Effectiveness and Safety of First-Line Pembrolizumab in Older Adults with PD-L1 Positive Non-Small Cell Lung Cancer: A Retrospective Cohort Study of the Alberta Immunotherapy Database
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes
3.3. Safety Data
3.4. Prognostic Factors of Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Rashdan, S.; Gerber, D.E. Immunotherapy for non-small cell lung cancer: From clinical trials to real-world practice. Transl. Lung Cancer Res. 2019, 8, 202–207. [Google Scholar] [CrossRef]
- Khozin, S.; Abernethy, A.P.; Nussbaum, N.C.; Zhi, J.; Curtis, M.; Tucker, M.; Lee, S.E.; Light, D.E.; Gossai, A.; Sorg, R.A.; et al. Characteristics of Real-World Metastatic Non-Small Cell Lung Cancer Patients Treated with Nivolumab and Pembrolizumab During the Year Following Approval. Oncologist 2018, 23, 328–336. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute SEER. Cancer Stat Facts: Lung and Bronchus Cancer; National Cancer Institute SEER: Bethesda, MD, USA. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 14 September 2021).
- Gridelli, C.; Sgambato, A. Elderly patients and PD-L1-positive advanced non-small cell lung cancer: Is pembrolizumab monotherapy effective and safe? Ann. Transl. Med. 2019, 7, S282. [Google Scholar] [CrossRef]
- Bhandari, S.; Gill, A.S.; Perez, C.A.; Jain, D. Management of immunotherapy toxicities in older adults. Semin. Oncol. 2018, 45, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; de Toma, A.; Pagani, F.; Randon, G.; Trevisan, B.; Prelaj, A.; Ferrara, R.; Proto, C.; Signorelli, D.; Ganzinelli, M.; et al. Efficacy and safety of immunotherapy in elderly patients with non-small cell lung cancer. Lung Cancer 2019, 137, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Youn, B.; Trikalinos, N.A.; Mor, V.; Wilson, I.B.; Dahabreh, I.J. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non–small cell lung cancer. Cancer 2019, 126, 978–985. [Google Scholar] [CrossRef]
- Marur, S.; Singh, H.; Mishra-Kalyani, P.; Larkins, E.; Keegan, P.; Sridhara, R.; Blumenthal, G.M.; Pazdur, R. FDA analyses of survival in older adults with metastatic non–small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies. Semin. Oncol. 2018, 45, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, A.P.; Arunachalam, A.; Burke, T.; McKay, C.; Cao, X.; Sorg, R.; Carbone, D.P. Real-world first-line treatment and overall survival in non-small cell lung cancer without known EGFR mutations or ALK rearrangements in US community oncology setting. PLoS ONE 2017, 12, e0178420. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics: A 2020 Special Report on Lung Cancer; Canadian Cancer Society: Toronto, ON, Canada, 2020. [Google Scholar]
