Cytochrome 4Z1 Expression Is Correlated with Poor Prognosis in Patients with Cervical Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Specimens
2.2. Immunohistochemistry
2.3. Scoring
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinicopathologic Features
3.2. Prevalence of CYP4Z1 Expression
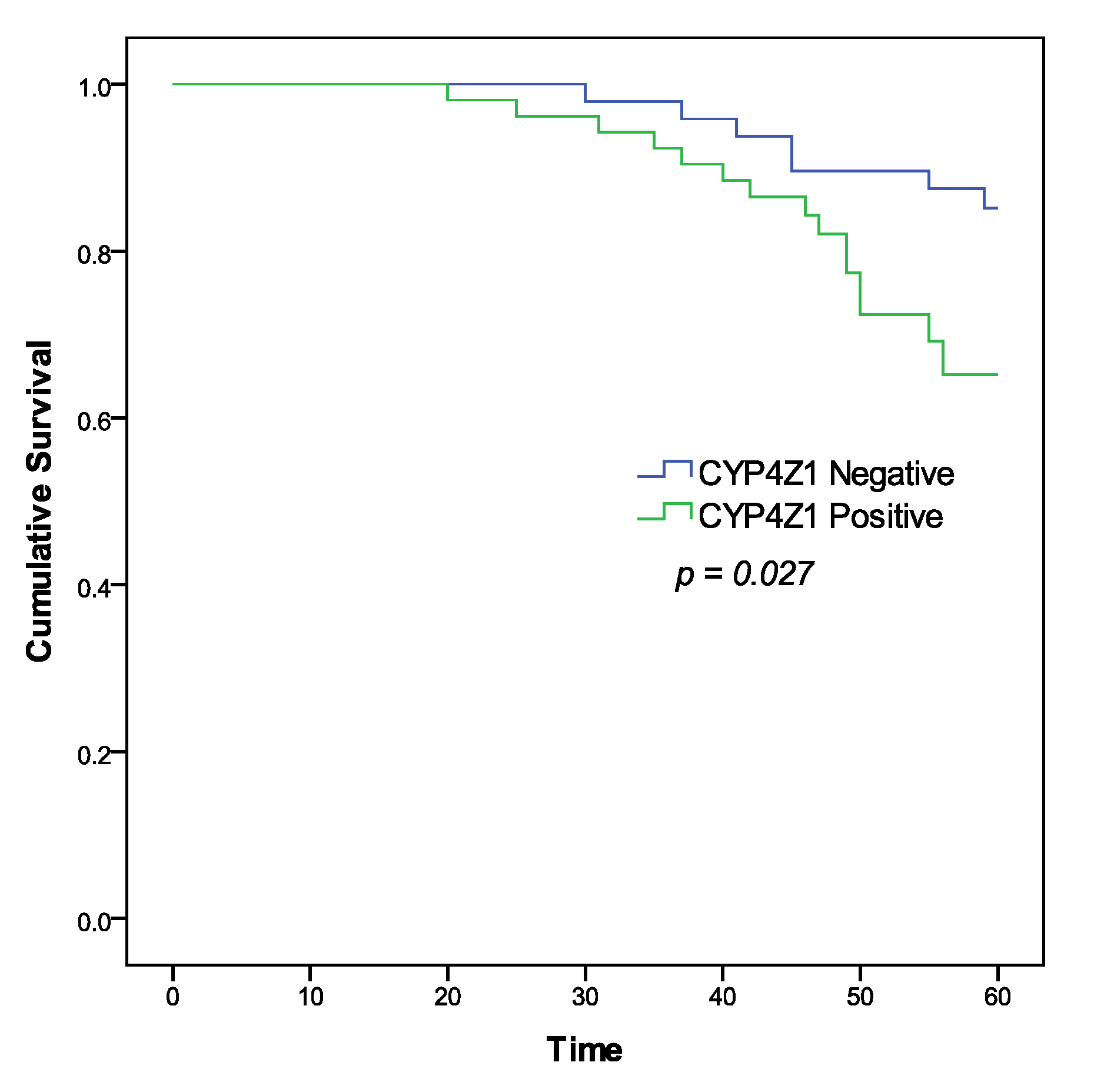
3.3. Survival Analysis and Prognostic Value of CYP4Z1 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalliala, I.; Athanasiou, A.; Veroniki, A.; Salanti, G.; Efthimiou, O.; Raftis, N.; Bowden, S.; Paraskevaidi, M.; Aro, K.; Arbyn, M. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: A systematic review and meta-analysis of the literature. Ann. Oncol. 2020, 31, 213–227. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Obeid, D.A.; Almatrrouk, S.A.; Alfageeh, M.B.; Al-Ahdal, M.N.; Alhamlan, F.S. Human papillomavirus epidemiology in populations with normal or abnormal cervical cytology or cervical cancer in the Middle East and North Africa: A systematic review and meta-analysis. J. Infect. Public Health 2020, 13, 1304–1313. [Google Scholar] [CrossRef]
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human papillomavirus infection and cervical cancer: Epidemiology, screening, and vaccination—review of current perspectives. J. Oncol. 2019, 2019, 3257939. [Google Scholar] [CrossRef] [PubMed]
- Alsbeih, G. HPV infection in cervical and other cancers in Saudi Arabia: Implication for prevention and vaccination. Front. Oncol. 2014, 4, 65. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Rajendran, P. The multifarious link between cytochrome P450s and cancer. Oxidative Med. Cell. Longev. 2020, 2020, 3028387. [Google Scholar] [CrossRef]
- Rieger, M.A.; Ebner, R.; Bell, D.R.; Kiessling, A.; Rohayem, J.; Schmitz, M.; Temme, A.; Rieber, E.P.; Weigle, B. Identification of a novel mammary-restricted cytochrome P450, CYP4Z1, with overexpression in breast carcinoma. Cancer Res. 2004, 64, 2357–2364. [Google Scholar] [CrossRef]
