Human Cytomegalovirus Is Associated with Lower HCC Recurrence in Liver Transplant Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Data Collection
2.2. Liver Transplant Protocol and HCC Patients Selection
2.3. CMV Serological Study and Standardization of CMV Surveillance
2.4. Definition of CMV pp65 Antigenemia and CMV Disease
2.5. Preemptive Treatment Protocol for CMV
2.6. Post-Transplant Outcome Assessment
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Entire Population
3.2. Comparison between CMV Antigenemia-Positive and Negative Patients
3.3. Univariate and Multivariate Logistic Regression for Predictors of HCC Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzaferro, V.M.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef]
- Roayaie, S.; Schwartz, J.D.; Sung, M.W.; Emre, S.H.; Miller, C.M.; Gondolesi, G.E.; Krieger, N.R.; Schwartz, M.E. Recurrence of hepatocellular carcinoma after liver transplant: Patterns and prognosis. Liver Transplant. 2004, 10, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, N.A. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J. Hepatol. 2019, 11, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, A.; Bustamante, J.; Gastaca, M.; Uriarte, J.G.; Ventoso, A.; Ruiz, P.; Ernandez, J.R.; Pijoan, I.; Testillano, M.; Suarez, M.J.; et al. Management of Hepatocellular Carcinoma Recurrence After Liver Transplantation. Transplant. Proc. 2010, 42, 660–662. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Ghobrial, R.M.; Tong, M.J.; Hiatt, J.R.; Cameron, A.M.; Hong, J.; Busuttil, R.W. Recurrence of Hepatocellular Carcinoma Fol-lowing Liver Transplantation: A Review of Preoperative and Postoperative Prognostic Indicators. Arch. Surg. 2008, 143, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Linares, L.; Sanclemente, G.; Cervera, C.; Hoyo, I.; Cofán, F.; Ricart, M.; Pérez-Villa, F.; Navasa, M.; Marcos, M.; Antón, A.; et al. Influence of Cytomegalovirus Disease in Outcome of Solid Organ Transplant Patients. Transplant. Proc. 2011, 43, 2145–2148. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.J.; Joh, J.W.; Kwon, C.H.D.; Song, S.; Shin, M.; Lee, S.K.; Moon, J.I.; Kim, G.S.; Hong, S.H. Is cytomegalovirus infection dangerous in cytomegalovi-rus-seropositive recipients after liver transplantation? Liver Transpl. 2011, 17, 446–455. [Google Scholar] [CrossRef]
