutational burden: Patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur. J. Cancer 2019, 116, 137–147. [Google Scholar] [CrossRef]
- Reck, M.; Taylor, F.; Penrod, J.R.; DeRosa, M.; Morrissey, L.; Dastani, H.; Orsini, L.; Gralla, R.J. Impact of Nivolumab versus Docetaxel on Health-Related Quality of Life and Symptoms in Patients with Advanced Squamous Non–Small Cell Lung Cancer: Results from the CheckMate 017 Study. J. Thorac. Oncol. 2018, 13, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Reni, M.; Zanon, S.; Balzano, G.; Passoni, P.; Pircher, C.; Chiaravalli, M.; Fugazza, C.; Ceraulo, D.; Nicoletti, R.; Arcidiacono, P.G.; et al. A randomised phase 2 trial of nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in locally advanced or borderline resectable pancreatic adenocarcinoma. Eur. J. Cancer 2018, 102, 95–102. [Google Scholar] [CrossRef]
- Reni, M.; Zanon, S.; Peretti, U.; Chiaravalli, M.; Barone, D.; Pircher, C.; Balzano, G.; Macchini, M.; Romi, S.; Gritti, E.; et al. Nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in metastatic pancreatic adenocarcinoma (PACT-19): A randomised phase 2 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 691–697. [Google Scholar] [CrossRef]
- Reyners, A.K.L.; de Munck, L.; Erdkamp, F.L.G.; Smit, W.M.; Hoekman, K.; Lalisang, R.I.; de Graaf, H.; Wymenga, A.N.M.; Polee, M.; Hollema, H.; et al. A randomized phase II study investigating the addition of the specific COX-2 inhibitor celecoxib to docetaxel plus carboplatin as first-line chemotherapy for stage IC to IV epithelial ovarian cancer, Fallopian tube or primary peritoneal carcinomas: The DoCaCel study. Ann. Oncol. 2012, 23, 2896–2902. [Google Scholar] [CrossRef]
- Rimawi, M.F.; Aleixo, S.B.; Rozas, A.A.; Neto, J.N.D.M.; Caleffi, M.; Figueira, A.C.; Souza, S.C.; Reiriz, A.B.; Gutierrez, C.; Arantes, H.; et al. A Neoadjuvant, Randomized, Open-Label Phase II Trial of Afatinib versus Trastuzumab versus Lapatinib in Patients with Locally Advanced HER2-Positive Breast Cancer. Clin. Breast Cancer 2014, 15, 101–109. [Google Scholar] [CrossRef]
- Rimawi, M.F.; Niravath, P.; Wang, T.; Rexer, B.N.; Forero, A.; Wolff, A.C.; Nanda, R.; Storniolo, A.M.; Krop, I.; Goetz, M.P.; et al. TBCRC023: A Randomized Phase II Neoadjuvant Trial of Lapatinib Plus Trastuzumab without Chemotherapy for 12 versus 24 Weeks in Patients with HER2-Positive Breast Cancer. Clin. Cancer Res. 2019, 26, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Rini, I.B.; Pal, S.K.; Escudier, B.J.; Atkins, M.B.; Hutson, T.; Porta, C.; Verzoni, E.; Needle, M.N.; McDermott, D.F. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): A phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2019, 21, 95–104. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Rini, I.B.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2016, 389, 255–265. [Google Scholar] [CrossRef]
- Rivera, F.; Benavides, M.; Gallego, J.; Guillen-Ponce, C.; Lopez-Martin, J.; Küng, M. Tumor treating fields in combination with gemcitabine or gemcitabine plus nab-paclitaxel in pancreatic cancer: Results of the PANOVA phase 2 study. Pancreatology 2019, 19, 64–72. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.-L.; Philco-Salas, M.J.; et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet 2016, 388, 2997–3005. [Google Scholar] [CrossRef]
- Robertson, J.F.; Cheung, K.-L.; Noguchi, S.; Shao, Z.; Degboe, A.; Lichfield, J.; Thirlwell, J.; Fazal, M.; Ellis, M.J. Health-related quality of life from the FALCON phase III randomised trial of fulvestrant 500 mg versus anastrozole for hormone receptor-positive advanced breast cancer. Eur. J. Cancer 2018, 94, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Rozeman, A.E.; Menzies, A.M.; van Akkooi, A.; Adhikari, C.; Bierman, C.; van de Wiel, A.B.; Scolyer, A.R.; Krijgsman, O.; Sikorska, K.; Eriksson, H.; et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019, 20, 948–960. [Google Scholar] [CrossRef]
- Ruíz-Borrego, M.; Guerrero-Zotano, A.; Bermejo, B.; Ramos, M.; Cruz, J.; Baena-Cañada, J.M.; Cirauqui, B.; Rodríguez-Lescure, Á.; Alba, E.; Martínez-Jáñez, N.; et al. Phase III evaluating the addition of fulvestrant (F) to anastrozole (A) as adjuvant therapy in postmenopausal women with hormone receptor-positive HER2-negative (HR+/HER2−) early breast cancer (EBC): Results from the GEICAM/2006–10 study. Breast Cancer Res. Treat. 2019, 177, 115–125. [Google Scholar] [CrossRef]
- Ruzsa, A.; NA EMD 1201081 Study Group; Sen, M.; Evans, M.; Lee, L.W.; Hideghety, K.; Rottey, S.; Klimak, P.; Holeckova, P.; Fayette, J.; et al. Phase 2, open-label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second-line cetuximab-naïve patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Investig. New Drugs 2014, 32, 1278–1284. [Google Scholar] [CrossRef]
