Novel Therapeutic Method for Unresectable Pancreatic Cancer—The Impact of the Long-Term Research in Therapeutic Effect of High-Intensity Focused Ultrasound (HIFU) Therapy
Abstract
:1. Introduction
2. Results
2.1. General and Clinical Data
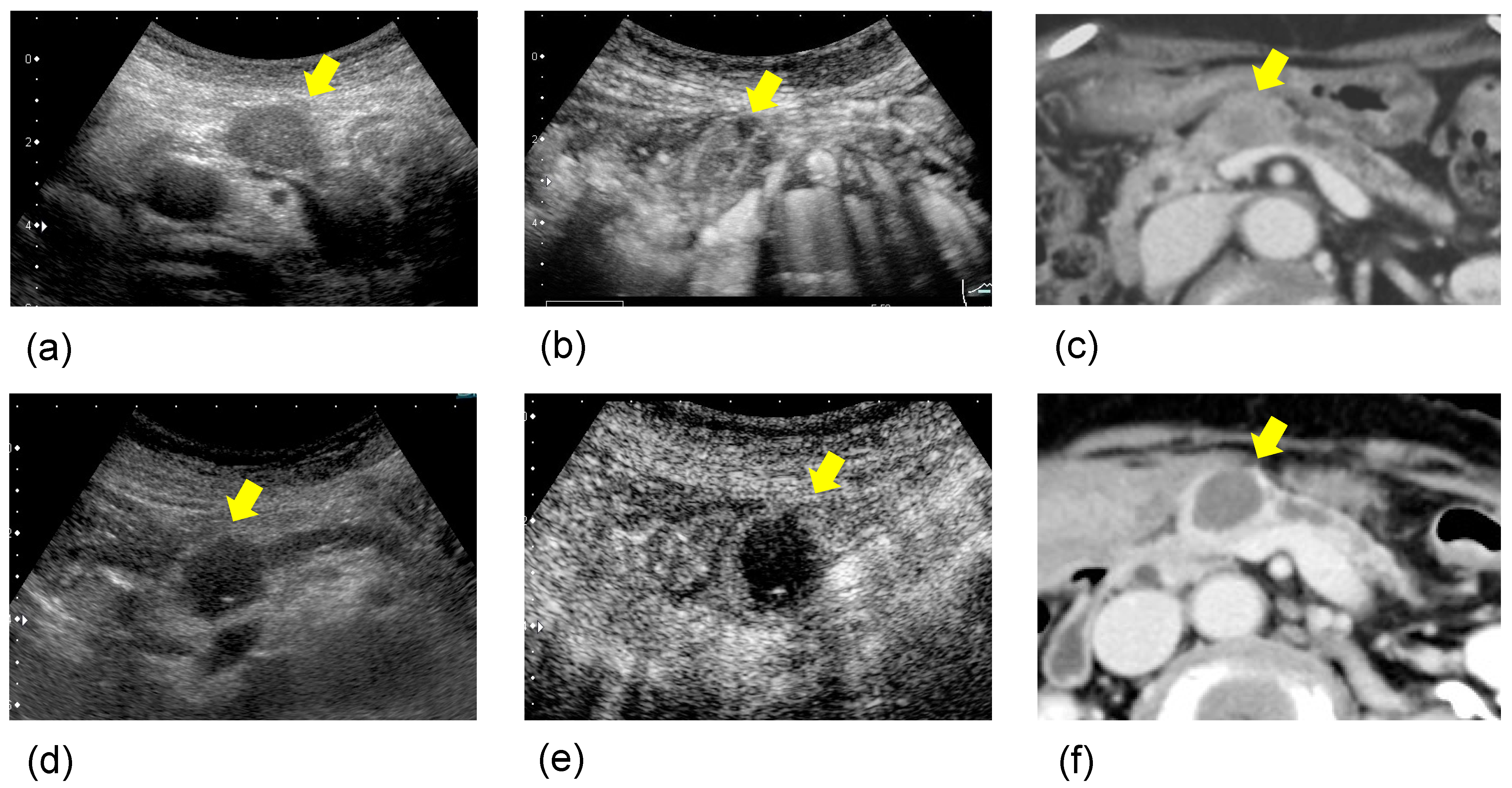
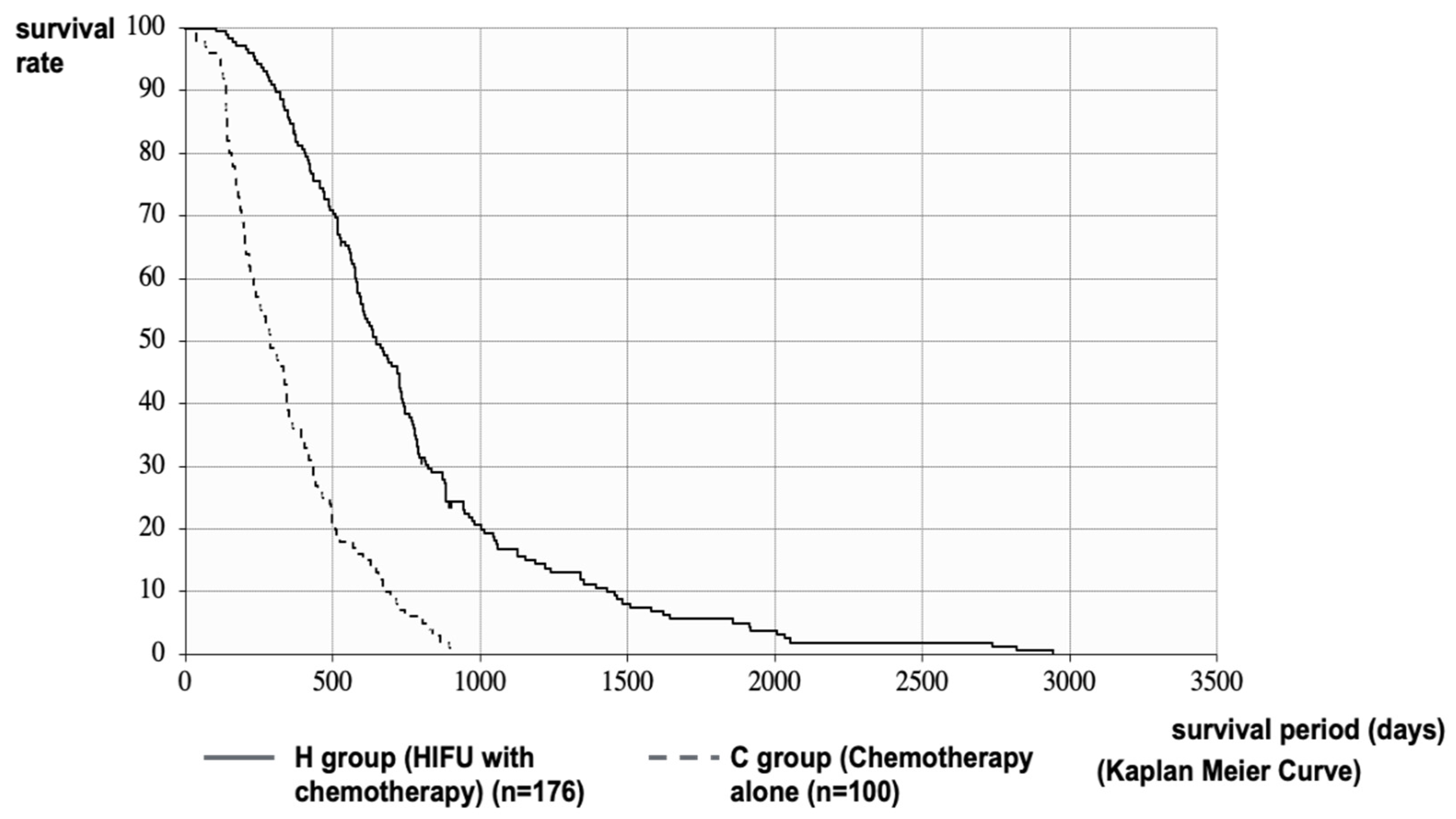
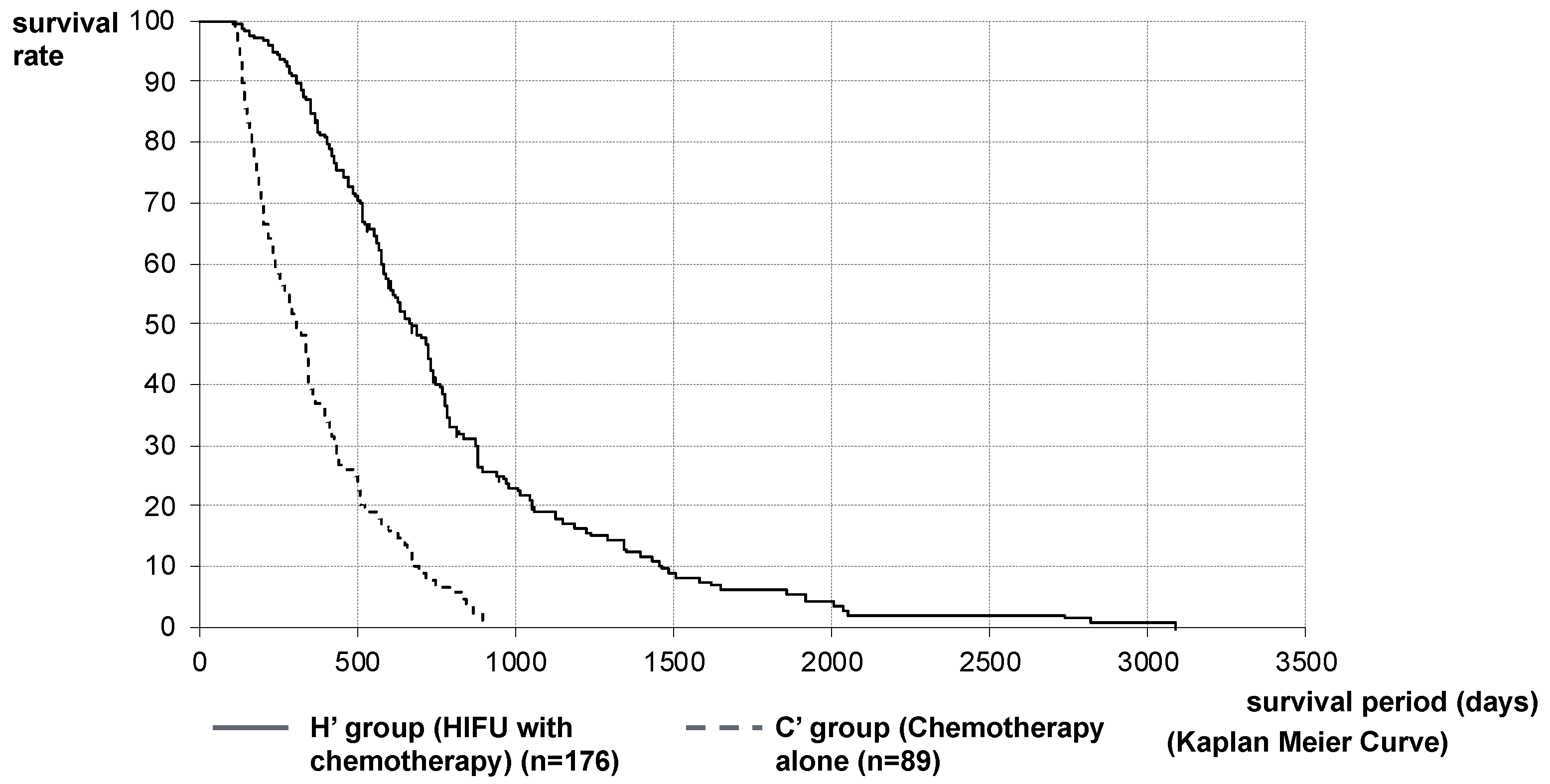
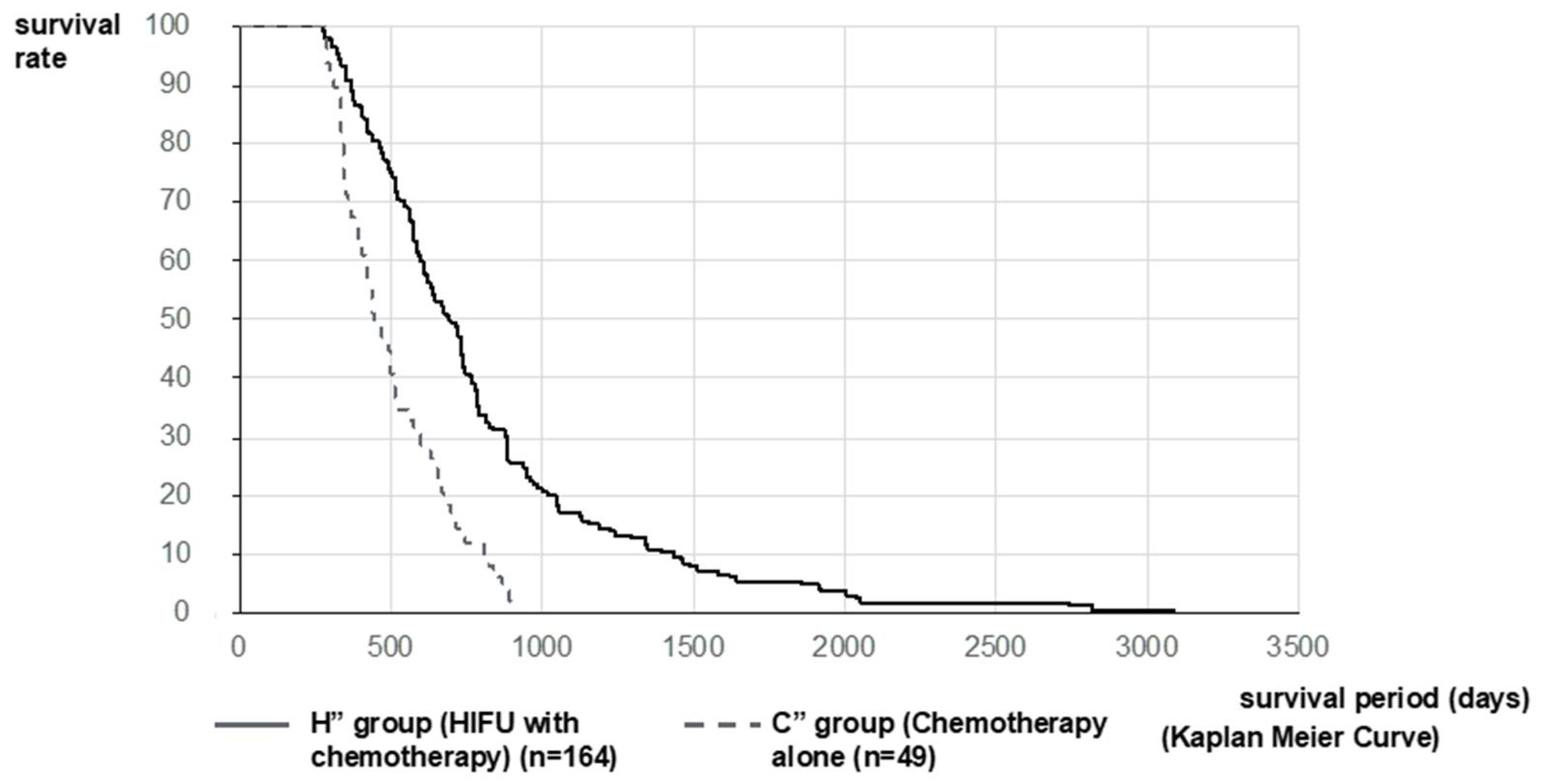
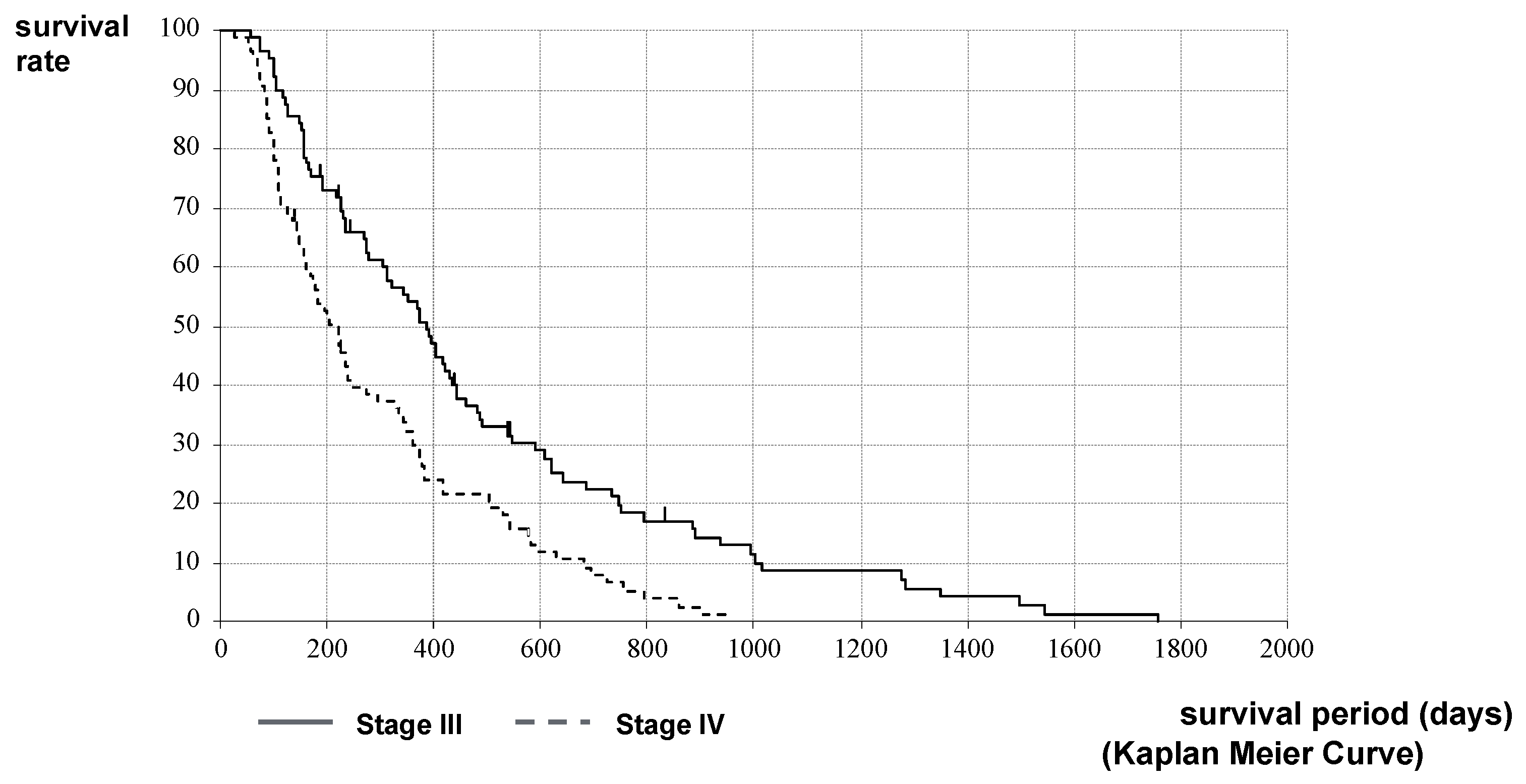
2.2. Therapeutic Effect of HIFU Therapy
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Inclusion Criteria of the H Group
4.1.2. Exclusion Criteria of the H Group
4.1.3. Inclusion Criteria of the C Group
4.1.4. Exclusion Criteria of the C Group
4.2. Methods
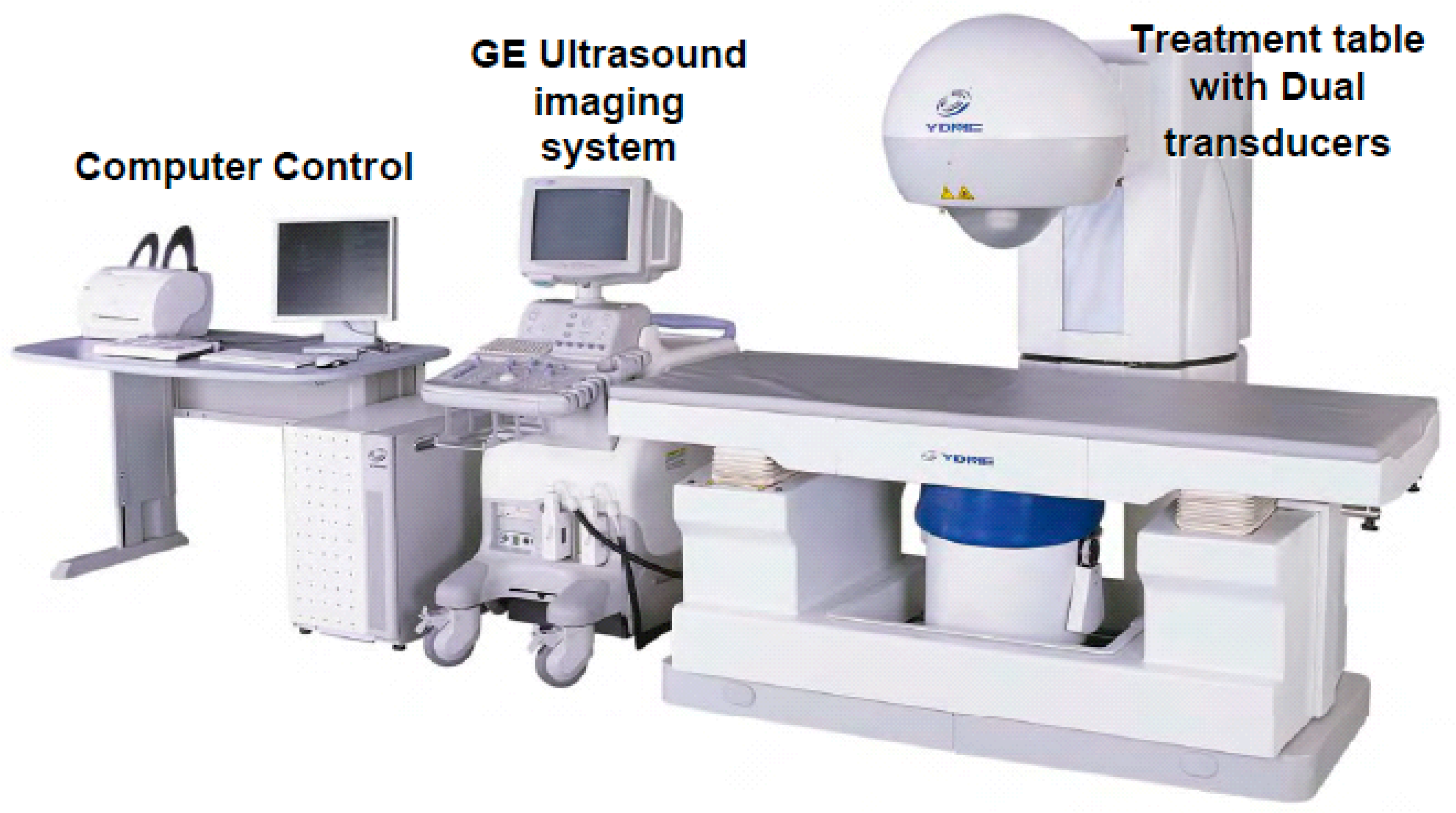
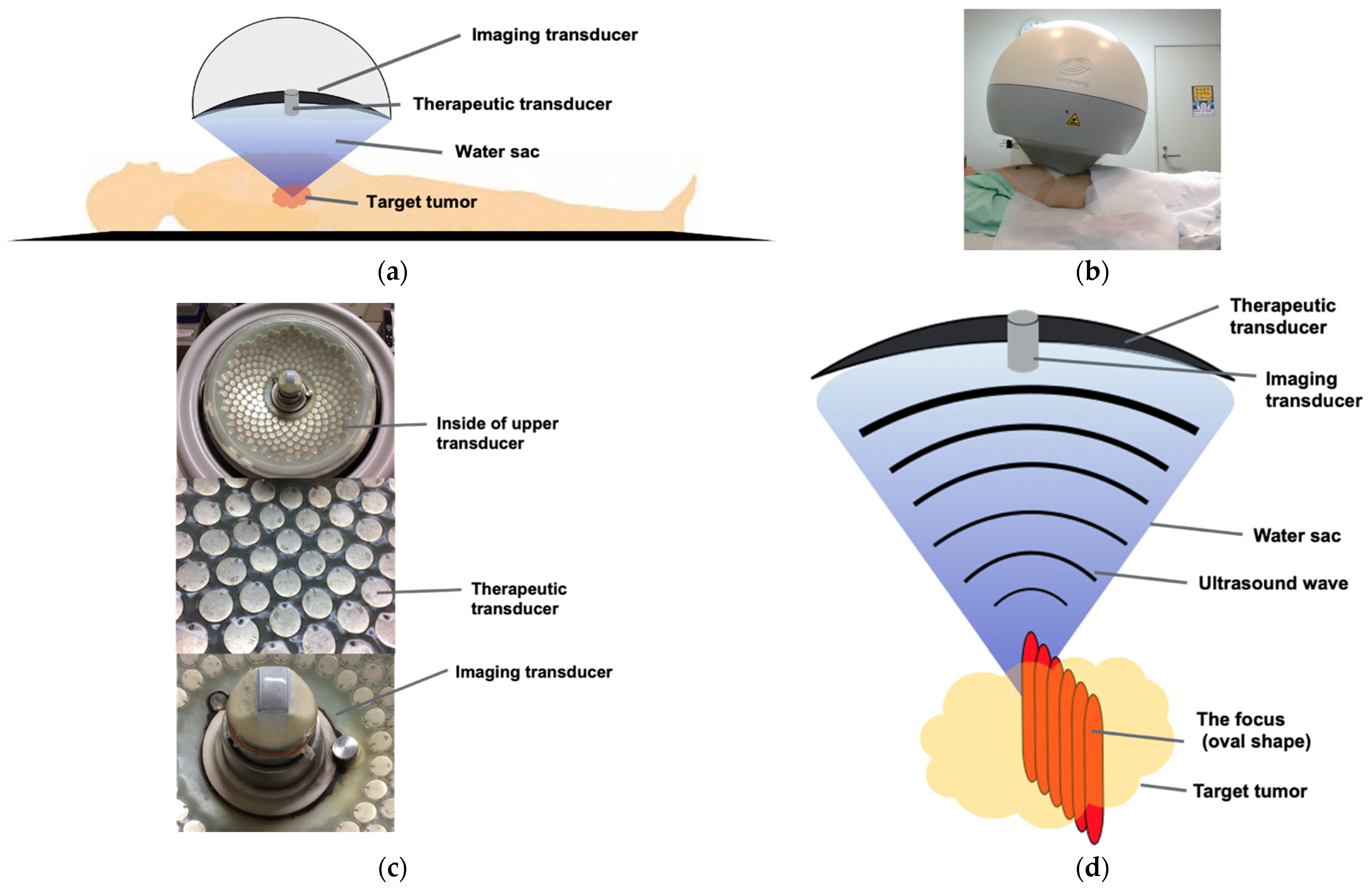
4.3. Devices
4.4. Definition
4.5. Outcome
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. World Health Report; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Sofuni, A.; Moriyasu, F.; Sano, T.; Yamada, K.; Itokawa, F.; Tsuchiya, T.; Tsuji, S.; Kurihara, T.; Ishii, K.; Itoi, T. The Current Potential of High-Intnsity Focused Ultrasound for Pancreatic Carcinoma. J. Hepatobil. Pancreat. Sci. 2011, 18, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, T.A.; Kennedy, J.E. High intensity focused ultrasound in the treatment of abdominal and gynaecological diseases. Int. J. Hyperth. 2007, 23, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Sofuni, A.; Moriyasu, F.; Sano, T.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R.; et al. Safety Trial of High-Intensity Focused Ultrasound Therapy for Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 9570–9577. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J. High-Intensity Focused Ultrasound in Patients With Late-Stage Pancreatic Carcinoma. Chin. Med. J. (Engl.) 2002, 115, 1332–1335. [Google Scholar] [PubMed]
- Wu, F.; Wang, Z.B.; Zhu, H.; Chen, W.Z.; Zou, J.Z.; Bai, J.; Li, K.Q.; Jin, C.B.; Xie, F.L.; Su, H.B. Feasibility of US-Guided High-Intensity Focused Ultrasound Treatment in Patients With Advanced Pancreatic Cancer: Initial Experience. Radiology 2005, 236, 1034–1040. [Google Scholar] [CrossRef]
- Dubinsky, T.J.; Cuevas, C.; Dighe, M.K.; Kolokythas, O.; Hwang, J.H. High-Intensity Focused Ultrasound: Current Potential and Oncologic Applications. Am. J. Roentgenol. 2008, 190, 191–199. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, G.; Wang, D.; Yu, X.; Zhang, Y.; Zhu, J.; Ji, Y.; Zhong, B.; Zhao, W.; Yang, Z.; et al. Concurrent Gemcitabine and High-Intensity Focused Ultrasound Therapy in Patients With Locally Advanced Pancreatic Cancer. Anticancer Drugs 2010, 21, 447–452. [Google Scholar] [CrossRef]
- Jung, S.E.; Cho, S.H.; Jang, J.H.; Han, J.Y. High-Intensity Focused Ultrasound Ablation in Hepatic and Pancreatic Cancer: Complications. Abdom. Imaging 2011, 36, 185–195. [Google Scholar] [CrossRef]