- Hung, H.; Hsu, P.; Lee, J.; Wang, Y.; Cheng, C.H.; Wu, T.-J.; Wu, T.-H.; Chou, H.; Chan, K.; Lee, W.; et al. Plasma cytomegalovirus DNA load predicts outcomes in liver transplant recipients. Immunity Inflamm. Dis. 2020, 9, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G.; Nehme, Z. Tumor Control by Cytomegalovirus: A Door Open for Oncolytic Virotherapy? Mol. Ther.-Oncolytics 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Klyushnenkova, E.N.; Kouiavskaia, D.V.; Parkins, C.J.; Caposio, P.; Botto, S.; Alexander, R.B.; Jarvis, M.A. A Cytomegalovirus-based Vaccine Expressing a Single Tumor-specific CD8+ T-cell Epitope Delays Tumor Growth in a Murine Model of Prostate. Cancer J. Immunother. 2012, 35, 390–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Coquard, L.; Pasquereau, S.; Russo, L.; Valmary-Degano, S.; Borg, C.; Pothier, P.; Herbein, G. Tumor control by human cytomegalovirus in a murine model of hepatocellular carcinoma. Mol. Ther.-Oncolytics 2016, 3, 16012. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Huang, H.; Grenier, J.M.; Perez, O.A.; Smilowitz, H.M.; Adler, B.; Khanna, K.M. Cytomegalovirus-Based Vaccine Expressing a Modified Tumor Antigen Induces Potent Tumor-Specific CD8+ T-cell Response and Protects Mice from Melanoma. Cancer Immunol. Res. 2015, 3, 536–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.J.; Dahiya, D.; Lee, C.S.; Lee, C.F.; Chou, H.S.; Chan, K.M.; Lee, W.C. Impact of portal venous hemodynamics on indices of liver function and graft regeneration after right lobe living donor liver transplantation. Liver Transpl. 2011, 17, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Cheng, C.H.; Wu, T.H.; Wu, T.J.; Chou, H.S.; Lee, C.S.; Lee, W.C. Clinical strategy for the reconstruction of middle hepatic vein tributaries in right liver living donor liver transplantation. World J. Surg. 2014, 38, 2927–2933. [Google Scholar] [CrossRef]
- Lee, W.; Lee, C.; Wu, T.J.; Soong, R.; Cheng, C.; Chou, H.S.; Chan, K. Adult Living Donor Liver Transplantation Across ABO-Incompatibility. Medicine 2015, 94, e1796. [Google Scholar] [CrossRef]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Roberts, J.P.; Ascher, N.L. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Ljungman, P.; Griffiths, P.; Paya, C. Definitions of Cytomegalovirus Infection and Disease in Transplant Recipients. Clin. Infect. Dis. 2002, 34, 1094–1097. [Google Scholar] [CrossRef]
- McBride, J.M.; Sheinson, D.; Jiang, J.; Lewin-Koh, N.; Werner, B.G.; Chow, J.K.L.; Wu, X.; Tavel, J.A.; Snydman, D.R. Correlation of Cytomegalovirus (CMV) Disease Severity and Mortality With CMV Viral Burden in CMV-Seropositive Donor and CMV-Seronegative Solid Organ Transplant Recipients. Open Forum Infect. Dis. 2019, 6, ofz003. