Mismatch Repair Protein Expression and Microsatellite Instability in Cutaneous Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry
2.3. Microscopic Evaluation
2.4. Multiplex-PCR and High-Resolution Capillary Electrophoresis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Lesiak, A. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Susok, L. Fortgeschrittene Basalzell- und Plattenepithelkarzinome der Haut. Best Pract. Onkol. 2019, 6, 262–271. [Google Scholar] [CrossRef]
- Wessely, A.; Steeb, T.; Leiter, U.; Garbe, C.; Berking, C.; Heppt, M.V. Immune Checkpoint Blockade in Advanced Cutaneous Squamous Cell Carcinoma: What Do We Currently Know in 2020? Int. J. Mol. Sci. 2020, 21, 9300. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.; Merlin, J.L.; Harlé, A. Detection of Microsatellite Instability: State of the Art and Future Applications in Circulating Tumour DNA (ctDNA). Cancers 2021, 13, 1491. [Google Scholar] [CrossRef] [PubMed]
- Perrett, C.M.; Harwood, C.A.; McGregor, J.M.; Warwick, J.; Cerio, R.; Karran, P. Expression of DNA mismatch repair proteins and MSH2 polymorphisms in nonmelanoma skin cancers of organ transplant recipients. Br. J. Dermatol. 2010, 162, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Saetta, A.A.; Stamatelli, A.; Karlou, M.; Michalopoulos, N.V.; Patsouris, E.; Aroni, K. Mutations of microsatellite instability target genes in sporadic basal cell carcinomas. Pathol. Res. Pract. 2007, 203, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Borie, C.; Euvrard, S.; Vérola, O.; Buhard, O.; Barete, S.; Molina, J.M.; Kanitakis, J.; Kérob, D.; Lebbé, C.; Duval, A. No evidence for microsatellite instability in immunodeficiency-related skin cancers. Am. J. Transplant. 