Complete and Durable Response to Nivolumab in Recurrent Poorly Differentiated Pancreatic Neuroendocrine Carcinoma with High Tumor Mutational Burden
Abstract
:1. Introduction
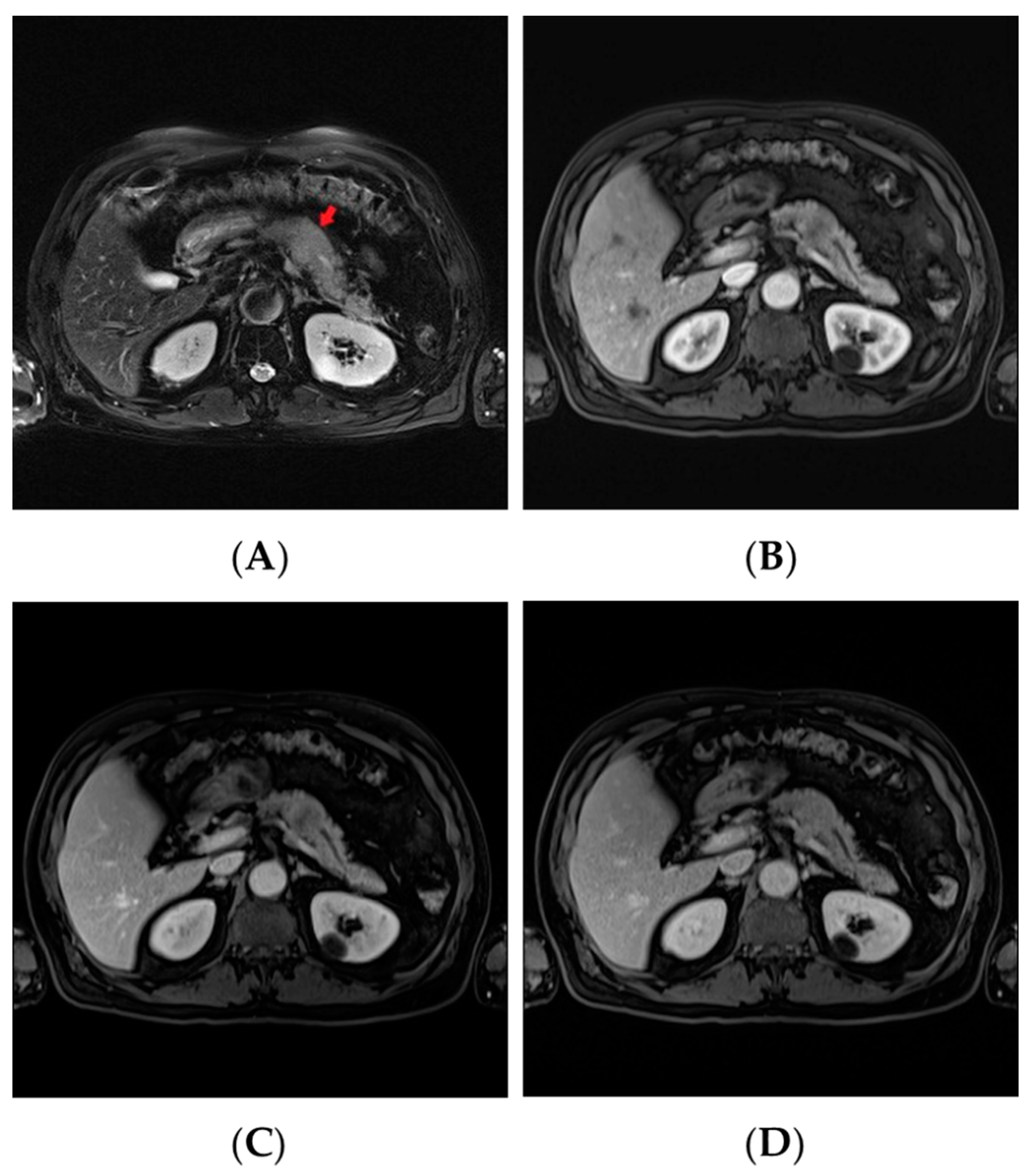
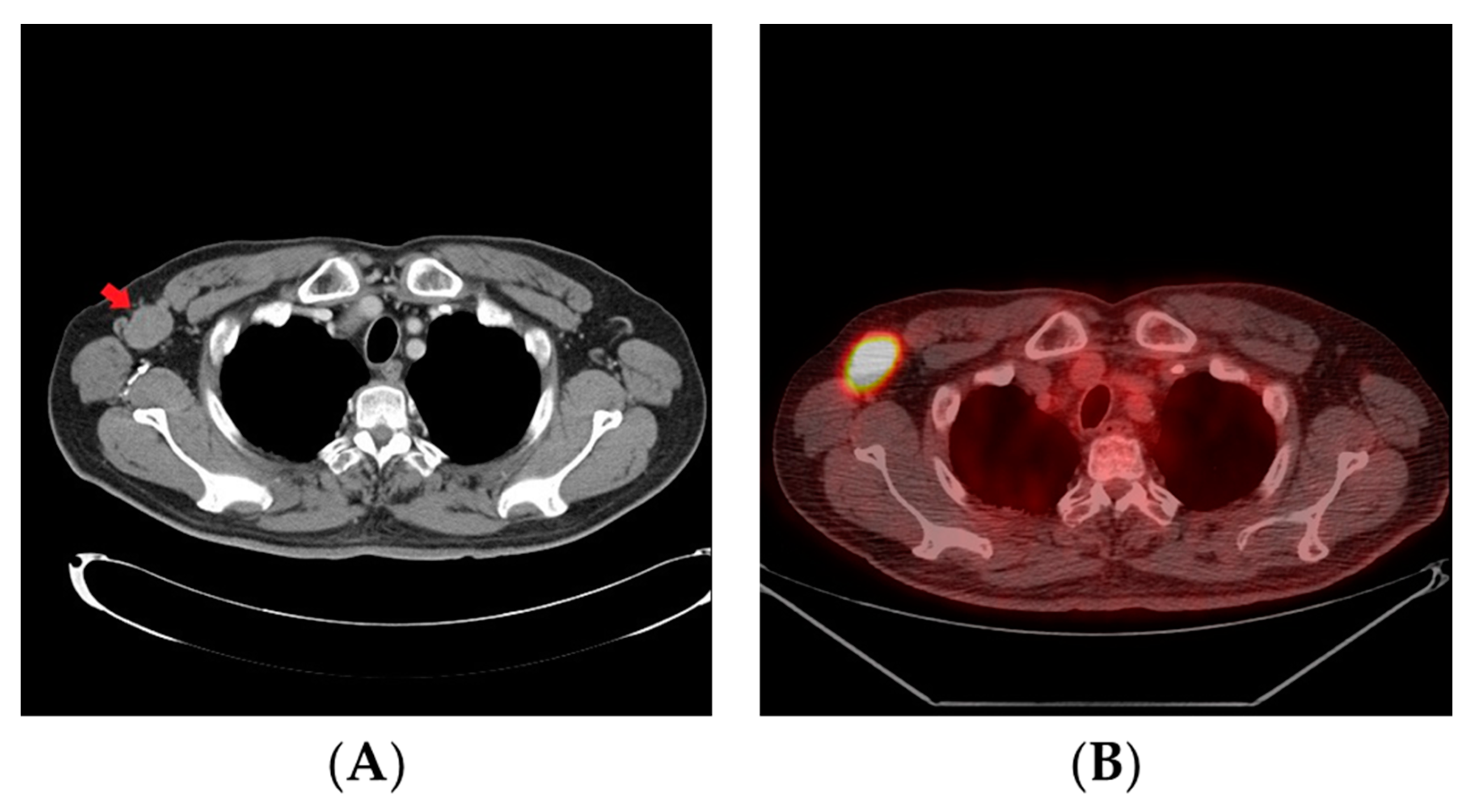
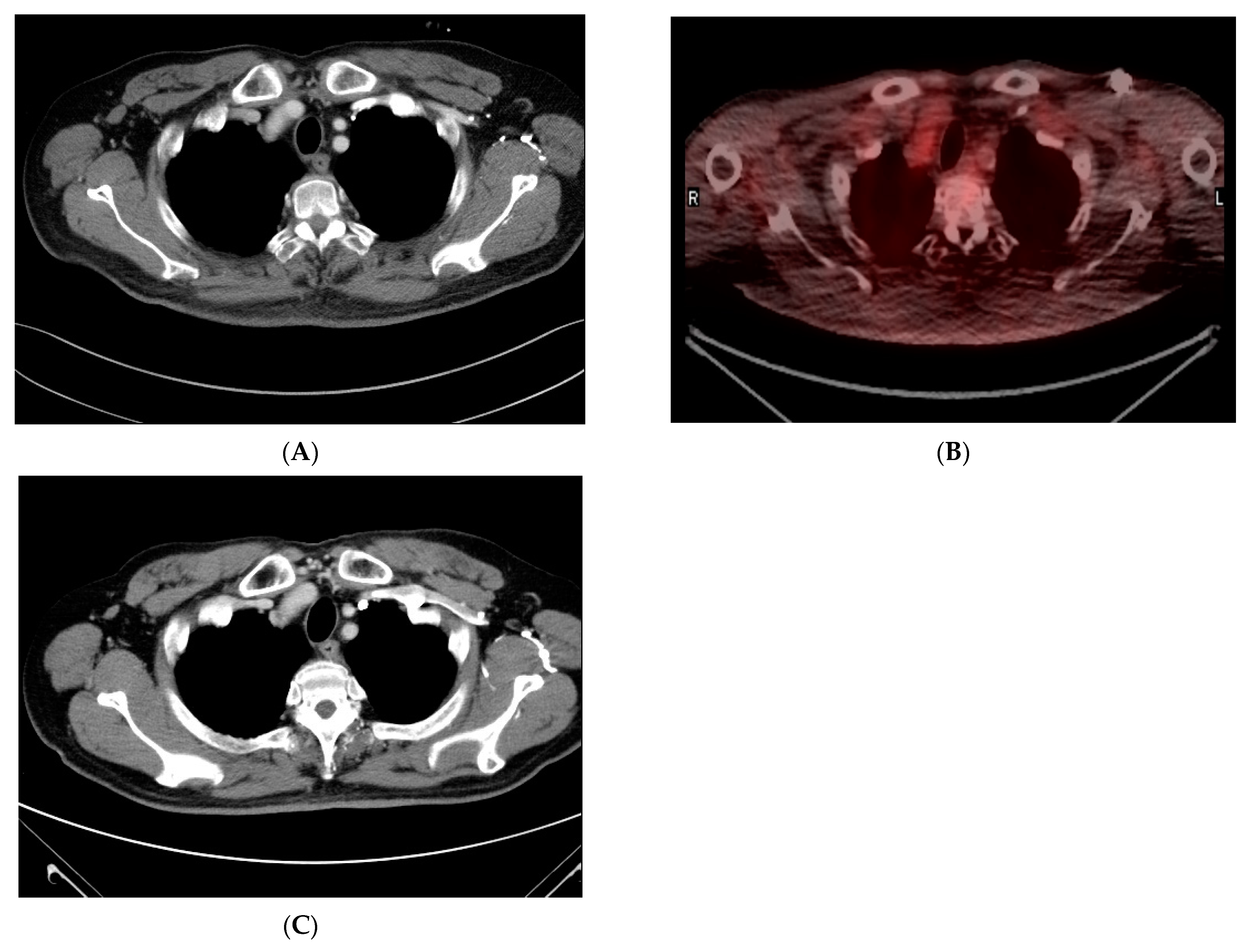
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
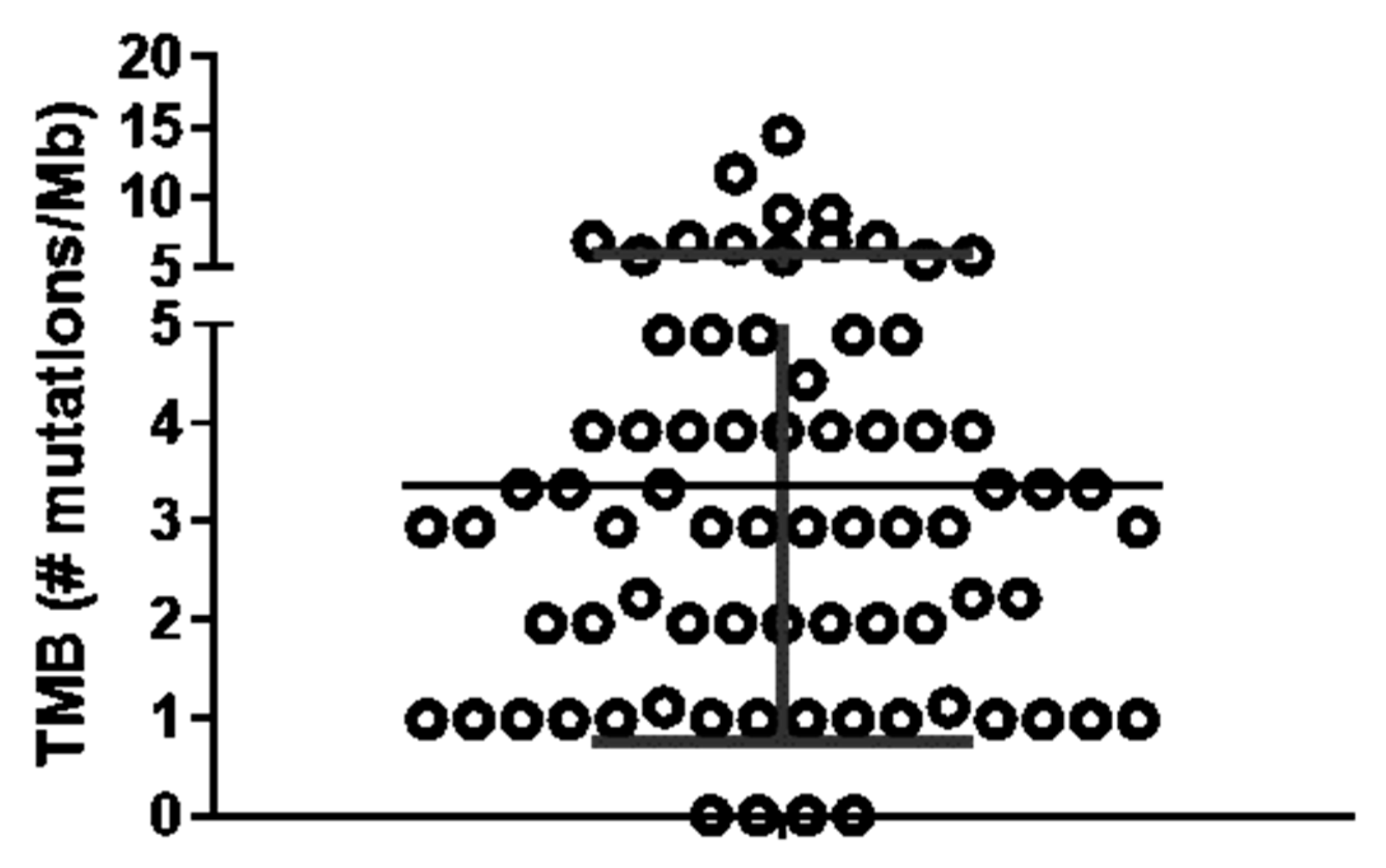
References
- Fesinmeyer, M.D.; Austin, M.A.; Li, C.I.; De Roos, A.J.; Bowen, D.J. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1766–1773. [Google Scholar] [CrossRef] [Green Version]
- Eads, J.R. Poorly Differentiated Neuroendocrine Tumors. Hematol. Oncol. Clin. N. Am. 2016, 30, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Coppola, D.; Klimstra, D.S.; Phan, A.T.; Kulke, M.H.; Wiseman, G.A.; Kvols, L.K. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas 2010, 39, 799–800. [Google Scholar] [CrossRef] [Green Version]
- Hentic, O.; Hammel, P.; Couvelard, A.; Rebours, V.; Zappa, M.; Palazzo, M.; Maire, F.; Goujon, G.; Gillet, A.; Lévy, P.; et al. FOLFIRI regimen: An effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr. Relat. Cancer 2012, 19, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, I.H.; Knigge, U.; Federspiel, B.; Hansen, C.P.; Skov, A.; Kjær, A.; Langer, S.W. Topotecan monotherapy in heavily pretreated patients with progressive advanced stage neuroendocrine carcinomas. J. Cancer 2014, 5, 628–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welin, S.; Sorbye, H.; Sebjornsen, S.; Knappskog, S.; Busch, C.; Oberg, K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011, 117, 4617–4622. [Google Scholar] [CrossRef] [PubMed]