- Sabbatini, P.; Harter, P.; Scambia, G.; Sehouli, J.; Meier, W.; Wimberger, P.; Baumann, K.H.; Kurzeder, C.; Schmalfeldt, B.; Cibula, D.; et al. Abagovomab As Maintenance Therapy in Patients with Epithelial Ovarian Cancer: A Phase III Trial of the AGO OVAR, COGI, GINECO, and GEICO—The MIMOSA Study. J. Clin. Oncol. 2013, 31, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Sadahiro, S.; Suzuki, T.; Okada, K.; Saito, G.; Miyakita, H.; Ogimi, T.; Chan, L.F.; Kamei, Y. Oral S-1 with 24-h Infusion of Irinotecan plus Bevacizumab versus FOLFIRI plus Bevacizumab as First-Line Chemotherapy for Metastatic Colorectal Cancer: An Open-Label Randomized Phase II Trial. Oncology 2020, 98, 637–642. [Google Scholar] [CrossRef]
- Salama, J.K.; Haraf, D.J.; Stenson, K.M.; Blair, E.A.; Witt, M.E.; Williams, R.; Kunnavakkam, R.; Cohen, E.E.W.; Seiwert, T.; Vokes, E.E. A randomized phase II study of 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy compared with bevacizumab plus 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy for intermediate-stage and T4N0-1 head and neck cancers. Ann. Oncol. 2011, 22, 2304–2309. [Google Scholar] [CrossRef]
- Santo, A.; Pilotto, S.; Galetta, D.; Grossi, F.; Fasola, G.; Romano, G.; Bonanno, L.; Bearz, A.; Papi, M.; Roca, E.; et al. Maintenance with lanreotide in small-cell lung cancer expressing somatostatine receptors: A multicenter, randomized, phase 3 trial. Lung Cancer 2019, 134, 121–126. [Google Scholar] [CrossRef]
- Saura, C.; Hlauschek, D.; Oliveira, M.; Zardavas, D.; Jallitsch-Halper, A.; de la Peña, L.; Nuciforo, P.; Ballestrero, A.; Dubsky, P.; Lombard, J.M.; et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2019, 20, 1226–1238. [Google Scholar] [CrossRef]
- Scagliotti, G.V.; Gaafar, R.; Nowak, A.; Nakano, T.; van Meerbeeck, J.; Popat, S.; Vogelzang, N.J.; Grosso, F.; EL Hassan, R.A.; Jakopovic, M.; et al. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir. Med. 2019, 7, 569–580. [Google Scholar] [CrossRef]
- Scagliotti, G.V.; Germonpré, P.; Bosquée, L.; Vansteenkiste, J.; Gervais, R.; Planchard, D.; Reck, M.; De Marinis, F.; Lee, J.S.; Park, K.; et al. A randomized phase II study of bortezomib and pemetrexed, in combination or alone, in patients with previously treated advanced non-small-cell lung cancer. Lung Cancer 2010, 68, 420–426. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Wright, G.S.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 21, 44–59. [Google Scholar] [CrossRef]
- Schwandt, A.; Von Gruenigen, V.E.; Wenham, R.M.; Frasure, H.; Eaton, S.; Fusco, N.; Fu, P.; Wright, J.J.; Dowlati, A.; Waggoner, S. Randomized phase II trial of sorafenib alone or in combination with carboplatin/paclitaxel in women with recurrent platinum sensitive epithelial ovarian, peritoneal, or fallopian tube cancer. Investig. New Drugs 2014, 32, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Sculier, J.-P.; Lafitte, J.-J.; Berghmans, T.; Meert, A.-P.; Scherpereel, A.; Roelandts, M.; Van Cutsem, O.; Colinet, B.; Bonduelle, Y.; Giner, V.; et al. A phase III randomised study comparing concomitant radiochemotherapy with cisplatin and docetaxel as induction versus consolidation treatment in patients with locally advanced unresectable non-small cell lung cancer. Lung Cancer 2018, 117, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Fayette, J.; Cupissol, D.; del Campo, J.M.; Clement, P.M.; Hitt, R.; Degardin, M.; Zhang, W.; Blackman, A.; Ehrnrooth, E.; et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann. Oncol. 2014, 25, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Melotek, J.M.; Blair, E.A.; Stenson, K.M.; Salama, J.K.; Witt, M.E.; Brisson, R.J.; Chawla, A.; Dekker, A.; Lingen, M.W.; et al. Final Results of a Randomized Phase 2 Trial Investigating the Addition of Cetuximab to Induction Chemotherapy and Accelerated or Hyperfractionated Chemoradiation for Locoregionally Advanced Head and Neck Cancer. Int. J. Radiat. Oncol. 2016, 96, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Si, X.; Zhang, L.; Wang, H.; Zhang, X.; Wang, M.; Han, B.; Li, K.; Wang, Q.; Shi, J.; Wang, Z.; et al. Quality of life results from a randomized, double-blinded, placebo-controlled, multi-center phase III trial of anlotinib in patients with advanced non-small cell lung cancer. Lung Cancer 2018, 122, 32–37. [Google Scholar] [CrossRef]
- Sim, S.H.; Park, I.H.; Jung, K.H.; Kim, S.-B.; Ahn, J.-H.; Lee, K.-H.; Im, S.-A.; Im, Y.-H.; Park, Y.H.; Sohn, J.; et al. Randomised Phase 2 study of lapatinib and vinorelbine vs vinorelbine in patients with HER2 + metastatic breast cancer after lapatinib and trastuzumab treatment (KCSG BR11-16). Br. J. Cancer 2019, 121, 985–990. [Google Scholar] [CrossRef]