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, D.; Lee, C.F.; Chan, K.M.; Wu, T.J.; Chou, H.S.; Cheng, S.S.; Lee, W.C. A short-term preemptive treatment for cytomegalovirus in-fection in seropositive patients after liver transplantation. J. Hepatobiliary Pancreat. Sci. 2011, 18, 32–38. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; Makuuchi, M.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Yao, F.Y.; Xiao, L.; Bass, N.M.; Kerlan, R.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Validation of the UCSF-Expanded Criteria Based on Preoperative Imaging. Arab. Archaeol. Epigr. 2007, 7, 2587–2596. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, E.M.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Erkes, D.A.; Wilski, N.A.; Snyder, C.M. Intratumoral infection by CMV may change the tumor environment by directly interacting with tumor-associated macrophages to promote cancer immunity. Hum. Vaccines Immunother. 2017, 13, 1778–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejad, E.B.; Ratts, R.B.; Panagioti, E.; Meyer, C.; Oduro, J.D.; Cicin-Sain, L.; Arens, R.; Früh, K.; van der Burg, S.H. Demarcated thresholds of tumor-specific CD8 T cells elicited by MCMV-based vaccine vectors provide robust correlates of protection. J. Immunother. Cancer 2019, 7, 25. [Google Scholar] [CrossRef]
- Klenerman, P. The (gradual) rise of memory inflation. Immunol. Rev. 2018, 283, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Sato, N.; Shiraki, A.; Yanagita, M.; Yoshida, Y.; Takemura, Y.; Shiraki, K. Everolimus delayed and suppressed cytomegalovirus DNA synthesis, spread of the infection, and alleviated cytomegalovirus infection. Antivir. Res. 2018, 162, 30–38. [Google Scholar] [CrossRef]
- Kang, I.; Lee, J.G.; Choi, S.H.; Kim, H.J.; Han, D.H.; Choi, G.H.; Kim, M.S.; Choi, J.S.; Kim, S.I.; Joo, D.J. Impact of everolimus on survival after liver transplantation for hepatocellular carcinoma. Clin. Mol. Hepatol. 2021, 27, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Jeng, L.-B.; Saliba, F.; Soin, A.S.; Lee, W.-C.; De Simone, P.; Nevens, F.; Suh, K.-S.; Fischer, L.; Joo, D.J.; et al. Efficacy and Safety of Everolimus With Reduced Tacrolimus in Liver Transplant Recipients: 24-month Results From the Pooled Analysis of 2 Randomized Controlled Trials. Transplantation 2020, 105, 1564–1575. [Google Scholar] [CrossRef]
- Gane, E.; Saliba, F.; Valdecasas, G.J.; O’Grady, J.; Pescovitz, M.D.; Lyman, S.; Robinson, A.C. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplantation Study Group [corrected]. Lancet 1997, 350, 1729–1733. [Google Scholar] [CrossRef]