- Gan, C.L.; Stukalin, I.; Meyers, D.E.; Dudani, S.; Grosjean, H.A.I.; Dolter, S.; Ewanchuk, B.W.; Goutam, S.; Sander, M.; Wells, C.; et al. Outcomes of patients with solid tumour malignancies treated with first-line immuno-oncology agents who do not meet eligibility criteria for clinical trials. Eur. J. Cancer 2021, 151, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, K.; Gill, R.R.; Widick, P.; Bindal, P.; McDonald, D.C.; Shea, M.; Rangachari, D.; Costa, D.B. Association of Performance Status With Survival in Patients With Advanced Non–Small Cell Lung Cancer Treated With Pembrolizumab Monotherapy. JAMA Netw. Open 2021, 4, e2037120. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; de Giglio, A.; Cannita, K.; Cortinovis, D.L.; Cornelissen, R.; Baldessari, C.; Giusti, R.; D’Argento, E.; Grossi, F.; Santoni, M.; et al. Smoking status during first-line immunotherapy and chemotherapy in NSCLC patients: A case–control matched analysis from a large multicenter study. Thorac. Cancer 2021, 12, 880–889. [Google Scholar] [CrossRef]
- Norum, J.; Nieder, C. Tobacco smoking and cessation and PD-L1 inhibitors in non-small cell lung cancer (NSCLC): A review of the literature. ESMO Open 2018, 3, e000406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, J.; Hu, X.; Gu, L.; Chen, B.; Khadaroo, P.A.; Shen, Z.; Dong, L.; Lv, Y.; Chitumba, M.N.; Liu, J. Smokers or non-smokers: Who benefits more from immune checkpoint inhibitors in treatment of malignancies? An up-to-date meta-analysis. World J. Surg. Oncol. 2020, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Meyers, D.E.; Stukalin, I.; Vallerand, I.A.; Lewinson, R.T.; Suo, A.; Dean, M.; North, S.; Pabani, A.; Cheng, T.; Heng, D.Y.C.; et al. The Lung Immune Prognostic Index Discriminates Survival Outcomes in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors. Cancers 2019, 11, 1713. [Google Scholar] [CrossRef] [Green Version]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Obesity Paradox in Patients with Cancer: A Systematic Review and Meta-Analysis of 6,320,365 Patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Lalani, A.-K.A.; Bakouny, Z.; Farah, S.; Xie, W.; Flippot, R.; Steinharter, J.; Nuzzo, P.; Fleischer, J.; Pal, S.; Rathi, N.; et al. Efficacy of immune checkpoint inhibitors (ICI) and genomic alterations by body mass index (BMI) in advanced renal cell carcinoma (RCC). Ann. Oncol. 2019, 30, v396. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