- Smith, I.; Robertson, J.; Kilburn, L.; Wilcox, M.; Evans, A.; Holcombe, C.; Horgan, K.; Kirwan, C.; Mallon, E.; Sibbering, M.; et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): An open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1443–1454, Erratum in 2020, 21, e553. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Soria, J.-C.; Wu, Y.-L.; Nakagawa, K.; Kim, S.-W.; Yang, J.-J.; Ahn, M.-J.; Wang, J.; Yang, J.C.-H.; Lu, Y.; Atagi, S.; et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): A phase 3 randomised trial. Lancet Oncol. 2015, 16, 990–998. [Google Scholar] [CrossRef]
- Soulières, D.; Faivre, S.; Mesía, R.; Remenár, É.; Li, S.-H.; Karpenko, A.; Dechaphunkul, A.; Ochsenreither, S.; Kiss, L.A.; Lin, J.-C.; et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017, 18, 323–335. [Google Scholar] [CrossRef]
- Spigel, D.R.; Luft, A.; Depenbrock, H.; Ramlau, R.; Khalil, M.; Kim, J.-H.; Mayo, C.; Chao, G.Y.; Obasaju, C.; Natale, R. An Open-Label, Randomized, Controlled Phase II Study of Paclitaxel-Carboplatin Chemotherapy with Necitumumab versus Paclitaxel-Carboplatin Alone in First-Line Treatment of Patients with Stage IV Squamous Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2017, 18, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Shipley, D.L.; Waterhouse, D.M.; Jones, S.F.; Ward, P.J.; Shih, K.C.; Hemphill, B.; McCleod, M.; Whorf, R.C.; Page, R.D.; et al. A Randomized, Double-Blinded, Phase II Trial of Carboplatin and Pemetrexed with or without Apatorsen (OGX-427) in Patients with Previously Untreated Stage IV Non-Squamous-Non-Small-Cell Lung Cancer: The SPRUCE Trial. Oncology 2019, 24, e1409–e1416. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.P.; Cook, A.; Brown, J.M.; Brundage, M.D.; Embleton-Thirsk, A.; Kaplan, R.S.; Raja, F.A.; Swart, A.M.W.; Velikova, G.; Qian, W.; et al. Quality of life with cediranib in relapsed ovarian cancer: The ICON6 phase 3 randomized clinical trial. Cancer 2017, 123, 2752–2761. [Google Scholar] [CrossRef]
- Sun, J.-M.; Lee, K.H.; Kim, B.-S.; Kim, H.-G.; Min, Y.J.; Yi, S.Y.; Yun, H.J.; Jung, S.-H.; Lee, S.-H.; Ahn, J.S.; et al. Pazopanib maintenance after first-line etoposide and platinum chemotherapy in patients with extensive disease small-cell lung cancer: A multicentre, randomised, placebo-controlled Phase II study (KCSG-LU12-07). Br. J. Cancer 2018, 118, 648–653. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, W.; Bai, Y.; Ge, M.; Hu, C.; Wu, S.; Hao, J.; Gao, M.; Pan, J.; Dong, P.; et al. Neoadjuvant dose-modified docetaxel in squamous cell carcinoma of the head and neck: A phase 3 study. Oral Dis. 2019, 26, 285–294. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W.-F.; Chen, N.-Y.; Zhang, N.; Hu, G.-Q.; Xie, F.-Y.; Sun, Y.; Chen, X.-Z.; Li, J.-G.; Zhu, X.-D.; et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016, 17, 1509–1520. [Google Scholar] [CrossRef]
- Suntharalingam, M.; Winter, K.; Ilson, D.; Dicker, A.P.; Kachnic, L.; Konski, A.; Chakravarthy, A.B.; Anker, C.J.; Thakrar, H.; Horiba, N.; et al. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients with Esophageal Cancer. JAMA Oncol. 2017, 3, 1520–1528. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Steele, J.P.; Nolan, L.; Gilligan, D.; Taylor, P.; Spicer, J.; Lind, M.; Mitra, S.; Shamash, J.; Phillips, M.M.; et al. Arginine Deprivation with Pegylated Arginine Deiminase in Patients with Argininosuccinate Synthetase 1–Deficient Malignant Pleural Mesothelioma. JAMA Oncol. 2017, 3, 58–66. [Google Scholar] [CrossRef]
- Takayama, K.; Ichiki, M.; Tokunaga, S.; Inoue, K.; Kawasaki, M.; Uchino, J.; Nakanishi, Y. Randomized Phase II Study of Weekly Paclitaxel plus Carboplatin versus Biweekly Paclitaxel plus Carboplatin for Patients with Previously Untreated Advanced Non-Small Cell Lung Cancer. Oncology 2019, 24, 1420.e1010. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Imamura, C.K.; Takano, T.; Saji, S.; Yamanaka, T.; Yonemori, K.; Takahashi, M.; Tsurutani, J.; Nishimura, R.; Sato, K.; et al. CYP2D6 Genotype–Guided Tamoxifen Dosing in Hormone Receptor–Positive Metastatic Breast Cancer (TARGET-1): A Randomized, Open-Label, Phase II Study. J. Clin. Oncol. 2020, 38, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.R.; Wright, G.S.; Thummala, A.R.; Danso, A.M.; Popovic, L.; Pluard, T.J.; Han, H.S.; Vojnović, Ž.; Vasev, N.; Ma, L.; et al. Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: A multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2019, 20, 1587–1601. [Google Scholar] [CrossRef]
- Tan, T.; Lim, D.W.-T.; Fong, K.-W.; Cheah, S.-L.; Soong, Y.L.; Ang, M.-K.; Ng, Q.-S.; Tan, D.; Ong, W.-S.; Tan, S.H.; et al. Concurrent Chemo-Radiation with or without Induction Gemcitabine, Carboplatin, and Paclitaxel: A Randomized, Phase 2/3 Trial in Locally Advanced Nasopharyngeal Carcinoma. Int. J. Radiat. Oncol. 2015, 91, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Gelderblom, H.; Palmerini, E.; Desai, J.; Bauer, S.; Blay, J.-Y.; Alcindor, T.; Ganjoo, K.; Martin-Broto, J.; Ryan, C.W.; et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): A randomised phase 3 trial. Lancet 2019, 394, 478–487. [Google Scholar] [CrossRef]