2010, 10, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Wisgerhof, H.C.; Hameetman, L.; Tensen, C.P.; Morreau, H.; de Fijter, J.W.; Bouwes Bavinck, J.N.; Willemze, R.; de Gruijl, F.R. Azathioprine-induced microsatellite instability is not observed in skin carcinomas of organ transplant recipients. J. Investig. Dermatol. 2009, 129, 1307–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosterd, K.; Nellen, R.G.; van Engeland, M.; van Geel, M.; van Steensel, M.A. Defects in DNA mismatch repair do not account for early-onset basal cell carcinoma. Br. J. Dermatol. 2008, 159, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Vatrano, S.; Pettinato, A.; Randazzo, V.; Zagami, M.; Agueli, C.; Cannella, S.; Banna, G.L.; Fraggetta, F.; Santoro, A. Diagnostic test assessment. Validation study of an alternative system to detect microsatellite instability in colorectal carcinoma. Pathol. J. Ital. Soc. Anat. Pathol. Diagn. Cytopathol. 2020, 112, 178–183. [Google Scholar]
- Reuschenbach, M.; Sommerer, C.; Hartschuh, W.; Zeier, M.; Doeberitz Mv Kloor, M. Absence of mismatch repair deficiency-related microsatellite instability in non-melanoma skin cancer. J. Investig. Dermatol. 2012, 132, 491–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, L.C.; Listgarten, J.; Trotter, M.J.; Andrew, S.E.; Tron, V.A. Evidence that dysregulated DNA mismatch repair characterizes human nonmelanoma skin cancer. Br. J. Dermatol. 2008, 158, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hatta, N.; Takata, A.; Ishizawa, S.; Niida, Y. Family with MSH2 mutation presenting with keratoacanthoma and precancerous skin lesions. J. Dermatol. 