| Characteristic | Age | p-Value | |
|---|---|---|---|
| <70 (n = 158) | ≥70 (n = 169) | ||
| Sex–no. (%) | 0.19 | ||
| Female | 88 (55.7) | 82 (48.5) | |
| Male | 70 (44.3) | 87 (51.5) | |
| Smoking status—no. (%) | 0.87 | ||
| Never | 12 (7.9) | 12 (7.4) | |
| Ever | 140 (92.1) | 150 (92.6) | |
| Histology—no. (%) | 0.06 | ||
| Adenocarcinoma | 125 (79.6) | 118 (69.8) | |
| Squamous | 22 (14.0) | 41 (24.3) | |
| Other | 10 (6.4) | 10 (5.9) | |
| Stage at Diagnosis—no. (%) | 0.03 | ||
| I/II | 7 (4.4) | 21 (12.4) | |
| III | 43 (27.2) | 46 (27.2) | |
| IV | 108 (68.4) | 102 (60.4) | |
| KRAS Status—no. (%) | 0.10 | ||
| Wildtype | 23 (14.6) | 14 (8.3) | |
| Mutated | 23 (14.6) | 19 (11.2) | |
| Unknown | 112 (70.9) | 136 (80.5) | |
| Autoimmune Disease—no. (%) | 0.06 | ||
| No | 128 (81.5) | 122 (72.6) | |
| Yes | 29 (18.5) | 46 (27.4) | |
| ECOG PS—no. (%) | 0.84 | ||
| <2 | 116 (73.4) | 125 (74.4) | |
| ≥2 | 42 (26.6) | 43 (25.6) | |
| Brain Metastases—no. (%) | 0.06 | ||
| No | 129 (82.7) | 152 (89.9) | |
| Yes | 27 (17.3) | 17 (10.1) | |
| Liver Metastases—no. (%) | 0.23 | ||
| No | 127 (80.9) | 145 (85.8) | |
| Yes | 30 (19.1) | 24 (14.2) | |
| LIPI—no. (%) | 0.03 | ||
| Good | 39 (32.8%) | 60 (46.2%) | |
| Poor/intermediate | 80 (67.2%) | 70 (53.8%) | |
| Clinical Outcome | Overall | Age | p-Value | |
|---|---|---|---|---|
| <70 (n = 158) | ≥70 (n = 169) | |||
| ORR—no. (%) | 79 (32.0) | 38 (33.3) | 41 (30.8) | 0.67 |
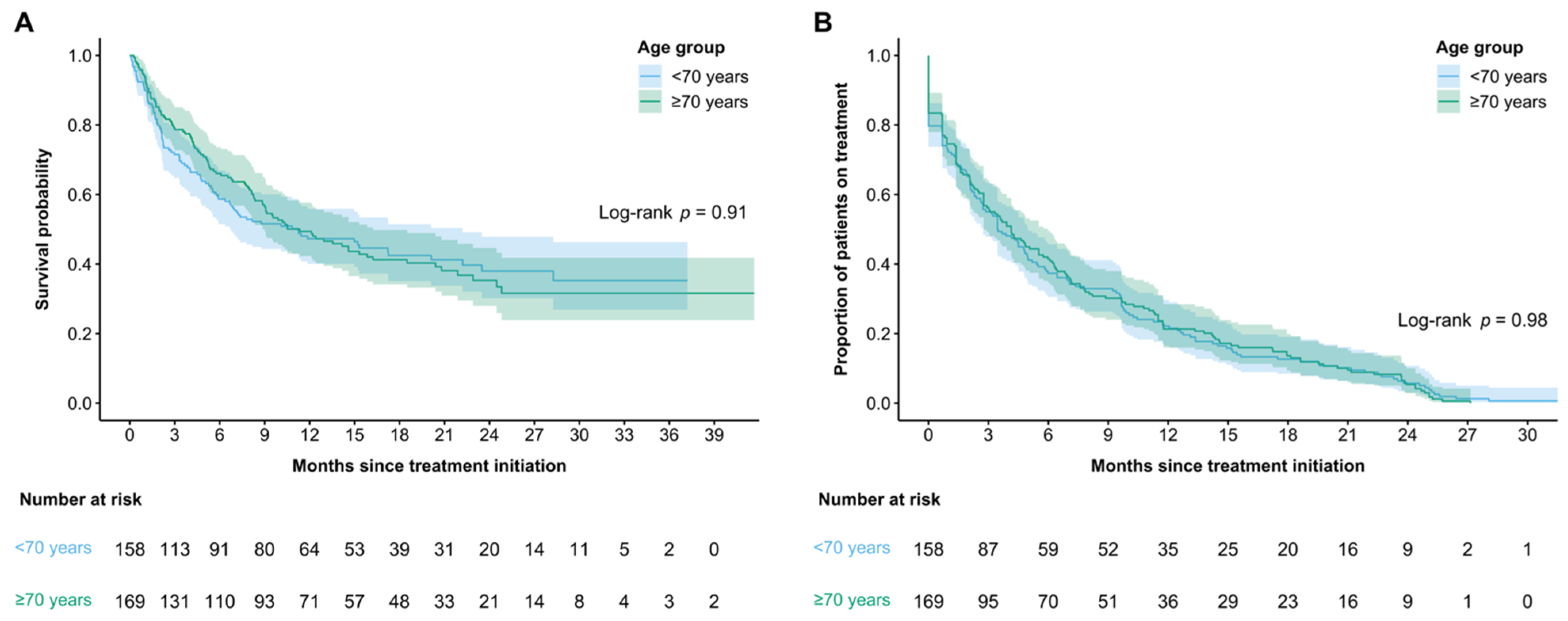
| mTTF—months (95% CI) | 3.91 (3.06–4.86) | 3.46 (2.73–4.99) | 4.14 (2.76–5.98) | 0.98 |
| mOS—months (95% CI) | 11.24 (8.77–15.31) | 11.2 (6.74–22.2) | 11.3 (8.87–16.2) | 0.91 |
| Landmark Analyses—no. (%) | ||||
| 3 month | 244 (74.6) | 113 (71.5) | 131 (77.5) | 0.21 |
| 12 month | 135 (41.3) | 64 (40.5) | 71 (42.0) | 0.78 |
| 24 month | 41 (12.5) | 20 (12.7) | 21 (12.4) | 0.95 |
| Subsequent Treatment—no. (%) | 61 (18.7) | 38 (24.1) | 23 (13.7) | 0.02 |
| Safety Outcomes | Age | p-Value | |
|---|---|---|---|
| <70 (n = 158) | ≥70 (n = 169) | ||
| Any Significant IrAE—no. (%) | 42 (26.6) | 45 (26.6) | 0.99 |
| Significant IrAE—no. (%) | 0.60 | ||
| Pneumonitis | 12 (28.6) | 16 (35.6) | |
| Colitis | 8 (19.0) | 5 (11.1) | |
| Arthritis | 5 (11.9) | 7 (15.6) | |
| Dermatologic | 6 (14.3) | 3 (6.7) | |
| Hepatitis | 1 (2.4) | 3 (6.7) | |
| Thyroiditis | 1 (2.4) | 2 (4.4) | |
| Adrenal insufficiency | 0 (0.0) | 2 (4.4) | |