- Orsi, F.; Zhang, L.; Arnone, P.; Orgera, G.; Bonomo, G.; Vigna, P.D.; Monfardini, L.; Zhou, K.; Chen, W.; Wang, Z.; et al. High-Intensity Focused Ultrasound Ablation: Effective and Safe Therapy for Solid Tumors in Difficult Locations. Am. J. Roentgenol. 2010, 195, W245–W252. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Chen, Z.; Meng, Z.; Lin, J.; Zhou, Z.; Wang, P.; Chen, L.; Liu, L. Analgesic Effect of High Intensity Focused Ultrasound Therapy for Unresectable Pancreatic Cancer. Int. J. Hyperth. 2011, 27, 101–107. [Google Scholar] [CrossRef]
- Orsi, F.; Arnone, P.; Chen, W.; Zhang, L. High Intensity Focused Ultrasound Ablation: A New Therapeutic Option for Solid Tumors. J. Cancer Res. Ther. 2010, 6, 414–420. [Google Scholar] [PubMed]
- Orgera, G.; Monfardini, L.; Della Vigna, P.; Zhang, L.; Bonomo, G.; Arnone, P.; Padrenostro, M.; Orsi, F. High-Intensity Focused Ultrasound (HIFU) in Patients With Solid Malignancies: Evaluation of Feasibility, Local Tumour Response and Clinical Results. Radiol. Med. 2011, 116, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shen, H.; Hu, X.Y.; Gu, F.Y.; Li, M.D.; Zhong, X. Multidisciplinary Treatment With Chemotherapy, Targeted Drug, and High-Intensity Focused Ultrasound in Advanced Pancreatic Carcinoma. Med. Oncol. 2012, 29, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, B.I.; Ryu, J.K.; Kim, Y.T.; Hwang, J.H.; Kim, S.H.; Han, J.K. Concurrent Chemotherapy and Pulsed High-Intensity Focused Ultrasound Therapy for the Treatment of Unresectable Pancreatic Cancer: Initial Experiences. Korean J. Radiol. 2011, 12, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Meng, Z.; Chen, Z.; Wang, K.; Yu, B.; Zhan, H.; Liu, L. Metastatic Adenocarcinoma of the Epididymis From Pancreatic Cancer Successfully Treated by Chemotherapy and High-Intensity Focused Ultrasound Therapy: A Case Report and Review of the Literature. Pancreas 2011, 40, 1160–1162. [Google Scholar] [CrossRef]
- Sung, H.Y.; Jung, S.E.; Cho, S.H.; Zhou, K.; Han, J.Y.; Han, S.T.; Kim, J.I.; Kim, J.K.; Choi, J.Y.; Yoon, S.K.; et al. Long-Term Outcome of High-Intensity Focused Ultrasound in Advanced Pancreatic Cancer. Pancreas 2011, 40, 1080–1086. [Google Scholar] [CrossRef]
- Khokhlova, T.D.; Hwang, J.H. HIFU for Palliative Treatment of Pancreatic Cancer. J. Gastrointest. Oncol. 2011, 2, 175–184. [Google Scholar]
- Wang, K.; Chen, L.; Meng, Z.; Lin, J.; Zhou, Z.; Wang, P.; Chen, Z. High Intensity Focused Ultrasound Treatment for Patients With Advanced Pancreatic Cancer: A Preliminary Dosimetric Analysis. Int. J. Hyperth. 2012, 28, 645–652. [Google Scholar] [CrossRef]
- Li, P.Z.; Zhu, S.H.; He, W.; Zhu, L.Y.; Liu, S.P.; Liu, Y.; Wang, G.H.; Ye, F. High-Intensity Focused Ultrasound Treatment for Patients With Unresectable Pancreatic Cancer. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 655–660. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, H.; Meng, Z.; Chen, Z.; Lin, J.; Shen, Y.; Gao, H. Safety Evaluation of High-Intensity Focused Ultrasound in Patients With Pancreatic Cancer. Onkologie 2013, 36, 88–92. [Google Scholar] [CrossRef]