- Basturk, O.; Tang, L.; Hruban, R.H.; Adsay, V.; Yang, Z.; Krasinskas, A.M.; Vakiani, E.; La Rosa, S.; Jang, K.T.; Frankel, W.L.; et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: A clinicopathologic analysis of 44 cases. Am. J. Surg. Pathol. 2014, 38, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Reck, M.; Spigel, D.R. The Future of Immunotherapy in the Treatment of Small Cell Lung Cancer. Oncologist 2016, 21, 910–921. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef] [Green Version]
- Ott, P.A.; Elez, E.; Hiret, S.; Kim, D.W.; Morosky, A.; Saraf, S.; Piperdi, B.; Mehnert, J.M. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 3823–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef]
- Doan, N.V.; Duc, N.M.; Ngan, V.K.; Anh, N.V.; Khuyen, H.K.; Nhan, N.T.; Giang, B.V.; Thong, P.M. Hypovascular pancreatic neuroendocrine tumor with hepatic metastases: A case report and literature review. Radiol. Case Rep. 2021, 16, 1424–1427. [Google Scholar] [CrossRef]
- Rindi, G.R.A.; Bosman, F.; Capella, C.; Ds, K.G.K.; Komminoth, P.E.S. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In WHO Classification of Tumours of the Digestive System; WHO: Geneva, Switzerland, 2010; Volume 4. [Google Scholar]