2015, 42, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.B.; Furihata, M.; Takeuchi, T.; Sonobe, H.; Ohtsuki, Y. Reduced human mismatch repair protein expression in the development of precancerous skin lesions to squamous cell carcinoma. Virchows Arch. 2001, 439, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Korabiowska, M.; Cordon-Cardo, C.; Jaenckel, F.; Stachura, J.; Fischer, G.; Brinck, U. Application of in situ hybridization probes for MLH-1 and MSH-2 in tissue microarrays of paraffin-embedded malignant melanomas: Correlation with immunohistochemistry and tumor stage. Hum. Pathol. 2004, 35, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Pellacani, G.; Tomasi, A.; Depenni, R.; Maccaferri, M.; Maiorana, A.; Orsi, G.; Giusti, F.; Cascinu, S.; Manfredini, M. Immunohistochemical mismatch repair proteins expression as a tool to predict the melanoma immunotherapy response. Mol. Clin. Oncol. 2020, 12, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambichler, T.; Abu Rached, N.; Tannapfel, A.; Becker, J.C.; Vogt, M.; Skrygan, M.; Wieland, U.; Silling, S.; Susok, L.; Stücker, M.; et al. Expression of Mismatch Repair Proteins in Merkel Cell Carcinoma. Cancers 2021, 13, 2524. [Google Scholar] [CrossRef] [PubMed]
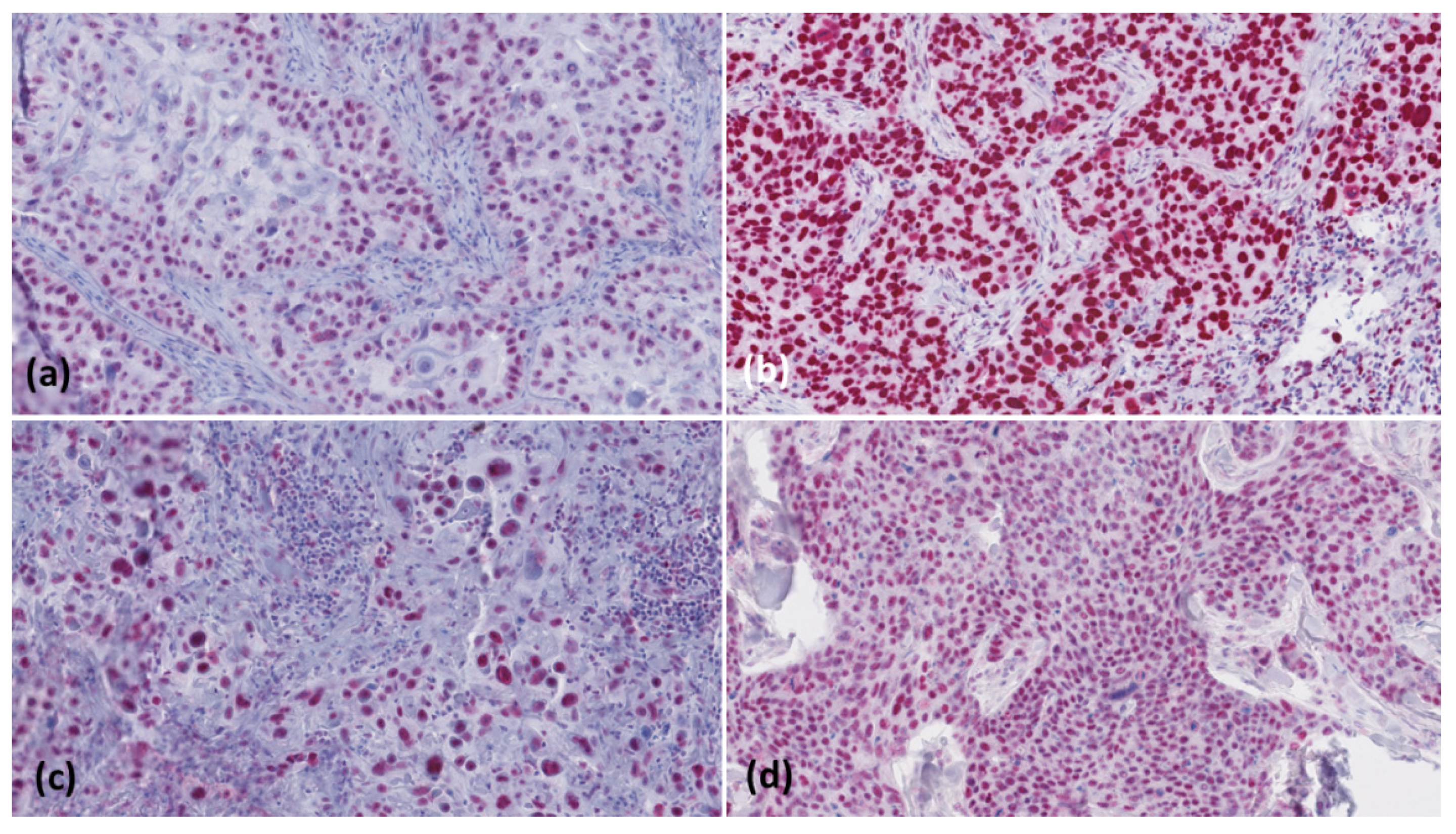
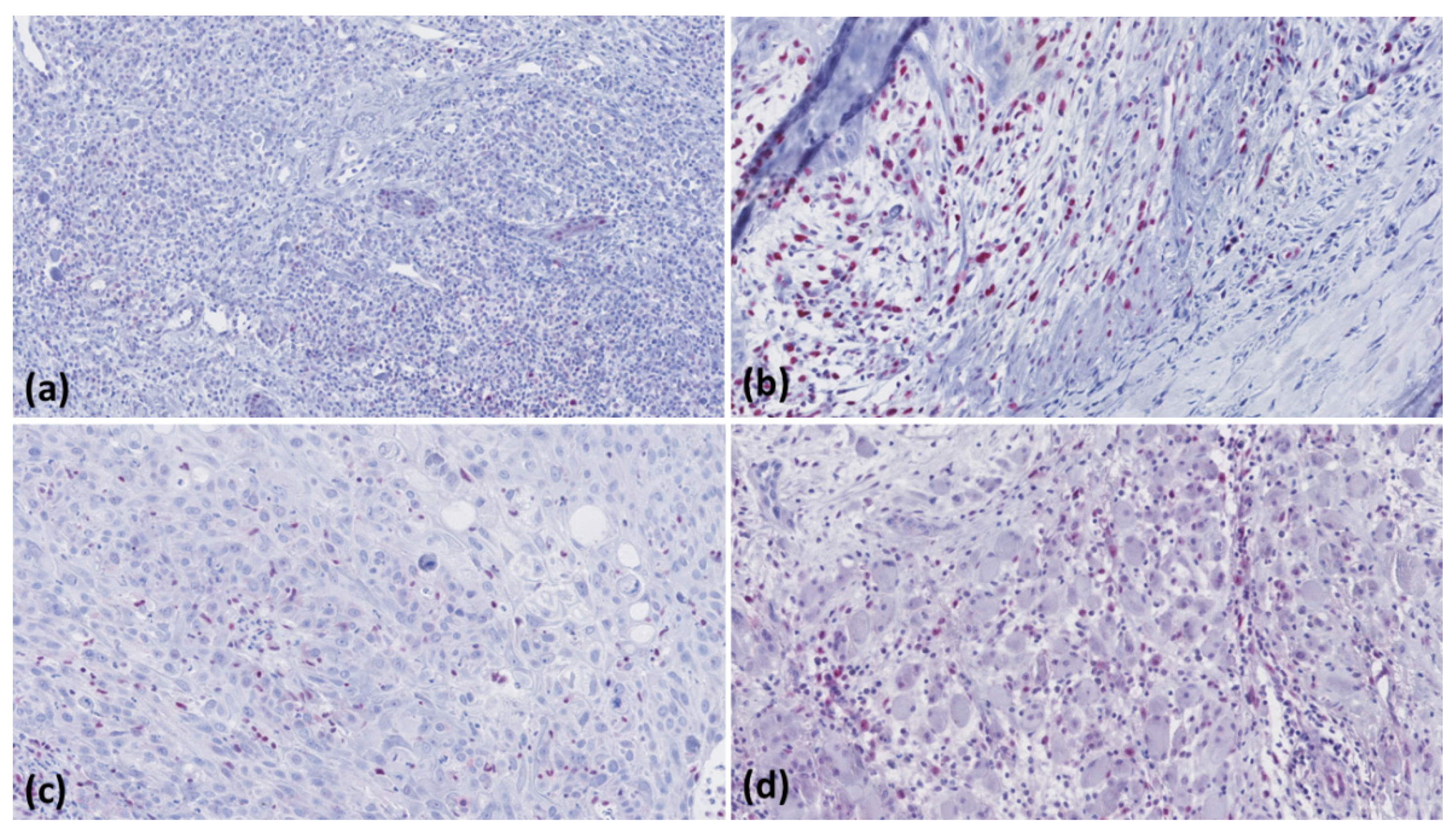
| Tumour | Actinic Keratosis | Bowen’s Disease | cSCC < 2 mm Tumour Thickness | cSCC > 6 mm Tumour Thickness | cSCC Metastases |
|---|---|---|---|---|---|
| n = | 20 | 21 | 20 | 21 | 20 |
| Case# | MLH1 | MSH2 | MSH6 | PMS2 |
|---|---|---|---|---|