- Yang, X.; Hutter, M.; Goh, W.W.B.; Bureik, M. CYP4Z1—A Human Cytochrome P450 Enzyme that Might Hold the Key to Curing Breast Cancer. Curr. Pharm. Des. 2017, 23, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.I.; Patimalla, S.; Stewart, K.N.; Miller, I.D.; Heys, S.D. Profiling the expression of cytochrome P450 in breast cancer. Histopathology 2009, 57, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alboaisa, N.S.; Alrawashdeh, H.M.; Hamdan, O.; Al-Sarayreh, S.; Al-Shuneigat, J.M.; Nofal, M.N. Screening of cytochrome 4Z1 expression in human non-neoplastic, pre-neoplastic and neoplastic tissues. Ecancermedicalscience 2020, 14, 1114. [Google Scholar] [CrossRef] [PubMed]
- Tradonsky, A.; Rubin, T.; Beck, R.; Ring, B.; Seitz, R.; Mair, S. A search for reliable molecular markers of prognosis in prostate cancer: A study of 240 cases. Am. J. Clin. Pathol. 2012, 137, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Downie, D.; McFadyen, M.C.; Rooney, P.H.; Cruickshank, M.E.; Parkin, D.E.; Miller, I.D.; Telfer, C.; Melvin, W.T.; Murray, G.I. Profiling cytochrome P450 expression in ovarian cancer: Identification of prognostic markers. Clin. Cancer Res. 2005, 11, 7369–7375. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alshammari, F.O.; Youssef, A.M.; Al-Sarayreh, S.; Almuhaisen, G.H.; Alnawaiseh, N.; Al Shuneigat, J.M.; Alrawashdeh, H.M. Profiling of CYP4Z1 and CYP1B1 expression in bladder cancers. Sci. Rep. 2021, 11, 5581. [Google Scholar] [CrossRef]
- Li, Y.; Steppi, A.; Zhou, Y.; Mao, F.; Miller, P.C.; He, M.M.; Zhao, T.; Sun, Q.; Zhang, J. Tumoral expression of drug and xenobiotic metabolizing enzymes in breast cancer patients of different ethnicities with implications to personalized medicine. Sci. Rep. 2017, 7, 4747. [Google Scholar] [CrossRef]
- Khayeka-Wandabwa, C.; Ma, X.; Cao, X.; Nunna, V.; Pathak, J.L.; Bernhardt, R.; Cai, P.; Bureik, M. Plasma membrane localization of CYP4Z1 and CYP19A1 and the detection of anti-CYP19A1 autoantibodies in humans. Int. Immunopharmacol. 2017, 73, 64–71. [Google Scholar] [CrossRef]