- Lloyd, R.V.O.R.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2014, 372, 320–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Paraghamian, S.E.; Longoria, T.C.; Eskander, R.N. Metastatic small cell neuroendocrine carcinoma of the cervix treated with the PD-1 inhibitor, nivolumab: A case report. Gynecol. Oncol. Res. Pract. 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Wang, V.E.; Urisman, A.; Albacker, L.; Ali, S.; Miller, V.; Aggarwal, R.; Jablons, D. Checkpoint inhibitor is active against large cell neuroendocrine carcinoma with high tumor mutation burden. J. Immunother. Cancer 2017, 5, 75. [Google Scholar] [CrossRef]
- Roberts, J.A.; Gonzalez, R.S.; Das, S.; Berlin, J.; Shi, C. Expression of PD-1 and PD-L1 in poorly differentiated neuroendocrine carcinomas of the digestive system: A potential target for anti-PD-1/PD-L1 therapy. Hum. Pathol. 2017, 70, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Ha, S.Y.; Lee, S.; Ahn, S.; Lee, J.; Park, S.H.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Kim, K.M.; et al. The Impact of PD-L1 Expression in Patients with Metastatic GEP-NETs. J. Cancer 2016, 7, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Foppen, M.H.G.; Goldinger, S.M.; et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015, 350, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef] [Green Version]