- Tap, W.D.; Papai, Z.; Van Tine, A.B.; Attia, S.; Ganjoo, K.N.; Jones, R.L.; Schuetze, S.; Reed, D.; Chawla, S.P.; Riedel, R.F.; et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): An international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2017, 18, 1089–1103. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Lee, S.J.; Hodi, F.S.; Rao, U.N.M.; Cohen, G.I.; Hamid, O.; Hutchins, L.F.; Sosman, J.A.; Kluger, H.M.; Eroglu, Z.; et al. Phase III Study of Adjuvant Ipilimumab (3 or 10 mg/kg) versus High-Dose Interferon Alfa-2b for Resected High-Risk Melanoma: North American Intergroup E1609. J. Clin. Oncol. 2020, 38, 567–575. [Google Scholar] [CrossRef]
- Tew, W.P.; Colombo, N.; Ray-Coquard, I.; Del Campo, J.M.; Oza, A.; Pereira, D.; Mammoliti, S.; Matei, D.; Scambia, G.; Tonkin, K.; et al. Intravenous aflibercept in patients with platinum-resistant, advanced ovarian cancer: Results of a randomized, double-blind, phase 2, parallel-arm study. Cancer 2013, 120, 335–343. [Google Scholar] [CrossRef]
- Thatcher, N.; Goldschmidt, J.H.; Thomas, M.; Schenker, M.; Pan, Z.; Rodriguez, L.P.-A.; Breder, V.; Ostoros, G.; Hanes, V. Efficacy and Safety of the Biosimilar ABP 215 Compared with Bevacizumab in Patients with Advanced Nonsquamous Non–small Cell Lung Cancer (MAPLE): A Randomized, Double-blind, Phase III Study. Clin. Cancer Res. 2019, 25, 2088–2095. [Google Scholar] [CrossRef]
- Thomas, M.; Ponce-Aix, S.; Navarro, A.; Riera-Knorrenschild, J.; Schmidt, M.; Wiegert, E.; Kapp, K.; Wittig, B.; Mauri, C.; Gómez, M.D.; et al. Immunotherapeutic maintenance treatment with toll-like receptor 9 agonist lefitolimod in patients with extensive-stage small-cell lung cancer: Results from the exploratory, controlled, randomized, international phase II IMPULSE study. Ann. Oncol. 2018, 29, 2076–2084. [Google Scholar] [CrossRef]
- Tomita, N.; Kunieda, K.; Maeda, A.; Hamada, C.; Yamanaka, T.; Sato, T.; Yoshida, K.; Boku, N.; Nezu, R.; Yamaguchi, S.; et al. Phase III randomised trial comparing 6 vs. 12-month of capecitabine as adjuvant chemotherapy for patients with stage III colon cancer: Final results of the JFMC37-0801 study. Br. J. Cancer 2019, 120, 689–696. [Google Scholar] [CrossRef]
- Tomita, Y.; Fukasawa, S.; Shinohara, N.; Kitamura, H.; Oya, M.; Eto, M.; Tanabe, K.; Saito, M.; Kimura, G.; Yonese, J.; et al. Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup 3-year follow-up analysis from the Phase III CheckMate 025 study. Jpn. J. Clin. Oncol. 2019, 49, 506–514. [Google Scholar] [CrossRef]
- Tortochaux, J.; Tao, Y.; Tournay, E.; Lapeyre, M.; Lesaunier, F.; Bardet, E.; Janot, F.; Lusinchi, A.; Benhamou, E.; Bontemps, P.; et al. Randomized phase III trial (GORTEC 98-03) comparing re-irradiation plus chemotherapy versus methotrexate in patients with recurrent or a second primary head and neck squamous cell carcinoma, treated with a palliative intent. Radiother. Oncol. 2011, 100, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Toulmonde, M.; Pulido, M.; Ray-Coquard, I.; Andre, T.; Isambert, N.; Chevreau, C.; Penel, N.; Bompas, E.; Saada, E.; Bertucci, F.; et al. Pazopanib or methotrexate–vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): A non-comparative, randomised, open-label, multicentre, phase 2 study. Lancet Oncol. 2019, 20, 1263–1272. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Tsao, A.S.; Miao, J.; Wistuba, I.I.; Vogelzang, N.J.; Heymach, J.V.; Fossella, F.V.; Lu, C.; Velasco, M.R.; Box-Noriega, B.; Hueftle, J.G.; et al. Phase II Trial of Cediranib in Combination with Cisplatin and Pemetrexed in Chemotherapy-Naïve Patients with Unresectable Malignant Pleural Mesothelioma (SWOG S0905). J. Clin. Oncol. 2019, 37, 2537–2547. [Google Scholar] [CrossRef]
- Tsukahara, K.; Kubota, A.; Hasegawa, Y.; Takemura, H.; Terada, T.; Taguchi, T.; Nagahara, K.; Nakatani, H.; Yoshino, K.; Higaki, Y.; et al. Randomized Phase III Trial of Adjuvant Chemotherapy with S-1 after Curative Treatment in Patients with Squamous-Cell Carcinoma of the Head and Neck (ACTS-HNC). PLoS ONE 2015, 10, e0116965. [Google Scholar] [CrossRef]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Vaklavas, C.; on behalf of the Translational Breast Cancer Research Consortium (TBCRC); Roberts, B.S.; Varley, K.E.; Lin, N.U.; Liu, M.C.; Rugo, H.S.; Puhalla, S.; Nanda, R.; Storniolo, A.M.; et al. TBCRC 002: A phase II, randomized, open-label trial of preoperative letrozole with or without bevacizumab in postmenopausal women with newly diagnosed stage 2/3 hormone receptor-positive and HER2-negative breast cancer. Breast Cancer Res. 2020, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Bajetta, E.; Valle, J.; Köhne, C.-H.; Hecht, J.R.; Moore, M.; Germond, C.; Berg, W.; Chen, B.-L.; Jalava, T.; et al. Randomized, Placebo-Controlled, Phase III Study of Oxaliplatin, Fluorouracil, and Leucovorin with or without PTK787/ZK 222584 in Patients with Previously Treated Metastatic Colorectal Adenocarcinoma. J. Clin. Oncol. 2011, 29, 2004–2010. [Google Scholar] [CrossRef]