- Ge, H.Y.; Miao, L.Y.; Wang, J.R.; Xiong, L.L.; Yan, F.; Zheng, C.S.; Jia, J.W.; Cui, L.G.; Chen, W. Correlation Between Ultrasound Reflection Intensity and Tumor Ablation Ratio of Late-Stage Pancreatic Carcinoma in HIFU Therapy: Dynamic Observation on Ultrasound Reflection Intensity. Sci. World J. 2013, 2013, 852874. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.F.; Wang, K.; Meng, Z.Q.; Chen, Z.; Lin, J.H.; Zhou, Z.H.; Wang, P.; Shi, W.D.; Sheng, Y.H. High Intensity Focused Ultrasound Treatment for Patients With Local Advanced Pancreatic Cancer. Hepatogastroenterology 2013, 60, 1906–1910. [Google Scholar]
- Xiaoping, L.; Leizhen, Z. Advances of High Intensity Focused Ultrasound (HIFU) for Pancreatic Cancer. Int. J. Hyperth. 2013, 29, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.G.; Bramis, K.; Pereira, S.P.; Fusai, G.K. Systematic Review of Novel Ablative Methods in Locally Advanced Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. High-Intensity Focused Ultrasound Treatment for Advanced Pancreatic Cancer. Gastroenterol. Res. Pract. 2014, 2014, 205325. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.Y.; Miao, L.Y.; Xiong, L.L.; Yan, F.; Zheng, C.S.; Wang, J.R.; Jia, J.W.; Cui, L.G.; Chen, W. High-Intensity Focused Ultrasound Treatment of Late-Stage Pancreatic Body Carcinoma: Optimal Tumor Depth for Safe Ablation. Ultrasound Med. Biol. 2014, 40, 947–955. [Google Scholar] [CrossRef]
- Wu, F. High Intensity Focused Ultrasound: A Noninvasive Therapy for Locally Advanced Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 16480–16488. [Google Scholar] [CrossRef]
- Li, C.C.; Wang, Y.Q.; Li, Y.P.; Li, X.L. High-Intensity Focused Ultrasound for Treatment of Pancreatic Cancer: A Systematic Review. J. Evid. Based Med. 2014, 7, 270–281. [Google Scholar] [CrossRef]
- Rombouts, S.J.; Vogel, J.A.; van Santvoort, H.C.; van Lienden, K.P.; van Hillegersberg, R.; Busch, O.R.; Besselink, M.G.; Molenaar, I.Q. Systematic Review of Innovative Ablative Therapies for the Treatment of Locally Advanced Pancreatic Cancer. Br. J. Surg. 2015, 102, 182–193. [Google Scholar] [CrossRef]
- Dimitrov, D.; Andreev, T.; Feradova, H.; Ignatov, B.; Zhou, K.; Johnson, C.; Delijski, T.; Gortchev, G.; Tomov, S. Multimodality Treatment by FOLFOX Plus HIFU in a Case of Advanced Pancreatic Carcinoma. A Case Report. JOP 2015, 16, 66–69. [Google Scholar]
- Luo, J.; Ren, X.; Yu, T. Efficacy of Extracorporeal Ultrasound-Guided High Intensity Focused Ultrasound: An Evaluation Based on Controlled Trials in China. Int. J. Radiat. Biol. 2015, 91, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Jove, J.; Perich, E.; Del Castillo, M.A. Ultrasound Guided High Intensity Focused Ultrasound for Malignant Tumors: The Spanish Experience of Survival Advantage in stage III and IV Pancreatic Cancer. Ultrason. Sonochem. 2015, 27, 703–706. [Google Scholar] [CrossRef]