- George, S.; Miao, D.; Demetri, G.D.; Adeegbe, D.; Rodig, S.J.; Shukla, S.; Lipschitz, M.; Amin-Mansour, A.; Raut, C.P.; Carter, S.L.; et al. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity 2017, 46, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Biton, J.; Mansuet-Lupo, A.; Pécuchet, N.; Alifano, M.; Ouakrim, H.; Arrondeau, J.; Boudou-Rouquette, P.; Goldwasser, F.; Leroy, K.; Goc, J.; et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti-PD-1 in Lung Adenocarcinoma. Clin. Cancer Res. 2018, 24, 5710–5723. [Google Scholar] [CrossRef] [Green Version]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, H.; Sanchez-Vega, F.; La, K.; Chatila, W.; Jonsson, P.; Halpenny, D.; Plodkowski, A.; Long, N.; Sauter, J.L.; Rekhtman, N.; et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J. Clin. Oncol. 2018, 36, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Aref, A.R.; Skoulidis, F.; Herter-Sprie, G.S.; Buczkowski, K.A.; Liu, Y.; Awad, M.M.; Denning, W.L.; et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res. 2016, 76, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Riaz, N.; Havel, J.J.; Kendall, S.M.; Makarov, V.; Walsh, L.A.; Desrichard, A.; Weinhold, N.; Chan, T.A. Recurrent SERPINB3 and SERPINB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nat. Genet. 2016, 48, 1327–1329. [Google Scholar] [CrossRef] [PubMed]
- Bracci, L.; Schiavoni, G.; Sistigu, A.; Belardelli, F. Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014, 21, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roselli, M.; Cereda, V.; di Bari, M.G.; Formica, V.; Spila, A.; Jochems, C.; Farsaci, B.; Donahue, R.; Gulley, J.L.; Schlom, J.; et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology 2013, 2, e27025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, L.; Mansfield, A.S.; Szczesna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]

| Gene | Chr | Exon | Accession Number | cDNA Change | Amino Acid Change | Coverage | Variant Allele Frequency (VAF) | COSMIC ID |
|---|---|---|---|---|---|---|---|---|
| ADAMTS6 | 5 | 15 | NM_197941 | c.1861G > T | p.Glu621Ter | 463 | 18.9% | - |
| ADAMTSL1 | 9 | - | NM_001040272 | c.4643 + 1G > T | splice donor | 1110 | 26.8% | - |
| AMER1 | X | 2 | NM_152424 | c.2265G > T | p.Glu755Asp | 422 | 63.8% | - |
| AXIN1 | 16 | - | NM_003502 | c.1020-5G > T | splice region | 1435 | 29.2% | - |
| AXL | 19 | 20 | NM_021913 | c.2446C > A | p.Pro816Thr | 788 | 39.6% | - |
| BCL9 | 1 | 8 | NM_004326 | c.2151G > T | p.Lys717Asn | 298 | 19.1% | - |
| BRD4 | 19 | 10 | NM_058243 | c.1895G > A | p.Arg632His | 1497 | 56.7% | COSM4666105 |
| CDC73 | 1 | 3 | NM_024529 | c.306G > A | p.Ala102= | 436 | 33.0% | COSM4026318 |
| DICER1 | 14 | 11 | NM_177438 | c.1808C > A | p.Pro603His | 586 | 60.9% | - |
| DNMT3A | 2 | 14 | NM_175629 | c.1607A > G | p.Tyr536Cys | 705 | 37.1% | - |
| DNMT3A | 2 | - | NM_175629 | c.639 + 8G > A | splice region | 1394 | 16.8% | - |
| EP300 | 22 | 31 | NM_001429 | c.7223A > T | p.Gln2408Leu | 533 | 45.5% | - |
| EPHA7 | 6 | - | NM_004440 | c.2173-1delG | splice acceptor | 515 | 16.3% | - |
| ERBB2 | 17 | 27 | NM_004448 | c.3616C > T | p.Gln1206Ter | 564 | 16.8% | - |