- Nunna, V.; Jalal, N.; Bureik, M. Anti-CYP4Z1 autoantibodies detected in breast cancer patients. Cell Mol. Immunol. 2017, 14, 572–574. [Google Scholar] [CrossRef]
- Savas, U.; Hsu, M.H.; Griffin, K.J.; Bell, D.R.; Johnson, E.F. Conditional regulation of the human CYP4X1 and CYP4Z1 genes. Arch. Biochem. Biophys. 2005, 436, 377–385. [Google Scholar] [CrossRef]
- Yu, W.; Chai, H.; Li, Y.; Zhao, H.; Xie, X.; Zheng, H.; Wang, C.; Wang, X.; Yang, G.; Cai, X.; et al. Increased expression of CYP4Z1 promotes tumor angiogenesis and growth in human breast cancer. Toxicol. Appl. Pharm. 2012, 264, 73–83. [Google Scholar] [CrossRef]
- Zheng, L.; Li, X.; Gu, Y.; Lv, X.; Xi, T. The 3′UTR of the pseudogene CYP4Z2P promotes tumor angiogenesis in breast cancer by acting as a ceRNA for CYP4Z1. Breast Cancer Res. Treat. 2015, 150, 105–118. [Google Scholar] [CrossRef]
- Zollner, A.; Dragan, C.A.; Pistorius, D.; Muller, R.; Bode, H.B.; Peters, F.T.; Maurer, H.H.; Bureik, M. Human CYP4Z1 catalyzes the in-chain hydroxylation of lauric acid and myristic acid. Biol. Chem. 2009, 390, 313–317. [Google Scholar] [CrossRef]
- McDonald, M.G.; Ray, S.; Amorosi, C.J.; Sitko, K.A.; Kowalski, J.P.; Paco, L.; Nath, A.; Gallis, B.; Totah, R.A.; Dunham, M.J.; et al. Expression and Functional Characterization of Breast Cancer-Associated Cytochrome P450 4Z1 in Saccharomyces cerevisiae. Drug. Metab. Dispos. 2017, 45, 1364–1371. [Google Scholar] [CrossRef]
- Evangelista, E.A.; Cho, C.W.; Aliwarga, T.; Totah, R.A. Expression and function of eicosanoid-producing cytochrome P450 enzymes in solid tumors. Front. Pharmacol. 2020, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Guo, Q.; Xiang, C.; Liu, S.; Jiang, Y.; Gao, L.; Ni, H.; Wang, T.; Zhao, Q.; Liu, H.; et al. Transcriptional factor six2 promotes the competitive endogenous RNA network between CYP4Z1 and pseudogene CYP4Z2P responsible for maintaining the stemness of breast cancer cells. J. Hematol. Oncol. 