| Other | 9 (21.4) | 7 (15.6) | |
| irAE Hospitalization—no. (%) | 13 (30.2) | 11 (25.6) | 0.63 |
| Any Hospitalization—no. (%) | 71 (44.9) | 79 (46.7) | 0.74 |
| Characteristic | OS HR (95% CI) | p-Value |
|---|---|---|
| Age | ||
| <70 (reference) versus ≥70 | 0.89 (0.62–1.26) | 0.50 |
| ECOG | ||
| <2 (reference) versus ≥2 | 2.49 (1.74–3.57) | <0.001 |
| Liver Metastases | ||
| No (reference) versus Yes | 1.06 (0.69–1.62) | 0.79 |
| BMI | ||
| <30 (reference) versus ≥30 | 1.63 (1.01–2.60) | 0.04 |
| PLR | ||
| <180 (reference) versus ≥180 | 0.85 (0.56–1.30) | 0.45 |
| LIPI | ||
| Good (reference) versus Int./Poor | 1.84 (1.26–2.68) | 0.001 |
| Albumin | ||
| ≥33 (reference) versus <33 | 1.48 (1.03–2.14) | 0.04 |
| Hemoglobin | ||
| ≥130 (reference) versus <130 | 1.45 (1.01–2.07) | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosjean, H.A.I.; Dolter, S.; Meyers, D.E.; Ding, P.Q.; Stukalin, I.; Goutam, S.; Kong, S.; Chu, Q.; Heng, D.Y.C.; Bebb, D.G.; et al. Effectiveness and Safety of First-Line Pembrolizumab in Older Adults with PD-L1 Positive Non-Small Cell Lung Cancer: A Retrospective Cohort Study of the Alberta Immunotherapy Database. Curr. Oncol. 2021, 28, 4213-4222. https://doi.org/10.3390/curroncol28050357
Grosjean HAI, Dolter S, Meyers DE, Ding PQ, Stukalin I, Goutam S, Kong S, Chu Q, Heng DYC, Bebb DG, et al. Effectiveness and Safety of First-Line Pembrolizumab in Older Adults with PD-L1 Positive Non-Small Cell Lung Cancer: A Retrospective Cohort Study of the Alberta Immunotherapy Database. Current Oncology. 2021; 28(5):4213-4222. https://doi.org/10.3390/curroncol28050357
Chicago/Turabian StyleGrosjean, Heidi A. I., Samantha Dolter, Daniel E. Meyers, Philip Q. Ding, Igor Stukalin, Siddhartha Goutam, Shiying Kong, Quincy Chu, Daniel Y. C. Heng, D. Gwyn Bebb, and et al. 2021. "Effectiveness and Safety of First-Line Pembrolizumab in Older Adults with PD-L1 Positive Non-Small Cell Lung Cancer: A Retrospective Cohort Study of the Alberta Immunotherapy Database" Current Oncology 28, no. 5: 4213-4222. https://doi.org/10.3390/curroncol28050357
APA StyleGrosjean, H. A. I., Dolter, S., Meyers, D. E., Ding, P. Q., Stukalin, I., Goutam, S., Kong, S., Chu, Q., Heng, D. Y. C., Bebb, D. G., Morris, D. G., Cheung, W. Y., & Pabani, A. (2021). Effectiveness and Safety of First-Line Pembrolizumab in Older Adults with PD-L1 Positive Non-Small Cell Lung Cancer: A Retrospective Cohort Study of the Alberta Immunotherapy Database. Current Oncology, 28(5), 4213-4222. https://doi.org/10.3390/curroncol28050357