- Van Der Valk, M.J.; Hilling, D.; Kranenbarg, E.M.-K.; Peeters, K.C.; Kapiteijn, E.; Tsonaka, R.; Van De Velde, C.J.; de Mheen, P.M.-V. Quality of Life after Curative Resection for Rectal Cancer in Patients Treated with Adjuvant Chemotherapy Compared with Observation: Results of the Randomized Phase III SCRIPT Trial. Dis. Colon Rectum 2019, 62, 711–720. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Van Leeuwen, J.H.S.; Schreuder, H.W.R.; Hermans, R.H.M.; De Hingh, I.H.J.T.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, R.; Lorente, D.; Murthy, V.; Thomas, K.; Parker, L.; Ahiabor, R.; Dearnaley, D.; Huddart, R.; De Bono, J.; Parker, C. A Randomised Phase 2 Trial of Dexamethasone versus Prednisolone in Castration-resistant Prostate Cancer. Eur. Urol. 2015, 67, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Armstrong, D.; Scambia, G.; Teneriello, M.; Sehouli, J.; Schweizer, C.; Weil, S.C.; Bamias, A.; Fujiwara, K.; Ochiai, K.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Study to Assess Efficacy and Safety of Weekly Farletuzumab in Combination with Carboplatin and Taxane in Patients with Ovarian Cancer in First Platinum-Sensitive Relapse. J. Clin. Oncol. 2016, 34, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Bergfeldt, K.; Franquet, A.; Lisyanskaya, A.; Bjermo, H.; Heldring, N.; Buyse, M.; Brize, A. A randomized phase III trial in patients with recurrent platinum sensitive ovarian cancer comparing efficacy and safety of paclitaxel micellar and Cremophor EL-paclitaxel. Gynecol. Oncol. 2019, 156, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; du Bois, A.; Floquet, A.; Rau, J.; Kim, J.-W.; del Campo, J.; Friedlander, M.; Pignata, S.; Fujiwara, K.; Colombo, N.; et al. Overall survival results of AGO-OVAR16: A phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol. Oncol. 2019, 155, 186–191. [Google Scholar] [CrossRef]
- Vergote, I.; Heitz, F.; Buderath, P.; Powell, M.; Sehouli, J.; Lee, C.M.; Hamilton, A.; Fiorica, J.; Moore, K.N.; Teneriello, M.; et al. A randomized, double-blind, placebo-controlled phase 1b/2 study of ralimetinib, a p38 MAPK inhibitor, plus gemcitabine and carboplatin versus gemcitabine and carboplatin for women with recurrent platinum-sensitive ovarian cancer. Gynecol. Oncol. 2020, 156, 23–31. [Google Scholar] [CrossRef]
- Vergote, I.; Scambia, G.; O’Malley, D.M.; Van Calster, B.; Park, S.-Y.; del Campo, J.M.; Meier, W.; Bamias, A.; Colombo, N.; Wenham, R.M.; et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 862–876. [Google Scholar] [CrossRef]
- Vergote, I.B.; Garcia, A.; Micha, J.; Pippitt, C.; Bendell, J.; Spitz, D.; Reed, N.; Dark, G.; Fracasso, P.M.; Ibrahim, E.N.; et al. Randomized Multicenter Phase II Trial Comparing Two Schedules of Etirinotecan Pegol (NKTR-102) in Women with Recurrent Platinum-Resistant/Refractory Epithelial Ovarian Cancer. J. Clin. Oncol. 2013, 31, 4060–4066. [Google Scholar] [CrossRef]
- Vergote, I.B.; Jimeno, A.; Joly, F.; Katsaros, D.; Coens, C.; Despierre, E.; Marth, C.; Hall, M.; Steer, C.; Colombo, N.; et al. Randomized Phase III Study of Erlotinib versus Observation in Patients with No Evidence of Disease Progression after First-Line Platin-Based Chemotherapy for Ovarian Carcinoma: A European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup Study. J. Clin. Oncol. 2014, 32, 320–326. [Google Scholar] [CrossRef]
- Verma, S.; O’Shaughnessy, J.; Burris, H.A.; Campone, M.; Alba, E.; Chandiwana, D.; Dalal, A.A.; Sutradhar, S.; Monaco, M.; Janni, W. Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: Results from MONALEESA-2. Breast Cancer Res. Treat. 2018, 170, 535–545. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Voss, M.H.; Bhatt, R.S.; Vogelzang, N.J.; Fishman, M.; Alter, R.S.; Rini, B.I.; Beck, J.T.; Joshi, M.; Hauke, R.; Atkins, M.B.; et al. A phase 2, randomized trial evaluating the combination of dalantercept plus axitinib in patients with advanced clear cell renal cell carcinoma. Cancer 2019, 125, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Members of Japan Clinical Oncology Group Brain Tumor Study Group (JCOG-BTSG); Natsume, A.; Mizusawa, J.; Katayama, H.; Fukuda, H.; Sumi, M.; Nishikawa, R.; Narita, Y.; Muragaki, Y.; et al. JCOG0911 INTEGRA study: A randomized screening phase II trial of interferonβ plus temozolomide in comparison with temozolomide alone for newly diagnosed glioblastoma. J. Neuro-Oncol. 