2019, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002, 82, 131–185. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Edson, K.Z.; Totah, R.A.; Rettie, A.E. Cytochrome P450 omega-Hydroxylases in Inflammation and Cancer. Adv. Pharm. 2015, 74, 223–262. [Google Scholar] [CrossRef]
- Chen, L.; Ackerman, R.; Guo, A.M. 20-HETE in neovascularization. Prostaglandins Other Lipid Mediat. 2012, 98, 63–68. [Google Scholar] [CrossRef]
- Cheranov, S.Y.; Karpurapu, M.; Wang, D.; Zhang, B.; Venema, R.C.; Rao, G.N. An essential role for SRC-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood 2008, 111, 5581–5591. [Google Scholar] [CrossRef] [PubMed]
- Sausville, L.N.; Williams, S.M.; Pozzi, A. Cytochrome P450 epoxygenases and cancer: A genetic and a molecular perspective. Pharmacol. Ther. 2019, 196, 183–194. [Google Scholar] [CrossRef]
- Stark, K.; Dostalek, M.; Guengerich, F.P. Expression and purification of orphan cytochrome P450 4X1 and oxidation of anandamide. FEBS J. 2008, 275, 3706–3717. [Google Scholar] [CrossRef]
- Lee, C.M.; Fuhrman, C.B.; Planelles, V.; Peltier, M.R.; Gaffney, D.K.; Soisson, A.P.; Dodson, M.K.; Tolley, H.D.; Green, C.L.; Zempolich, K.A. Phosphatidylinositol 3-kinase inhibition by LY294002 radiosensitizes human cervical cancer cell lines. Clin. Cancer Res. 2006, 12, 250–256. [Google Scholar] [CrossRef]
- McFarlane, M.; Graham, S.V. Human papillomavirus regulation of SR proteins. Biochem. Soc. Trans. 2010, 38, 1116–1121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Wu, J.; Ling, M.T.; Zhao, L.; Zhao, K.-N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer 2015, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Machalz, D.; Yan, Q.; Sorensen, E.J.; Wolber, G.; Bureik, M. Importance of asparagine-381 and arginine-487 for substrate recognition in CYP4Z1. Biochem. Pharmacol. 2020, 174, 113850. [Google Scholar] [CrossRef]