| FGFR4 | 5 | 3 | NM_213647 | c.184C > A | p.Arg62Ser | 2221 | 19.8% | - |
| FGFR4 | 5 | 17 | NM_213647 | c.2158G > T | p.Gly720Trp | 755 | 29.4% | - |
| HR | 8 | - | NM_005144 | c.2367 + 3G > C | splice region | 505 | 22.9% | - |
| KMT2A | 11 | - | NM_001197104 | c.5364-3C > T | splice region | 802 | 18.0% | - |
| KMT2D | 12 | 11 | NM_003482 | c.3308G > T | p.Cys1103Phe | 1128 | 32.0% | - |
| LRP1B | 2 | 89 | NM_018557 | c.13516G > A | p.Asp4506Asn | 471 | 19.1% | COSM3567099 |
| LRP1B | 2 | 67 | NM_018557 | c.10470dupC | p.Asp3491ArgfsTer6 | 484 | 41.1% | - |
| MAX | 14 | 4 | NM_002382 | c.172-1_172delGGinsTT | splice acceptor | 625 | 52.6% | - |
| MUC16 | 19 | 14 | NM_024690 | c.36746G > T | p.Arg12249Leu | 2158 | 57.9% | - |
| NSD1 | 5 | 23 | NM_022455 | c.6611A > C | p.Glu2204Ala | 876 | 20.7% | - |
| PTPRD | 9 | 38 | NM_002839 | c.5048C > A | p.Ser1683Tyr | 625 | 19.9% | COSM6961014 |
| PTPRD | 9 | - | NM_002839 | c.2350-1G > T | splice acceptor | 645 | 19.4% | - |
| PTPRT | 20 | 16 | NM_007050 | c.2435C > A | p.Thr812Asn | 1873 | 28.0% | - |
| PTPRT | 20 | 12 | NM_007050 | c.1948G > T | p.Val650Leu | 2553 | 41.5% | - |
| SERPINB3 | 18 | 8 | NM_006919 | c.1061C > A | p.Ser354Ter | 965 | 30.6% | COSM6149208 |
| STAG2 | X | 8 | NM_001042751 | c.568A > G | p.Ile190Val | 276 | 64.0% | - |
| TERT | 5 | 9 | NM_198253 | c.2476G > A | p.Val826Ile | 1040 | 21.8% | COSM6916168 |
| TET1 | 10 | 2 | NM_030625 | c.981A > G | p.Ile327Met | 1780 | 30.1% | - |