- Shi, Y.; Ying, X.; Hu, X.; Zhao, J.; Fang, X.; Wu, M.; Chen, T.Z.; Shen, H. Influence of High Intensity Focused Ultrasound (HIFU) Treatment to the Pancreatic Function in Pancreatic Cancer Patients. Pak. J. Pharm. Sci. 2015, 28 (Suppl. 3), 1097–1100. [Google Scholar] [PubMed]
- Wang, G.; Zhou, D. Preoperative Ultrasound Ablation for Borderline Resectable Pancreatic Cancer: A Report of 30 Cases. Ultrason. Sonochem. 2015, 27, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, T.D.; Hwang, J.H. HIFU for Palliative Treatment of Pancreatic Cancer. Adv. Exp. Med. Biol. 2016, 880, 83–95. [Google Scholar]
- Copelan, A.; Hartman, J.; Chehab, M.; Venkatesan, A.M. High-Intensity Focused Ultrasound: Current Status for Image-Guided Therapy. Semin. Intervent. Radiol. 2015, 32, 398–415. [Google Scholar] [CrossRef] [Green Version]
- Marinova, M.; Rauch, M.; Mücke, M.; Rolke, R.; Gonzalez-Carmona, M.A.; Henseler, J.; Cuhls, H.; Radbruch, L.; Strassburg, C.P.; Zhang, L.; et al. High-Intensity Focused Ultrasound (HIFU) for Pancreatic Carcinoma: Evaluation of Feasibility, Reduction of Tumour Volume and Pain Intensity. Eur. Radiol. 2016, 26, 4047–4056. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Huang, G.L.; Sun, X.L.; Zhao, X.C.; Li, Z.G. The Combination Therapy of High-Intensity Focused Ultrasound With Radiotherapy in Locally Advanced Pancreatic Carcinoma. World J. Surg. Oncol. 2016, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, K.; Zheng, L.; Meng, Z. Retrospective Analysis of High Intensity Focused Ultrasound Combined With S-1 in the Treatment of Metastatic Pancreatic Cancer After Failure of Gemcitabine. Am. J. Cancer Res. 2016, 6, 84–90. [Google Scholar] [PubMed]
- Strunk, H.M.; Henseler, J.; Rauch, M.; Mücke, M.; Kukuk, G.; Cuhls, H.; Radbruch, L.; Zhang, L.; Schild, H.H.; Marinova, M. Clinical Use of High-Intensity Focused Ultrasound (HIFU) for Tumor and Pain Reduction in Advanced Pancreatic Cancer. RoFo 2016, 188, 662–670. [Google Scholar] [CrossRef] [Green Version]
- Ning, Z.Y.; Cheng, C.S.; Xie, J.; Chen, Q.W.; Xu, L.T.; Zhuang, L.P.; Zhang, C.Y.; Song, L.B.; Shi, W.D.; Zhu, X.Y.; et al. A Retrospective Analysis of Survival Factors of High Intensity Focused Ultrasound (HIFU) Treatment for Unresectable Pancreatic Cancer. Discov. Med. 2016, 21, 435–445. [Google Scholar] [PubMed]
- Niu, S.; Cheng, L.; Qiao, Y.; Fu, Y.F.; Cao, C. Combined Stent Insertion and High-Intensity Focused Ultrasound Ablation for Patients With Malignant Obstructive Jaundice. Surg. Laparosc. Endosc. Percutan. Tech. 2016, 26, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Dababou, S.; Marrocchio, C.; Rosenberg, J.; Bitton, R.; Pauly, K.B.; Napoli, A.; Hwang, J.H.; Ghanouni, P. A Meta-Analysis of Palliative Treatment of Pancreatic Cancer With High Intensity Focused Ultrasound. J. Ther. Ultrasound 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Zhao, F.; Shi, Y.; Deng, Y.; Hu, X.; Shen, H. The Efficacy of a New High Intensity Focused Ultrasound Therapy for Locally Advanced Pancreatic Cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 2105–2111. [Google Scholar] [CrossRef]