- Yan, Q.; Machalz, D.; Zöllner, A.; Sorensen, E.J.; Wolber, G.; Bureik, M. Efficient substrate screening and inhibitor testing of human CYP4Z1 using permeabilized recombinant fission yeast. Biochem. Pharmacol. 2017, 146, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Machalz, D.; Li, H.; Du, W.; Sharma, S.; Liu, S.; Bureik, M.; Wolber, G. Discovery of a Novel Potent Cytochrome P450 CYP4Z1 Inhibitor. Eur. J. Med. Chem. 2021, 215, 113255. [Google Scholar] [CrossRef]
- Kowalski, J.P.; McDonald, M.G.; Pelletier, R.D.; Hanenberg, H.; Wiek, C.; Rettie, A.E. Design and Characterization of the First Selective and Potent Mechanism-Based Inhibitor of Cytochrome P450 4Z1. J. Med. Chem. 2020, 63, 4824–4836. [Google Scholar] [CrossRef] [PubMed]
- Buonaguro, F.M.; Pauza, D.; Tornesello, M.L.; Hainaut, P.; Franco, R.; Marincola, F.M. Cancer diagnostic and predictive biomarkers. BioMed Res. Int. 2014, 2014, 980163. [Google Scholar] [CrossRef]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Maruvada, P.; Wang, W.; Wagner, P.D.; Srivastava, S. Biomarkers in molecular medicine: Cancer detection and diagnosis. Biotechniques 2005, 38, S9–S15. [Google Scholar] [CrossRef] [PubMed]
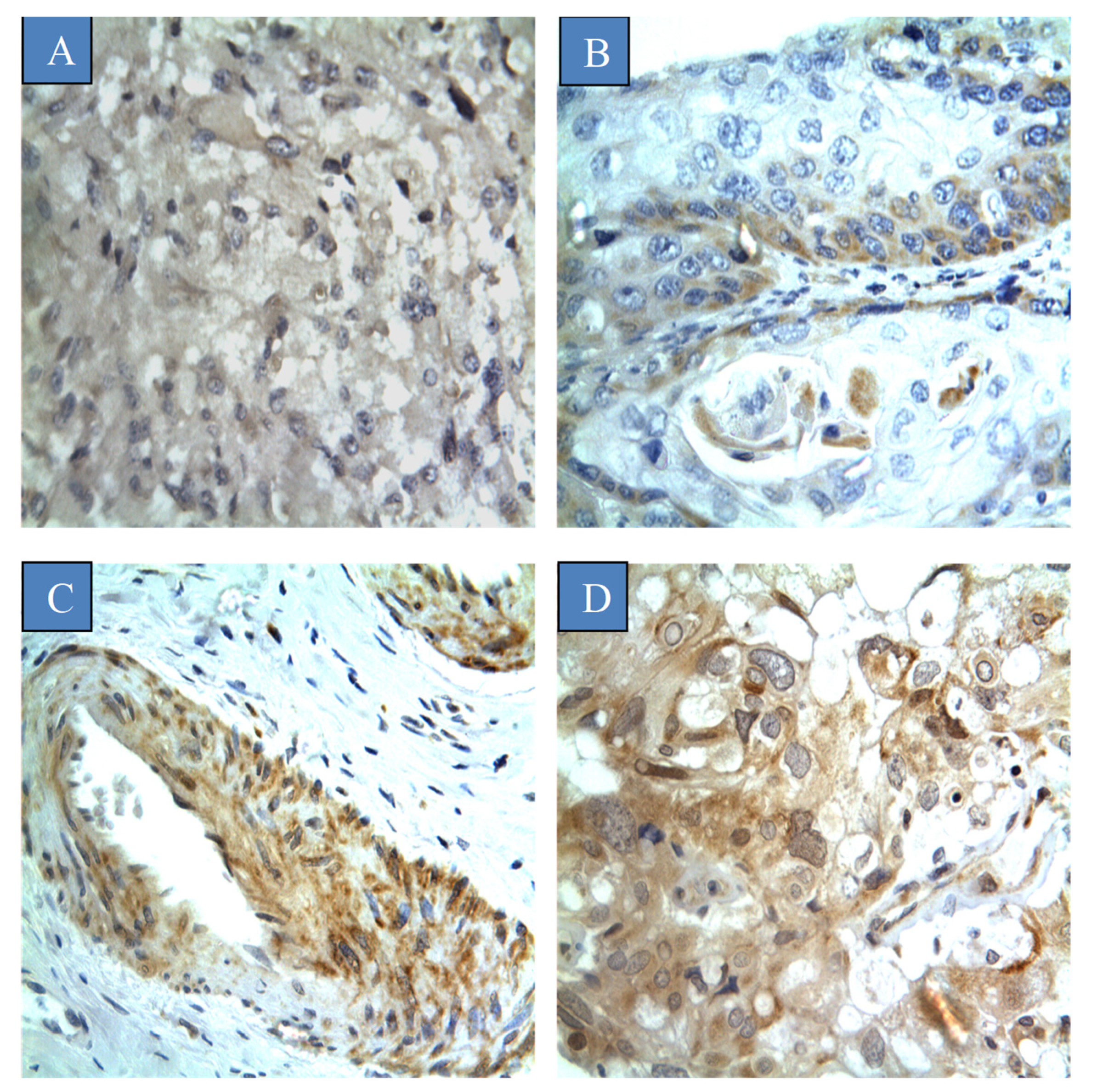
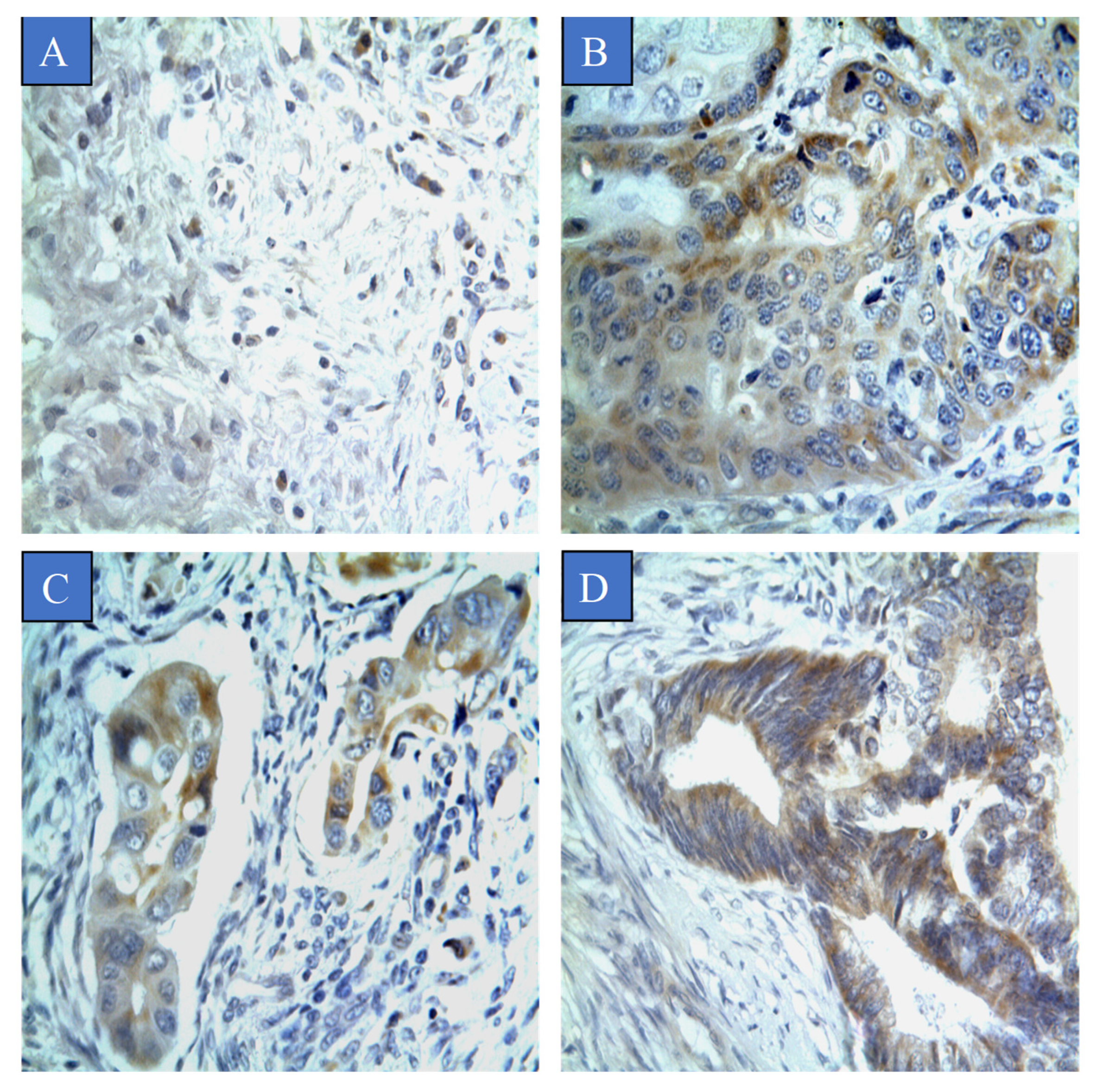
| CYP4Z1 Expression | |||||
|---|---|---|---|---|---|
| Characteristic | Negative | Low | Medium | High | p Value |
| Age: | |||||
| <50 (n = 69, 62.7%) | 30 (43.5%) | 12 (17.4%) | 19 (27.5%) | 8 (11.6%) | 0.104 |
| ≥50 (n = 41, 37.3%) | 25 (61%) | 3 (7.3%) | 12 (29.3%) | 1 (2.4%) | |
| Pathology subtype: | |||||
| Squamous cell carcinoma (n = 95, 86.4%) | 45 (47.4%) | 15 (15.8%) | 29 (30.5%) | 6 (6.3%) | 0.011 |
| Adenocarcinoma (n = 3, 2.7%) | 0 (0%) | 0 (0%) | 1 (33.3%) | 2 (66.7%) | |
| Endometrioid adenocarcinoma (n = 2, 1.8%) | 0 (0%) | 0 (0%) | 1 (50%) | 1 (50%) | |
| Normal (n = 10, 9.1%) | 10 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Histological grade: | |||||