- Singh, N.; Wagener, M.M. Strategies To Prevent Organ Disease by Cytomegalovirus in Solid Organ Transplant Recipients. Ann. Intern. Med. 2006, 144, 456–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paya, C.; Humar, A.; Dominguez, E.; Washburn, K.; Blumberg, E.; Alexander, B.; Freeman, R.; Heaton, N.; Pescovitz, M.D.; Valganciclovir Solid Organ Transplant Study Group. Efficacy and Safety of Valganciclovir vs. Oral Ganciclovir for Prevention of Cytomegalovirus Disease in Solid Organ Transplant Recipients. Am. J. Transplant. 2004, 4, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, P.I.; Mourtzoukou, E.G.; Varbobitis, I.C.; Falagas, E.M. Severe cytomegalovirus infection in apparently immunocompetent patients: A systematic review. Virol. J. 2008, 5, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razonable, R.R.; Emery, V. Management of CMV infection and disease in transplant patients. 27–29 February 2004. Herpes 2004, 11, 77–86. [Google Scholar] [PubMed]
- Peleg, A.Y.; Husain, S.; Qureshi, Z.A.; Silveira, F.P.; Sarumi, M.; Shutt, K.; Kwak, E.J.; Paterson, D. Risk Factors, Clinical Characteristics, and Outcome of Nocardia Infection in Organ Transplant Recipients: A Matched Case-Control Study. Clin. Infect. Dis. 2007, 44, 1307–1314. [Google Scholar] [CrossRef] [Green Version]
- Humar, A.; Kumar, D.; Raboud, J.; Caliendo, A.M.; Moussa, G.; Levy, G.; Mazzulli, T. Interactions between cytomegalovirus, human her-pesvirus-6, and the recurrence of hepatitis C after liver transplantation. Am. J. Transplant. 2002, 2, 461–466. [Google Scholar] [CrossRef]
- Razonable, R.R.; Paya, C.V.; Smith, T.F. Role of the laboratory in diagnosis and management of cytomegalovirus infection in hem-atopoietic stem cell and solid-organ transplant recipients. J. Clin. Microbiol. 2002, 40, 746–752. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Dummer, J.S.; Estes, W.R.; Meng, S.; Wright, P.F.; Tang, Y.-W. Measurement of Human Cytomegalovirus Loads by Quantitative Real-Time PCR for Monitoring Clinical Intervention in Transplant Recipients. J. Clin. Microbiol. 2003, 41, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Piiparinen, H.; Höckerstedt, K.; Grönhagen-Riska, C.; Lautenschlager, I. Comparison of two quantitative CMV PCR tests, Cobas Amplicor CMV Monitor and TaqMan assay, and pp65-antigenemia assay in the determination of viral loads from peripheral blood of organ transplant patients. J. Clin. Virol. 2004, 30, 258–266. [Google Scholar] [CrossRef] [PubMed]
| Factors | Median Value or Number (Percentage) | Mean ± SD | Range |
|---|---|---|---|
| General characteristics | |||
| Recipient age | 56 | 56 ± 7.1 | 33–70 |
| Recipient gender, male | 221 (78.9%) | ||
| Pre-LT characteristics | |||
| MELD score | 12 | 13.5 ± 6.1 | 5–40 |