2018, 138, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fan, W.; Peng, J.; Lu, Z.; Pan, Z.; Li, L.; Gao, Y.; Li, H.; Chen, G.; Wu, X.; et al. Total mesorectal excision with or without preoperative chemoradiotherapy for resectable mid/low rectal cancer: A long-term analysis of a prospective, single-center, randomized trial. Cancer Commun. 2018, 38, 73. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandalà, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, X.; Ye, X.; Feng, Q.; Xu, Y.; Zhang, L.; Sun, W.; Dong, Y.; Meng, Q.; Li, T.; et al. Microwave ablation plus chemotherapy versus chemotherapy in advanced non-small cell lung cancer: A multicenter, randomized, controlled, phase III clinical trial. Eur. Radiol. 2020, 30, 2692–2702. [Google Scholar] [CrossRef]
- Weide, B.; Eigentler, T.; Catania, C.; Ascierto, P.A.; Cascinu, S.; Becker, J.C.; Hauschild, A.; Romanini, A.; Danielli, R.; Dummer, R.; et al. A phase II study of the L19IL2 immunocytokine in combination with dacarbazine in advanced metastatic melanoma patients. Cancer Immunol. Immunother. 2019, 68, 1547–1559. [Google Scholar] [CrossRef]
- Weiss, J.; Csoszi, T.; Maglakelidze, M.; Hoyer, R.; Beck, J.; Gomez, M.D.; Lowczak, A.; Aljumaily, R.; Lima, C.R.; Boccia, R.; et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: A phase Ib/randomized phase II trial. Ann. Oncol. 2019, 30, 1613–1621. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Wildiers, H.; Tryfonidis, K.; Lago, L.D.; Vuylsteke, P.; Curigliano, G.; Waters, S.; Brouwers, B.; Altintas, S.; Touati, N.; Cardoso, F.; et al. Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer (EORTC 75111-10114): An open-label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group. Lancet Oncol. 2018, 19, 323–336. [Google Scholar] [CrossRef]
- Wirth, L.J.; Dakhil, S.; Kornek, G.; Axelrod, R.; Adkins, D.; Pant, S.; O’Brien, P.; Debruyne, P.R.; Oliner, K.S.; Dong, J.; et al. PARTNER: An open-label, randomized, phase 2 study of docetaxel/cisplatin chemotherapy with or without panitumumab as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2016, 61, 31–40. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Wu, S.-X.; Wang, L.-H.; Luo, H.-L.; Xie, C.-Y.; Zhang, X.-B.; Hu, W.; Zheng, A.-P.; Li, D.-J.; Zhang, H.-Y.; Lian, X.-L.; et al. Randomised phase III trial of concurrent chemoradiotherapy with extended nodal irradiation and erlotinib in patients with inoperable oesophageal squamous cell cancer. Eur. J. Cancer 2018, 93, 99–107. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Cheng, Y.; Zhou, J.; Lu, S.; Zhang, Y.; Zhao, J.; Kim, D.-W.; Soo, R.A.; Kim, S.-W.; Pan, H.; et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): An open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir. Med. 2020, 8, 1132–1143. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Chu, D.-T.; Han, B.; Liu, X.; Zhang, L.; Zhou, C.; Liao, M.; Mok, T.; Jiang, H.; Duffield, E.; et al. Phase III, randomized, open-label, first-line study in Asia of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer: Evaluation of patients recruited from mainland China. Asia-Pac. J. Clin. Oncol. 2012, 8, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Lu, S.; Cheng, Y.; Zhou, C.; Wang, J.; Mok, T.; Zhang, L.; Tu, H.-Y.; Wu, L.; Feng, J.; et al. Nivolumab versus Docetaxel in a Predominantly Chinese Patient Population with Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J. Thorac. Oncol. 2019, 14, 867–875. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lu, S.; Lu, Y.; Zhou, J.; Shi, Y.-K.; Sriuranpong, V.; Ho, J.; Ong, C.K.; Tsai, C.-M.; Chung, C.-H.; et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1539–1548. [Google Scholar] [CrossRef]
- Xu, J.; Shen, L.; Zhou, Z.; Li, J.; Bai, C.; Chi, Y.; Li, Z.; Xu, N.; Li, E.; Liu, T.; et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1500–1512. [Google Scholar] [CrossRef]
- Xu, T.; Hu, C.; Zhu, G.; He, X.; Wu, Y.; Ying, H. Preliminary results of a phase III randomized study comparing chemotherapy neoadjuvantly or concurrently with radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Med. Oncol. 2011, 29, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Yachnin, J.; Gilje, B.; Thon, K.; Johansson, H.; Brandberg, Y.; Panaretakis, T.; Ullén, A. Weekly versus 3-weekly cabazitaxel for the treatment of castration-resistant prostate cancer: A randomised phase II trial (ConCab). Eur. J. Cancer 2018, 97, 33–40. [Google Scholar] [CrossRef]
- Yamada, Y.; Denda, T.; Gamoh, M.; Iwanaga, I.; Yuki, S.; Shimodaira, H.; Nakamura, M.; Yamaguchi, T.; Ohori, H.; Kobayashi, K.; et al. S-1 and irinotecan plus bevacizumab versus mFOLFOX6 or CapeOX plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer (TRICOLORE): A randomized, open-label, phase III, noninferiority trial. Ann. Oncol. 2017, 29, 624–631. [Google Scholar] [CrossRef]