| 0.22 * | 99.45 | 99.81 | 68.83 |
| 99.78 | 0.59 * | 43.67 * | 98.81 |
| 98.69 | 95.76 | 99.67 | 2.32 * |
| 97.8 | 91.19 | 100 | 0.42 * |
| 95.34 | 0.64 * | 12.07 | 93.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambichler, T.; Ganjuur, N.; Tannapfel, A.; Vogt, M.; Scholl, L.; Abu Rached, N.; Bruckmüller, S.; Skrygan, M.; Becker, J.C.; Käfferlein, H.U.; et al. Mismatch Repair Protein Expression and Microsatellite Instability in Cutaneous Squamous Cell Carcinoma. Curr. Oncol. 2021, 28, 3316-3322. https://doi.org/10.3390/curroncol28050287
Gambichler T, Ganjuur N, Tannapfel A, Vogt M, Scholl L, Abu Rached N, Bruckmüller S, Skrygan M, Becker JC, Käfferlein HU, et al. Mismatch Repair Protein Expression and Microsatellite Instability in Cutaneous Squamous Cell Carcinoma. Current Oncology. 2021; 28(5):3316-3322. https://doi.org/10.3390/curroncol28050287
Chicago/Turabian StyleGambichler, Thilo, Nomun Ganjuur, Andrea Tannapfel, Markus Vogt, Lisa Scholl, Nessr Abu Rached, Stefanie Bruckmüller, Marina Skrygan, Jürgen C. Becker, Heiko U. Käfferlein, and et al. 2021. "Mismatch Repair Protein Expression and Microsatellite Instability in Cutaneous Squamous Cell Carcinoma" Current Oncology 28, no. 5: 3316-3322. https://doi.org/10.3390/curroncol28050287
APA StyleGambichler, T., Ganjuur, N., Tannapfel, A., Vogt, M., Scholl, L., Abu Rached, N., Bruckmüller, S., Skrygan, M., Becker, J. C., Käfferlein, H. U., Brüning, T., & Lang, K. (2021). Mismatch Repair Protein Expression and Microsatellite Instability in Cutaneous Squamous Cell Carcinoma. Current Oncology, 28(5), 3316-3322. https://doi.org/10.3390/curroncol28050287