| I (n = 9, 9%) | 4 (44.4%) | 1 (11.1%) | 3 (33.3%) | 1 (11.1%) | 0.594 |
| II (n = 27, 27%) | 10 (37%) | 3 (11.1%) | 12 (44.4%) | 2 (7.4%) | |
| III (n = 64, 64%) | 34 (53.1%) | 11 (17.2%) | 16 (25%) | 3 (4.7%) | |
| Histological stage: | |||||
| I (n = 56, 56%) | 47 (83.9%) | 9 (16.1%) | 0 (0%) | 0 (0%) | 0.001 |
| II (n = 38, 38%) | 1 (2.6%) | 6 (15.8%) | 31 (81.6%) | 0 (0%) | |
| III (n = 6, 6%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (100%) | |
| Tumour invasion: | |||||
| T1 (n = 59, 59%) | 47 (79.7%) | 9 (15.3%) | 0 (0%) | 3 (5.1%) | 0.007 |
| T2 (n = 38, 38%) | 1 (2.6%) | 6 (15.8%) | 31 (81.6%) | 0 (0%) | |
| T3 (n = 3, 3%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (100%) | |
| Lymph node metastasis: | |||||
| Negative (n = 95, 95%) | 48 (50.5%) | 15 (15.8%) | 31 (32.6%) | 1 (1.1%) | 0.003 |
| Positive (n = 5, 5%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (100%) | |
| Distant metastasis: | |||||
| Negative (n = 100, 100%) | 48 (48%) | 15 (15%) | 31 (31%) | 6 (6%) | 0.203 |
| Positive (n = 0, 0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| HPV16/18 status: | |||||
| Low (n = 22, 22%) | 13 (59.1%) | 1 (4.5%) | 8 (36.4%) | 0 (0%) | 0.231 |
| Moderate (n = 46, 46%) | 18 (39.1%) | 10 (21.7%) | 13 (28.3%) | 5 (10.9%) | |
| High (n = 32, 32%) | 17 (53.1%) | 4 (12.5%) | 10 (31.3%) | 1 (3.1%) | |
| Ki67 status: | |||||
| Low (n = 47, 47%) | 22 (46.8%) | 6 (12.8%) | 16 (34%) | 3 (6.4%) | 0.749 |
| Moderate (n = 35, 35%) | 19 (54.3%) | 6 (17.1%) | 8 (22.9%) | 2 (5.7%) | |
| High (n = 18, 18%) | 7 (38.9%) | 3 (16.7%) | 7 (28.9%) | 1 (5.6%) | |
| Prognostic Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95%Cl | p-Value | HR | 95% Cl | p-Value | |
| Age | 0.977 | 0.930–1.127 | 0.364 | 0.997 | 0.943–1.054 | 0.911 |
| Histological grade | 1.818 | 0.659–5.013 | 0.248 | 1.308 | 0.580–2.952 | 0.518 |
| Histological stage | 8.422 | 3.486–13.347 | 0.007 | 7.384 | 3.318–11.381 | 0.023 |