- Yamasaki, M.; Yasuda, T.; Yano, M.; Hirao, M.; Kobayashi, K.; Fujitani, K.; Tamura, S.; Kimura, Y.; Miyata, H.; Motoori, M.; et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann. Oncol. 2016, 28, 116–120. [Google Scholar] [CrossRef]
- Yamaue, H.; Tsunoda, T.; Tani, M.; Miyazawa, M.; Yamao, K.; Mizuno, N.; Okusaka, T.; Ueno, H.; Boku, N.; Fukutomi, A.; et al. Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC Study. Cancer Sci. 2015, 106, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Lee, S.J.; Cho, J.; Lee, K.H.; Park, K.U.; Kim, K.H.; Cho, E.K.; Choi, Y.H.; Kim, H.R.; Kim, H.-G.; et al. A Randomized, Open-Label, Phase II Study Comparing Pemetrexed Plus Cisplatin Followed by Maintenance Pemetrexed versus Pemetrexed Alone in Patients with Epidermal Growth Factor Receptor (EGFR)-Mutant Non-small Cell Lung Cancer after Failure of First-Line EGFR Tyrosine Kinase Inhibitor: KCSG-LU12-13. Cancer Res. Treat. 2019, 51, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kim, Y.H.; Sakamori, Y.; Nagai, H.; Ozasa, H.; Kaneda, T.; Yoshioka, H.; Nakagawa, H.; Tomii, K.; Okada, A.; et al. A Randomized Phase II Study of Maintenance Bevacizumab, Pemetrexed or Bevacizumab Plus Pemetrexed for Advanced Non-squamous Non-small Cell Lung Cancer. Anticancer Res. 2020, 40, 2981–2987. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Ishibashi, K.; Koda, K.; Yokomizo, H.; Oda, N.; Oshiro, M.; Kato, H.; Oya, M.; Nakajima, H.; Ooki, S.; et al. A Japanese multicenter phase II study of adjuvant chemotherapy with mFOLFOX6/CAPOX for stage III colon cancer treatment after D2/D3 lymphadenectomy. Surg. Today 2019, 49, 498–506. [Google Scholar] [CrossRef]
- Yoshioka, H.; Shimokawa, M.; Seto, T.; Morita, S.; Yatabe, Y.; Okamoto, I.; Tsurutani, J.; Satouchi, M.; Hirashima, T.; Atagi, S.; et al. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first-line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation-positive non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.; Ellard, S.L.; Hotte, S.J.; Gingerich, J.R.; Joshua, A.M.; Gleave, M.E.; Chi, K.N. A randomized phase 2 study of a HSP27 targeting antisense, apatorsen with prednisone versus prednisone alone, in patients with metastatic castration resistant prostate cancer. Investig. New Drugs 2017, 36, 278–287. [Google Scholar] [CrossRef]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2015, 387, 1405–1414. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Hong, S.; Yang, Y.; Yu, G.; Jia, J.; Peng, P.; Wu, X.; Lin, Q.; Xi, X.; et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: A multicentre, randomised, open-label, phase 3 trial. Lancet 2016, 388, 1883–1892. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, Z.; Min, W.; Sui, X.; Kang, H.; Zhang, Y.; Ren, H.; Wang, X.J. Perioperative versus Preoperative Chemotherapy with Surgery in Patients with Resectable Squamous Cell Carcinoma of Esophagus. J. Thorac. Oncol. 2015, 10, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.-Z.; Chen, K.-N.; Chen, C.; Gu, C.-D.; Wang, J.; Yang, X.-N.; Mao, W.-M.; Wang, Q.; Qiao, G.-B.; Cheng, Y.; et al. Erlotinib versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non–Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. J. Clin. Oncol. 2019, 37, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Bai, Y.; Song, Y.; Luo, H.; Ren, X.; Wang, X.; Shi, B.; Fu, C.; Cheng, Y.; Liu, J.; et al. Anlotinib versus Sunitinib as First-Line Treatment for Metastatic Renal Cell Carcinoma: A Randomized Phase II Clinical Trial. Oncology 2019, 24, e702–e708. [Google Scholar] [CrossRef]
- Zimmer, L.; Livingstone, E.; Hassel, J.C.; Fluck, M.; Eigentler, T.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1558–1568. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef]
- Ezeife, D.A.; Dionne, F.; Fares, A.F.; Cusano, E.L.R.; Fazelzad, R.; Ng, W.; Husereau, D.; Ali, F.; Sit, C.; Stein, B.; et al. Value assessment of oncology drugs using a weighted criterion-based approach. Cancer 2019, 126, 1530–1540. [Google Scholar] [CrossRef]
- Del Paggio, J.C.; Berry, J.S.; Hopman, W.M.; Eisenhauer, E.A.; Prasad, V.; Gyawali, B.; Booth, C.M. Evolution of the randomized clinical trial in the era of precision oncology. JAMA Oncol. 2021, 7, 728–734. [Google Scholar] [CrossRef]
- Unger, J.M.; Barlow, E.W.; Ramsey, S.D.; Leblanc, M.; Blanke, C.D.; Hershman, D.L.; Barlow, W.E. The Scientific Impact of Positive and Negative Phase 3 Cancer Clinical Trials. JAMA Oncol. 2016, 2, 875–881. [Google Scholar] [CrossRef]