| Hepatitis B infection | 173 (61.8%) | ||
| Hepatitis C infection | 79 (28.2%) | ||
| LDLT | 233 (83.2%) | ||
| Right lobe in LDLT | 220 (94.4%) | ||
| GRWR (%) in LDLT | 0.92 | 0.98 ± 0.22 | 0.57–1.79 |
| Local regional treatment before LT | 212 (75.7%) | ||
| Tumor status within Milan criteria (by radiologic assessment) | 234 (83.6%) | ||
| AFP | 13.4 | 213.5 ± 1168.2 | 1–18,250 |
| Explant pathology characteristics | |||
| Recipient with solitary tumor | 109 (38.9%) | ||
| Maximum tumor size(cm) | 2.4 | 2.8 ± 1.6 | 0–11 |
| Satellite nodules | 24 (8.6%) | ||
| Macroscopic vascular invasion | 17 (6.1%) | ||
| Microscopic vascular invasion | 52 (18.6%) | ||
| CMV study | |||
| Preoperative CMV IgG positive | 278 (99.3%) | ||
| PP65 antigenemia positive | 121 (43.2%) | ||
| PP65, maximum/per 500 × 103 PBL | 2 | 1–115 | |
| Persistent antigenemia > 2 weeks | 28/121 (23.1%) | ||
| Relapsed CMV antigenemia | 33/121 (27.3%) | ||
| Severe CMV disease | 6/121 (5%) | ||
| Clinical outcome | |||
| Follow-up period(months) | 82.5 | 84.3 ± 49.4 | 3–191 |
| Five-year recurrence free survival, cumulative | 83.7% | ||
| Five-year overall survival, cumulative | 73.1% | ||
| Major complications | 23 (8.2%) | ||
| Cause of mortality in 5 years | |||
| Other bacterial or fungal Infection | 30/75 (40.0%) | ||
| HCC-related | 26/75 (34.7%) | ||
| Rejection | 8/75 (10.7%) | ||
| Others | 11/75 (14.6%) | ||
| Factors | CMV Positive n = 121 | CMV Negative n = 159 | p-Value |
|---|---|---|---|
| General characteristic | |||
| Recipient age, year-old (>60) | 40 (33.1%) | 46 (28.9%) | 0.458 |
| Recipient gender, male | 94 (77.7%) | 127 (79.9%) | 0.656 |
| Pre-LT characteristic | |||
| MELD score >20 | 23 (19.0%) | 11 (6.9%) | 0.002 |
| Hepatitis B infection | 70 (57.9%) | 103 (64.8%) | 0.237 |
| Hepatitis C infection | 39 (32.2%) | 40 (25.2%) | 0.193 |
| LDLT | 96 (79.3%) | 137 (86.2%) | 0.130 |
| Right lobe in LDLT | 89/96 (92.7%) | 131/137 (95.6%) | 0.340 |
| GRWR ≤ 0.8% in LDLT | 20/96 (20.8%) | 27/137 (19.7%) | 0.833 |
| Local regional treatment before LT | 89 (73.6%) | 123 (77.4%) | 0.462 |
| Beyond Milan criteria | 21 (17.4%) | 25 (15.7%) | 0.715 |
| AFP > 200 ng/mL | 18 (14.9%) | 22 (13.8%) | 0.805 |
| Explant pathology characteristic | |||
| Recipients with multiple tumors | 77 (63.6%) | 91 (59.1%) | 0.443 |
| Maximum tumor size > 3 cm | 39 (32.2%) | 52 (32.7%) | 0.933 |
| Satellite nodules | 7 (5.8%) | 17 (10.7%) | 0.146 |
| Macroscopic vascular invasion | 6 (5.0%) | 11 (6.9%) | 0.496 |
| Microscopic vascular invasion | 23 (19%) | 29 (18.2%) | 0.870 |
| Beyond Milan criteria | 37 (30.6%) | 60 (37.7%) | 0.212 |