- Saccomandi, P.; Lapergola, A.; Longo, F.; Schena, E.; Quero, G. Thermal Ablation of Pancreatic Cancer: A Systematic Literature Review of Clinical Practice and Pre-Clinical Studies. Int. J. Hyperth. 2018, 35, 398–418. [Google Scholar] [CrossRef]
- Strunk, H.M.; Lützow, C.; Henseler, J.; Mücke, M.; Rauch, M.; Marx, C.; Schild, H.H.; Marinova, M. Mesenteric Vessel Patency Following HIFU Therapy in Patients With Locally Invasive Pancreatic Cancer. Ultraschall Med. 2018, 39, 650–658. [Google Scholar] [CrossRef]
- Marinova, M.; Huxold, H.C.; Henseler, J.; Mücke, M.; Conrad, R.; Rolke, R.; Ahmadzadehfar, H.; Rauch, M.; Fimmers, R.; Luechters, G.; et al. Clinical Effectiveness and Potential Survival Benefit of US-Guided High-Intensity Focused Ultrasound Therapy in Patients With Advanced-Stage Pancreatic Cancer. Ultraschall Med. 2019, 40, 625–637. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Zhu, J.; Zhu, L.; Zhu, Y.; Hu, K.; Zhao, H. Response of Patients With Locally Advanced Pancreatic Adenocarcinoma to High-Intensity Focused Ultrasound Treatment: A Single-Center, Prospective, Case Series in China. Cancer Manag. Res. 2018, 10, 4439–4446. [Google Scholar] [CrossRef] [Green Version]
- Marinova, M.; Wilhelm-Buchstab, T.; Strunk, H. Advanced Pancreatic Cancer: High-Intensity Focused Ultrasound (HIFU) and Other Local Ablative Therapies. RoFo 2019, 191, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Ning, Z.; Xie, J.; Chen, Q.; Zhang, C.; Xu, L.; Song, L.; Meng, Z. HIFU Is Safe, Effective, and Feasible in Pancreatic Cancer Patients: A Monocentric Retrospective Study Among 523 Patients. OncoTargets Ther. 2019, 12, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Li, J.; Diao, L.; Ma, K.; Fan, Y.; Yang, W. High-Intensity Focused Ultrasound Ablation for Advanced Pancreatic Cancer. J. Cancer Res. Ther. 2019, 15, 831–835. [Google Scholar]
- Tao, S.F.; Gu, W.H.; Gu, J.C.; Zhu, M.L.; Wang, Q.; Zheng, L.Z. A Retrospective Case Series of High-Intensity Focused Ultrasound (HIFU) in Combination With Gemcitabine and Oxaliplatin (Gemox) on Treating Elderly Middle and Advanced Pancreatic Cancer. Oncotargets Ther. 2019, 12, 9735–9745. [Google Scholar] [CrossRef] [Green Version]
- Sofuni, A.; Fujita, M.; Asai, Y.; Tsuchiya, T.; Ishii, K.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Nagai, K.; et al. A Case of Unresectable Pancreatic Cancer With Long-Term Survival in High-Intensity Focused Ultrasound (HIFU) Therapy. Ultrasound Int. Open 2019, 5, E89–E92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; Liu, F.; Liu, Y.; Xia, F.F.; Fu, Y.F. Stent Insertion With High-Intensity Focused Ultrasound Ablation for Distal Biliary Obstruction Secondary to Pancreatic Carcinoma. Medicine 2020, 99, e19099. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.; Stanislavova, N.; Yotsov, T.; Zhou, K. Recurrent Pancreatic Cancer Patient Treated by Chemotherapy and Focused Ultrasound Surgery. A Case Report. Med. Ultrason. 2020, 22, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, H.; Zhou, K.; Jin, C.; Yang, Y.; Zhang, J.; Yang, W.; Ran, L.; Dimitrov, D.D. Effects of High-Intensity Focused Ultrasound Treatment on Peripancreatic Arterial and Venous Blood Vessels in Pancreatic Cancer. Oncol. Lett. 2020, 19, 3839–3850. [Google Scholar] [CrossRef] [Green Version]