- Morris, Z.; Wooding, S.; Grant, J. The answer is 17 years, what is the question: Understanding time lags in translational research. J. R. Soc. Med. 2011, 104, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Ezeife, D.A.; Truong, T.H.; Heng, D.Y.C.; Bourque, S.; Welch, S.A.; Tang, P.A. Comparison of oncology drug approval between Health Canada and the US Food and Drug Administration. Cancer 2015, 121, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Fojo, T.; Mailankody, S. An Appraisal of Clinically Meaningful Outcomes Guidelines for Oncology Clinical Trials. JAMA Oncol. 2016, 2, 1238–1240. [Google Scholar] [CrossRef] [PubMed][Green Version]
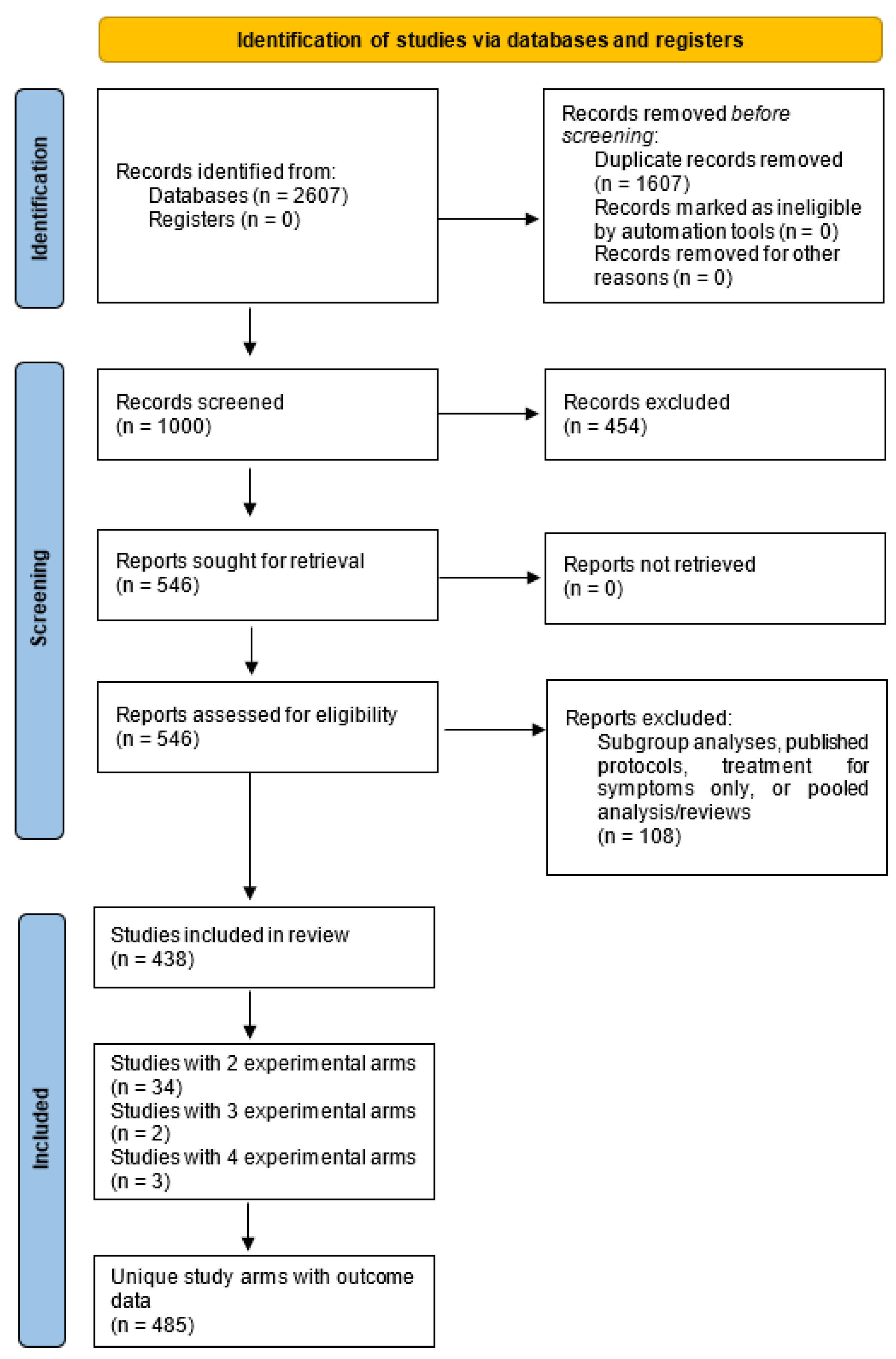
| Characteristic | Pre-Value Frameworks: 2010–2014 (n = 60) | Post-Value Frameworks: 2015–2020 (n = 425) | Total (n = 485) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Primary endpoint 1 | 0.011 a | ||||||
| Overall survival | 9 | (14.5%) | 96 | (20.6%) | 105 | (19.8%) | |
| Progression-free survival | 28 | (45.2%) | 233 | (49.9%) | 261 | (49.3%) | |
| Response rate | 17 | (27.4%) | 48 | (10.3%) | 65 | (12.3%) | |
| Quality of life | 2 | (3.2%) | 31 | (6.6%) | 33 | (6.2%) | |
| Other | 6 | (9.7%) | 59 | (12.6%) | 65 | (12.3%) | |
| Quality of life | 0.745 a | ||||||
| Not reported | 45 | (75%) | 321 | (75.5%) | 366 | (75.5%) | |
| Improved | 3 | (5%) | 35 | (8.24%) | 38 | (7.8%) | |
| Worse | 1 | (1.67%) | 9 | (2.12%) | 10 | (2.1%) | |
| Comparable | 11 | (18.3%) | 60 | (14.1%) | 71 | (14.6%) | |
| Type of therapy | 0.007 a | ||||||
| Immunotherapy/cancer vaccines | 1 | (1.67%) | 72 | (16.9%) | 73 | (15.1%) | |
| Cytotoxic therapy | 21 | (35%) | 109 | (25.6%) | 130 | (26.8%) | |
| Other | 7 | (11.7%) | 43 | (10.1%) | 50 | (10.3%) | |
| Small molecule kinase inhibitor | 22 | (36.7%) | 128 | (30.1%) | 150 | (30.9%) | |
| Targeted monoclonal antibody | 9 | (15%) | 73 | (17.2%) | 82 | (16.9%) | |
| Line of therapy | <0.001 a | ||||||
| First | 27 | (45%) | 205 | (48.2%) | 232 | (47.8%) | |
| Second or more | 32 | (53.3%) | 144 | (33.9%) | 176 | (36.3%) | |
| Any | 1 | (1.67%) | 76 | (17.9%) | 77 | (15.9%) | |
| Disease setting | <0.001 b | ||||||
| Curative | 35 | (58.3%) | 134 | (31.5%) | 169 | (34.8%) | |
| Palliative | 25 | (41.7%) | 291 | (68.5%) | 316 | (65.2%) | |
| Trial design | 0.112 a | ||||||
| Randomized phase 2 | 36 | (60%) | 217 | (51.1%) | 253 | (52.2%) | |
| Randomized phase 3 | 22 | (36.7%) | 203 | (47.8%) | 225 | (46.4%) | |
| Randomized phase 2/3 | 2 | (3.3%) | 5 | (1.2%) | 7 | (1.4%) | |
| Secondary analysis | |||||||
| Yes | 3 | (5.0%) | 61 | (14.4%) | 64 | (13.2%) | 0.043 a |
| No | 57 | (95%) | 364 | (85.6%) | 421 | (86.8%) | |
| Combination therapy | |||||||
| Yes | 38 | (63.3%) | 287 | (67.5%) | 325 | (67%) | 0.558 b |
| No | 22 | (36.7%) | 138 | (32.5%) | 160 | (33%) | |
| Endpoint | Pre-Value Frameworks 2010–2014 | Post-Value Frameworks 2015–2020 | p-Value (WilCoxon) |
|---|---|---|---|
| Overall survival improvement ΔOS, months (n = 232) | Mean: −0.83 | Mean: 1.65 | 0.006 |
| Median (range): −0.2 (−28.4–28.2) | Median (range): 1.2 (−25.6–19.4) | ||
| Progression-free survival improvement ΔPFS, months (n = 284) | Mean: 0.75 | Mean: 2.00 | 0.023 |
| Median (range): 0.2 (−5.99–11.2) | Median (range): 1.4 (−20.7–36.3) | ||
| Response rate improvement, % (n = 242) | Mean: 6.4 | Mean: 9.19 | 0.281 |
| Median (range): 5.0 (−21.0–46.0) | Median (range): 7.4 (−32.7–52.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusano, E.; Wong, C.; Taguedong, E.; Vaska, M.; Abedin, T.; Nixon, N.; Karim, S.; Tang, P.; Heng, D.Y.C.; Ezeife, D. Impact of Value Frameworks on the Magnitude of Clinical Benefit: Evaluating a Decade of Randomized Trials for Systemic Therapy in Solid Malignancies. Curr. Oncol. 2021, 28, 4894-4928. https://doi.org/10.3390/curroncol28060412
Cusano E, Wong C, Taguedong E, Vaska M, Abedin T, Nixon N, Karim S, Tang P, Heng DYC, Ezeife D. Impact of Value Frameworks on the Magnitude of Clinical Benefit: Evaluating a Decade of Randomized Trials for Systemic Therapy in Solid Malignancies. Current Oncology. 2021; 28(6):4894-4928. https://doi.org/10.3390/curroncol28060412
Chicago/Turabian StyleCusano, Ellen, Chelsea Wong, Eddy Taguedong, Marcus Vaska, Tasnima Abedin, Nancy Nixon, Safiya Karim, Patricia Tang, Daniel Y. C. Heng, and Doreen Ezeife. 2021. "Impact of Value Frameworks on the Magnitude of Clinical Benefit: Evaluating a Decade of Randomized Trials for Systemic Therapy in Solid Malignancies" Current Oncology 28, no. 6: 4894-4928. https://doi.org/10.3390/curroncol28060412
APA StyleCusano, E., Wong, C., Taguedong, E., Vaska, M., Abedin, T., Nixon, N., Karim, S., Tang, P., Heng, D. Y. C., & Ezeife, D. (2021). Impact of Value Frameworks on the Magnitude of Clinical Benefit: Evaluating a Decade of Randomized Trials for Systemic Therapy in Solid Malignancies. Current Oncology, 28(6), 4894-4928. https://doi.org/10.3390/curroncol28060412