| Clinical outcome | |||
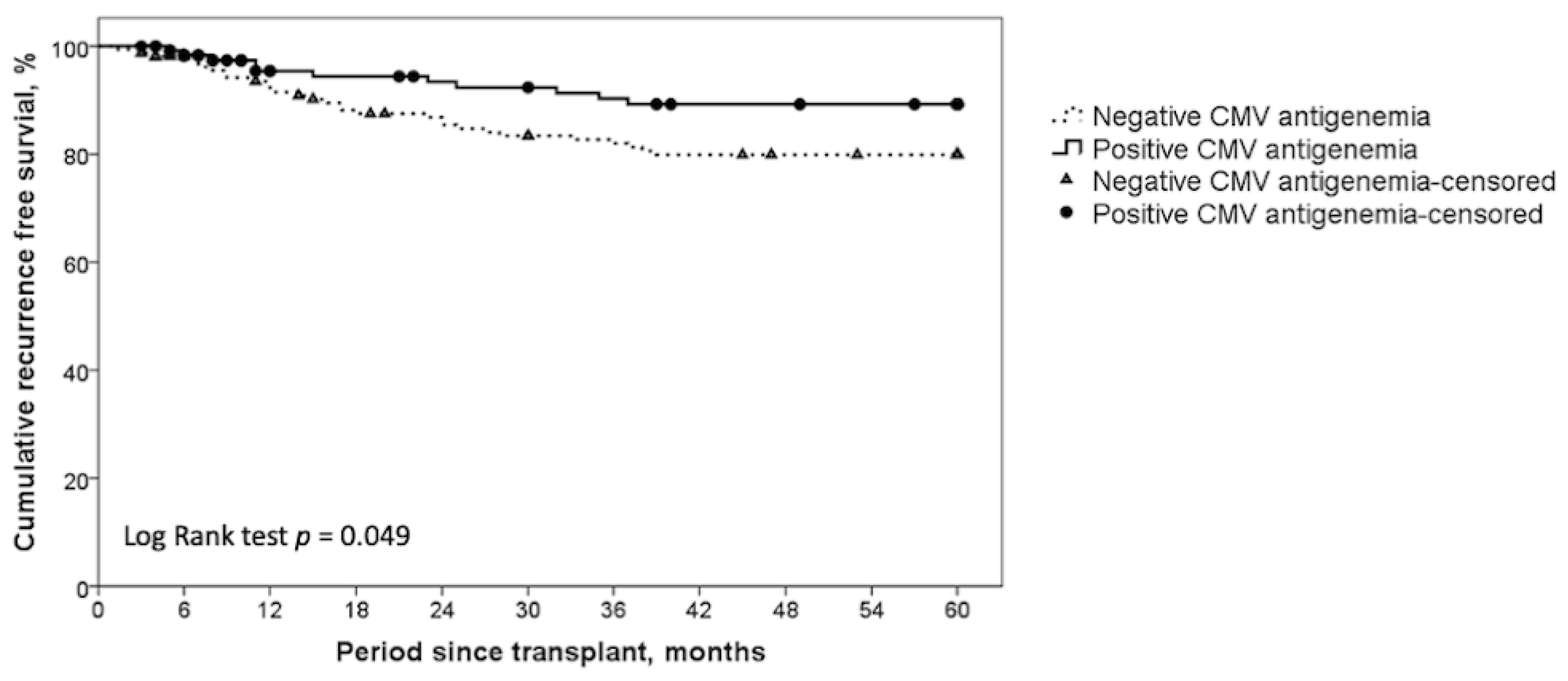
| Five-year recurrence free survival, cumulative | 89.2% | 79.9% | 0.049 |
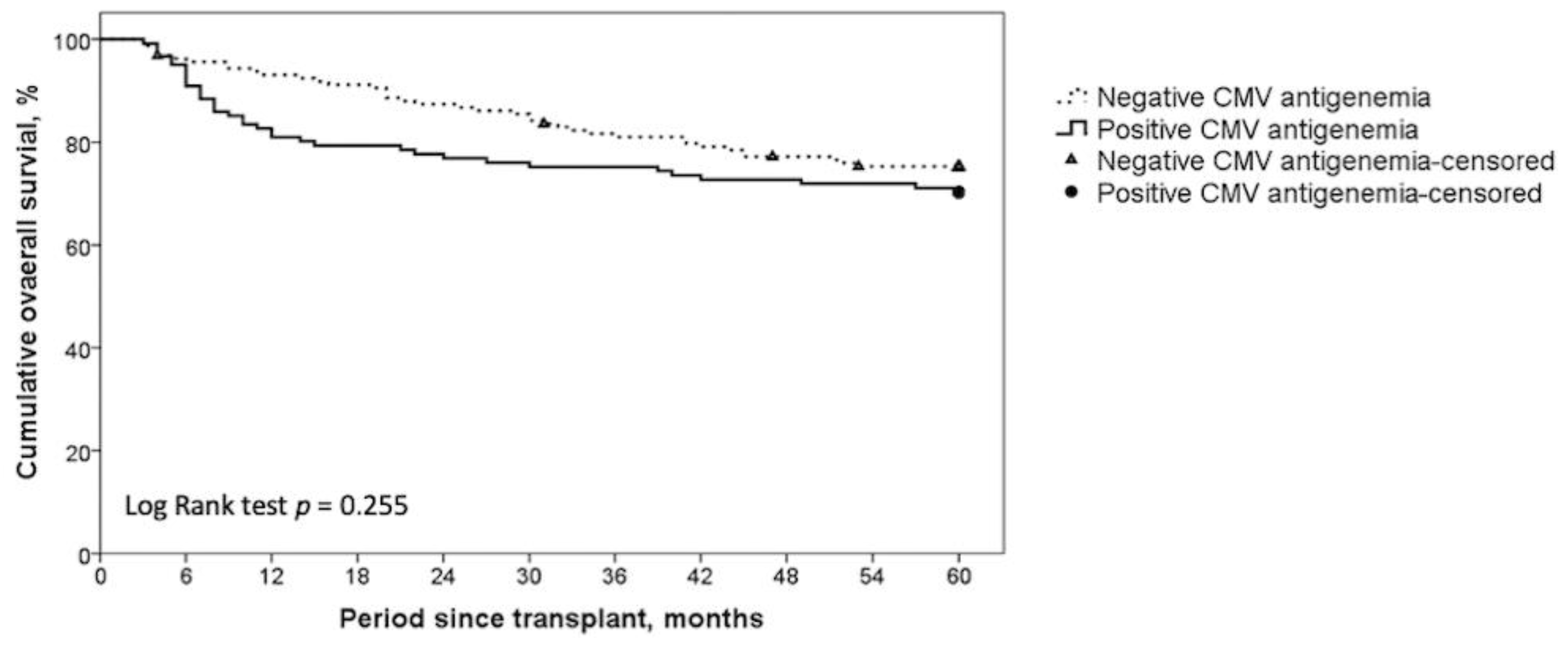
| Five-year overall survival, cumulative | 70.2% | 75.3% | 0.255 |
| Major complications | 18 (14.9%) | 5 (3.2%) | <0.001 |
| Causes of death in 5 years after LT | 0.004 | ||
| Other bacterial or fungal Infection | 21 (58.3%) | 9 (23.1%) | 0.002 |
| HCC related | 6 (16.7%) | 20 (51.3%) | 0.002 |
| Rejection | 5 (13.9%) | 3 (7.7%) | 0.385 |
| Others | 4 (11.1%) | 7 (17.9%) | 0.403 |
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Pre-LT characteristic | ||||||
| Beyond Milan criteria (by radiology) | 3.35 | 1.59–7.07 | 0.001 | |||
| CMV study | ||||||
| Positive CMV antigenemia | 0.43 | 0.21–0.90 | 0.025 | 0.44 | 0.20–0.97 | 0.042 |
| Explant pathology characteristic | ||||||
| Multiple tumor numbers | 2.56 | 1.17–5.60 | 0.019 | |||
| Maximum tumor size > 3 cm | 2.85 | 1.45–5.60 | 0.002 | |||
| Satellite nodule | 3.38 | 1.34–8.51 | 0.010 | |||
| Macroscopic vascular invasion | 3.55 | 1.24–10.22 | 0.019 | |||
| Microscopic vascular invasion | 4.72 | 2.31–9.65 | <0.001 | 3.86 | 1.78–8.36 | 0.001 |
| Beyond Milan criteria (by pathology) | 4.10 | 2.05–8.21 | <0.001 | 3.69 | 1.77–7.71 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, P.-J.; Hung, H.-C.; Lee, J.-C.; Wang, Y.-C.; Cheng, C.-H.; Wu, T.-H.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C.; et al. Human Cytomegalovirus Is Associated with Lower HCC Recurrence in Liver Transplant Patients. Curr. Oncol. 2021, 28, 4281-4290. https://doi.org/10.3390/curroncol28060364
Hsu P-J, Hung H-C, Lee J-C, Wang Y-C, Cheng C-H, Wu T-H, Wu T-J, Chou H-S, Chan K-M, Lee W-C, et al. Human Cytomegalovirus Is Associated with Lower HCC Recurrence in Liver Transplant Patients. Current Oncology. 2021; 28(6):4281-4290. https://doi.org/10.3390/curroncol28060364
Chicago/Turabian StyleHsu, Po-Jung, Hao-Chien Hung, Jin-Chiao Lee, Yu-Chao Wang, Chih-Hsien Cheng, Tsung-Han Wu, Ting-Jung Wu, Hong-Shiue Chou, Kun-Ming Chan, Wei-Chen Lee, and et al. 2021. "Human Cytomegalovirus Is Associated with Lower HCC Recurrence in Liver Transplant Patients" Current Oncology 28, no. 6: 4281-4290. https://doi.org/10.3390/curroncol28060364
APA StyleHsu, P.-J., Hung, H.-C., Lee, J.-C., Wang, Y.-C., Cheng, C.-H., Wu, T.-H., Wu, T.-J., Chou, H.-S., Chan, K.-M., Lee, W.-C., & Lee, C.-F. (2021). Human Cytomegalovirus Is Associated with Lower HCC Recurrence in Liver Transplant Patients. Current Oncology, 28(6), 4281-4290. https://doi.org/10.3390/curroncol28060364