- Thudium, M.; Bette, B.; Tonguc, T.; Ghaei, S.; Conrad, R.; Becher, M.U.; Mücke, M.; Luechters, G.; Strunk, H.; Marinova, M. Multidisciplinary Management and Outcome in Pancreatic Cancer Patients Treated With High-Intensity Focused Ultrasound. Int. J. Hyperth. 2020, 37, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Japanese Society of Ultrasonics, Instrument and Safety Committee. Materials Related to the Safety of Ultrasonic Diagnostic Equipment; Japanese Society of Ultrasonics: Tokyo, Japan, 2009; pp. 4–28. [Google Scholar]
- World Federation for Ultrasound in Medicine and Biology (WFUMB). Synopsis. In Proceedings of the WFUMB Symposium on Safety and Standardisation in Medical Ultrasound, Hornbaek, Denmark, 30 August–1 September 1991.
- Hwang, J.H.; Wang, Y.N.; Warren, C.; Upton, M.P.; Starr, F.; Zhou, Y.; Mitchell, S.B. Preclinical In Vivo Evaluation of an Extracorporeal HIFU Device for Ablation of Pancreatic Tumors. Ultrasound Med. Biol. 2009, 35, 967–975. [Google Scholar] [CrossRef]
- Xie, B.; Li, Y.Y.; Jia, L.; Nie, Y.Q.; Du, H.; Jiang, S.M. Experimental Ablation of the Pancreas With High Intensity Focused Ultrasound (HIFU) in a Porcine Model. Int. J. Med. Sci. 2010, 8, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Hu, B.; Guo, Q.; Chen, L. Treatment of Pancreatic Cancer in a Nude Mouse Model Using High-Intensity Focused Ultrasound. Exp. Ther. Med. 2013, 5, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Hassanuddin, A.; Choi, J.H.; Seo, D.W.; Ryu, C.H.; Kim, S.H.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Factors Affecting Tumor Ablation During High Intensity Focused Ultrasound Treatment. Gut Liver 2014, 8, 433–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupré, A.; Melodelima, D.; Pflieger, H.; Chen, Y.; Vincenot, J.; Kocot, A.; Langonnet, S.; Rivoire, M. Thermal Ablation of the Pancreas With Intraoperative High-Intensity Focused Ultrasound: Safety and Efficacy in a Porcine Model. Pancreas 2017, 46, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.S.; Liu, L.X.; Gu, Y.H.; Lin, Q.F.; Guo, R.H.; Shu, Y.Q. The Effect of Endostatin and Gemcitabine Combined With HIFU on the Animal Xenograft Model of Human Pancreatic Cancer. Biomed. Pharmacother. 2010, 64, 309–312. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, J.Y.; Kim, H.; Choi, Y.; Park, J.; Han, J.K.; Choi, B.I. Pulsed High-Intensity Focused Ultrasound Enhances Apoptosis of Pancreatic Cancer Xenograft With Gemcitabine. Ultrasound Med. Biol. 2013, 39, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.; Kim, Y.J.; Lee, J.Y.; Han, J.K.; Choi, B.I. Dynamic Contrast-Enhanced Ultrasonographic (DCE-US) Assessment of the Early Response After Combined Gemcitabine and HIFU With Low-Power Treatment for the Mouse Xenograft Model of Human Pancreatic Cancer. Eur. Radiol. 2014, 24, 2059–2068. [Google Scholar] [CrossRef]