| Tumour invasion | 3.445 | 1.382–8.589 | 0.008 | 0.363 | 0.267–1.621 | 0.084 |
| Lymph node metastasis | 7.995 | 4.778–12.253 | 0.003 | 6.543 | 3.979–11.416 | 0.061 |
| HPV16/18 | 1.334 | 0.690–2.619 | 0.385 | 1.135 | 0.535–2.405 | 0.741 |
| Ki67 | 0.730 | 0.371–1.438 | 0.363 | 0.649 | 0.334–1.261 | 0.202 |
| CYP4Z1 expression | 1.531 | 1.154–1.943 | 0.002 | 1.113 | 1.059–1.743 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-saraireh, Y.M.; Alshammari, F.O.F.O.; Youssef, A.M.M.; Al-sarayra, Y.M.; Al-saraireh, R.A.; Al-muhaisen, G.H.; Al-mahdy, Y.S.; Al-Kharabsheh, A.M.; Abufraijeh, S.M.; Alrawashdeh, H.M. Cytochrome 4Z1 Expression Is Correlated with Poor Prognosis in Patients with Cervical Cancer. Curr. Oncol. 2021, 28, 3573-3584. https://doi.org/10.3390/curroncol28050306
Al-saraireh YM, Alshammari FOFO, Youssef AMM, Al-sarayra YM, Al-saraireh RA, Al-muhaisen GH, Al-mahdy YS, Al-Kharabsheh AM, Abufraijeh SM, Alrawashdeh HM. Cytochrome 4Z1 Expression Is Correlated with Poor Prognosis in Patients with Cervical Cancer. Current Oncology. 2021; 28(5):3573-3584. https://doi.org/10.3390/curroncol28050306
Chicago/Turabian StyleAl-saraireh, Yousef M., Fatemah O. F. O. Alshammari, Ahmed M. M. Youssef, Yahya M. Al-sarayra, Renata A. Al-saraireh, Ghadeer H. Al-muhaisen, Yanal S. Al-mahdy, Ahlam M. Al-Kharabsheh, Seham M. Abufraijeh, and Hamzeh Mohammad Alrawashdeh. 2021. "Cytochrome 4Z1 Expression Is Correlated with Poor Prognosis in Patients with Cervical Cancer" Current Oncology 28, no. 5: 3573-3584. https://doi.org/10.3390/curroncol28050306
APA StyleAl-saraireh, Y. M., Alshammari, F. O. F. O., Youssef, A. M. M., Al-sarayra, Y. M., Al-saraireh, R. A., Al-muhaisen, G. H., Al-mahdy, Y. S., Al-Kharabsheh, A. M., Abufraijeh, S. M., & Alrawashdeh, H. M. (2021). Cytochrome 4Z1 Expression Is Correlated with Poor Prognosis in Patients with Cervical Cancer. Current Oncology, 28(5), 3573-3584. https://doi.org/10.3390/curroncol28050306