| TP53 | 17 | 7 | NM_000546 | c.774A > T | p.Glu258Asp | 1587 | 35.7% | COSM44962 |
| TP53 | 17 | 5 | NM_000546 | c.422G > A | p.Cys141Tyr | 1021 | 30.1% | COSM43708 |
| TSC2 | 16 | 12 | NM_000548 | c.1171G > A | p.Val391Met | 983 | 36.6% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, N.-W.; Tan, K.-T.; Li, C.-F.; Kuo, Y.-H. Complete and Durable Response to Nivolumab in Recurrent Poorly Differentiated Pancreatic Neuroendocrine Carcinoma with High Tumor Mutational Burden. Curr. Oncol. 2021, 28, 4587-4596. https://doi.org/10.3390/curroncol28060388
Kang N-W, Tan K-T, Li C-F, Kuo Y-H. Complete and Durable Response to Nivolumab in Recurrent Poorly Differentiated Pancreatic Neuroendocrine Carcinoma with High Tumor Mutational Burden. Current Oncology. 2021; 28(6):4587-4596. https://doi.org/10.3390/curroncol28060388
Chicago/Turabian StyleKang, Nai-Wen, Kien-Thiam Tan, Chien-Feng Li, and Yu-Hsuan Kuo. 2021. "Complete and Durable Response to Nivolumab in Recurrent Poorly Differentiated Pancreatic Neuroendocrine Carcinoma with High Tumor Mutational Burden" Current Oncology 28, no. 6: 4587-4596. https://doi.org/10.3390/curroncol28060388
APA StyleKang, N.-W., Tan, K.-T., Li, C.-F., & Kuo, Y.-H. (2021). Complete and Durable Response to Nivolumab in Recurrent Poorly Differentiated Pancreatic Neuroendocrine Carcinoma with High Tumor Mutational Burden. Current Oncology, 28(6), 4587-4596. https://doi.org/10.3390/curroncol28060388