- Li, T.; Chen, H.; Khokhlova, T.; Wang, Y.N.; Kreider, W.; He, X.; Hwang, J.H. Passive Cavitation Detection During Pulsed HIFU Exposures of Ex Vivo Tissues and In Vivo Mouse Pancreatic Tumors. Ultrasound Med. Biol. 2014, 40, 1523–1534. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.H.; Lee, J.Y.; Kim, H.R.; Kim, B.R.; Park, E.J.; Kim, H.S.; Han, J.K.; Choi, B.I. Therapeutic Effects of Microbubbles Added to Combined High-Intensity Focused Ultrasound and Chemotherapy in a Pancreatic Cancer Xenograft Model. Korean J. Radiol. 2016, 17, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Horise, Y.; Maeda, M.; Konishi, Y.; Okamoto, J.; Ikuta, S.; Okamoto, Y.; Ishii, H.; Yoshizawa, S.; Umemura, S.; Ueyama, T.; et al. Sonodynamic Therapy With Anticancer Micelles and High-Intensity Focused Ultrasound in Treatment of Canine Cancer. Front. Pharmacol. 2019, 10, 545. [Google Scholar] [CrossRef]
| Total | Stage III | Stage IV | p Value | |
|---|---|---|---|---|
| Total number of treatment cases | 332 | 158 | 174 | N/A |
| Number of new cases | 176 | 89 | 87 | N/A |
| Number of only one time of treatment cases | 93 | 44 | 49 | p = 0.360 |
| Number of repeated treatment cases | 83 | 45 | 38 | p = 0.360 |
| Mean number of repeated treatments | 2.94 (2–12) | 3.01 (2–12) | 3.32 (2–9) | p = 0.293 |
| Mean treatment sessions (times) | 2.0 (1–5) | 2.2 (1–5) | 1.9 (1–4) | p = 0.026 # |
| Mean total treatment time (min) | 89.4 (15–600) | 96.3 (15–280) | 82.2 (15–600) | p = 0.162 |
| Mean total number of irradiation (shots/1 session) | 1709.6 (280–6420) | 1905 (280–6420) | 1510 (280–4104) | p = 0.018 # |
| Rate of given anesthesia (sedation) (%) | 0 (0/176) | 0 (0/89) | 0 (0/87) | N/A |
| Rate of given pain-killer (%) | 0.6 (1/176) | 0 (0/89) | 1.1 (1/87) | p = 0.310 |
| Adverse event (%) | 2.8 (5/176) | 2.2 (2 */89) | 3.4 (3 **/87) | p = 0.632 |
| Total | Stage III | Stage IV | p Value | |
|---|---|---|---|---|
| Mean tumor size; before therapy | 33.3 (±10.9) | 33.0 (±10.2) | 33.5 (±11.6) | p = 0.738 |
| after therapy | 33.5 (±11.3) | 33.5 (±11.3) | 34.0 (±12.2) | p = 0.790 |
| The rate of complete tumor ablation (%) | 90.3 | 92.1 | 88.5 | p = 0.469 |
| The rate of symptom relief effect (%) | 66.7 | 64.4 | 44.9 | p = 0.029 * |
| Rate of no worsening change of CA19-9 level (%) | 48.1 | 63.2 | 34.9 | p = 0.011 * |
| The effectiveness of primary lesion (CR/PR/SD/PD) | 0/21/106/49 | 0/12/60/17 | 0/9/46/32 | N/A |
| Primary disease control rate (DCR) (%) | 72.2 | 80.9 | 63.2 | p = 0.009 * |
| Therapy after HIFU (operation/chemotherapy/arterial infusion chemotherapy/ radiation/immunotherapy/BSC) | 8/149/1/0/4/22 | 7/74/0/0/1/12 | 1/75/1/0/3/10 | N/A |
| Total (n = 176) | Stage III (n = 89) | Stage IV (n = 87) | |
|---|---|---|---|
| Age (range) | 64 (22–92) | 66 (22–92) | 63 (28–87) |
| Sex (male/female) | 90/86 | 41/48 | 49/38 |
| Performance status (0/1/2/3/4) | 85/87/4/0/0 | 49/39/1/0/0 | 36/48/3/0/0 |
| Location | |||
| (head/uncus/body/ | 49/21/73/ | 28/15/39/ | 21/6/34/ |
| body-tail/tail/others *) | 7/4/22 | 6/1/0 | 1/3/22 |
| Chemotherapy regimen | (n = 165) (%) | (n = 83) (%) | (n = 82) (%) |
| GEM | 90 (54.5) | 52 (62.7) | 38 (46.3) |
| S-1 | 28 (17.0) | 9 (10.8) | 19 (23.2) |
| GEM + S-1 | 20 (12.1) | 11 (13.3) | 9 (11.0) |
| GEM + nab-PTX | 22 (13.3) | 9 (10.8) | 13 (15.9) |
| m FOLFIRINOX | 5 (3.0) | 2 (2.4) | 3 (3.7) |
| Total (n = 100) | Stage III (n = 49) | Stage IV (n = 51) | |
|---|---|---|---|
| Age (range) | 63 (45–75) | 63 (45–74) | 64 (46–75) |
| Sex (male/female) | 57/43 | 27/25 | 30/18 |
| Performance status (0/1/2/3/4) | 35/31/23/11/0 | 20/18/11/3/0 | 15/13/12/8/0 |
| Location | |||
| (head/uncus/body/ | 46/7/27/ | 22/3/17/ | 24/4/10/ |
| body-tail/tail/others *) | 5/14/1 | 2/5/0 | 3/9/1 |
| Chemotherapy regimen | (n = 100) (%) | (n = 49) (%) | (n = 51) (%) |
| GEM | 66 (66.0) | 36 (73.5) | 30 (58.8) |
| S-1 | 10 (10.0) | 5 (10.2) | 5 (9.8) |
| GEM + S-1 | 18 (19.0) | 7 (14.3) | 11 (21.5) |
| GEM + erlotinib | 1 (1.0) | 0 (0) | 1 (2.0) |
| GEM + nab-PTX | 4 (4.0) | 1 (2.0) | 3 (5.9) |
| m FOLFIRINOX | 1 (1.0) | 0 (0) | 1 (2.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sofuni, A.; Asai, Y.; Tsuchiya, T.; Ishii, K.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Nagai, K.; Yamamoto, K.; et al. Novel Therapeutic Method for Unresectable Pancreatic Cancer—The Impact of the Long-Term Research in Therapeutic Effect of High-Intensity Focused Ultrasound (HIFU) Therapy. Curr. Oncol. 2021, 28, 4845-4861. https://doi.org/10.3390/curroncol28060409
Sofuni A, Asai Y, Tsuchiya T, Ishii K, Tanaka R, Tonozuka R, Honjo M, Mukai S, Nagai K, Yamamoto K, et al. Novel Therapeutic Method for Unresectable Pancreatic Cancer—The Impact of the Long-Term Research in Therapeutic Effect of High-Intensity Focused Ultrasound (HIFU) Therapy. Current Oncology. 2021; 28(6):4845-4861. https://doi.org/10.3390/curroncol28060409
Chicago/Turabian StyleSofuni, Atsushi, Yasutsugu Asai, Takayoshi Tsuchiya, Kentaro Ishii, Reina Tanaka, Ryosuke Tonozuka, Mitsuyoshi Honjo, Shuntaro Mukai, Kazumasa Nagai, Kenjiro Yamamoto, and et al. 2021. "Novel Therapeutic Method for Unresectable Pancreatic Cancer—The Impact of the Long-Term Research in Therapeutic Effect of High-Intensity Focused Ultrasound (HIFU) Therapy" Current Oncology 28, no. 6: 4845-4861. https://doi.org/10.3390/curroncol28060409
APA StyleSofuni, A., Asai, Y., Tsuchiya, T., Ishii, K., Tanaka, R., Tonozuka, R., Honjo, M., Mukai, S., Nagai, K., Yamamoto, K., Matsunami, Y., Kurosawa, T., Kojima, H., Homma, T., Minami, H., Nakatsubo, R., Hirakawa, N., Miyazawa, H., Nagakawa, Y., ... Itoi, T. (2021). Novel Therapeutic Method for Unresectable Pancreatic Cancer—The Impact of the Long-Term Research in Therapeutic Effect of High-Intensity Focused Ultrasound (HIFU) Therapy. Current Oncology, 28(6), 4845-4861. https://doi.org/10.3390/curroncol28060409







