Safety of Immune Checkpoint Inhibitors in Elderly Patients: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anisimov, V.N.; Sikora, E. Relationships between cancer and aging: A multilevel approach. Biogerontology 2009, 10, 323–338. [Google Scholar] [CrossRef]
- Saavedra, D.; Garcia, B. T Cell Subpopulations in Healthy Elderly and Lung Cancer Patients: Insights from Cuban Studies. Front. Immun. 2017, 8, 146. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- World Health Organization. Life Expectancy and Healthy Life Expectancy. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy (accessed on 23 July 2021).
- Balducci, L. Geriatric oncology: Challenges for the new century. Eur. J. Cancer 2000, 36, 1741–1754. [Google Scholar] [CrossRef]
- Valero, J.G.; Matas-Céspedes, A.; Arenas, F.; Rodriguez, V.; Carreras, J.; Serrat, N.; Guerrero-Hernández, M.; Yahiaoui, A.; Balagué, O.; Martin, S.; et al. The receptor of the colony-stimulating factor-1 (CSF-1R) is a novel prognostic factor and therapeutic target in follicular lymphoma. Leukemia 2021. [Google Scholar] [CrossRef]
- Carreras, J.; Kikuti, Y.Y.; Miyaoka, M.; Hiraiwa, S.; Tomita, S.; Ikoma, H.; Kondo, Y.; Ito, A.; Nakamura, N.; Hamoudi, R. A Combination of Multilayer Perceptron, Radial Basis Function Artificial Neural Networks and Machine Learning Image Segmentation for the Dimension Reduction and the Prognosis Assessment of Diffuse Large B-Cell Lymphoma. AI 2021, 2, 106–134. [Google Scholar] [CrossRef]
- Grossi, F.; Crinò, L.; Logroscino, A.; Canova, S.; Delmonte, A.; Melotti, B.; Proto, C.; Gelibter, A.; Cappuzzo, F.; Turci, D.; et al. Use of nivolumab in elderly patients with advanced squamous non-small-cell lung cancer: Results from the Italian cohort of an expanded access programme. Eur. J. Cancer 2018, 100, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Migliorino, M.R.; Gelibter, A.; Grossi, F.; Fagnani, D.; Bordi, P.; Franchina, T.; Turci, D.; Di Lauro, L.; Cascinu, S.; Calabro, L.; et al. Use of nivolumab in elderly patients with advanced non-squamous NSCLC: Results from the Italian expanded access program (EAP). Ann. Oncol. 2017, 28, v471. [Google Scholar] [CrossRef]
- Smit, H.J.M.; Aerts, J.; Heuvel, M.V.D.; Hiltermann, T.; Bahce, I.; Smit, E.; Dingemans, A.-M.; Hendriks, L.; Stigt, J.; Schramel, F.; et al. Effects of checkpoint inhibitors in advanced non-small cell lung cancer at population level from the National Immunotherapy Registry. Lung Cancer 2020, 140, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, O.; Imai, H.; Minemura, H.; Suzuki, K.; Wasamoto, S.; Umeda, Y.; Osaki, T.; Kasahara, N.; Uchino, J.; Sugiyama, T.; et al. Efficacy and safety of immune checkpoint inhibitor monotherapy in pretreated elderly patients with non-small cell lung cancer. Cancer Chemother. Pharmacol. 2020, 85, 761–771. [Google Scholar] [CrossRef] [PubMed]
- de Giorgi, U.; Cartenì, G.; Giannarelli, D.; Basso, U.; Galli, L.; Cortesi, E.; Caserta, C.; Pignata, S.; Sabbatini, R.; Bearz, A.; et al. Safety and efficacy of nivolumab for metastatic renal cell carcinoma: Real-world results from an expanded access programme. BJU Int. 2019, 123, 98–105. [Google Scholar] [CrossRef]
- Vitale, M.G.; Scagliarini, S.; Galli, L.; Pignata, S.; Re, G.L.; Berruti, A.; Defferrari, C.; Spada, M.; Masini, C.; Santini, D.; et al. Efficacy and safety data in elderly patients with metastatic renal cell carcinoma included in the nivolumab Expanded Access Program (EAP) in Italy. PLoS ONE 2018, 13, e0199642. [Google Scholar] [CrossRef]
- Youn, B.; Trikalinos, N.A. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non–small cell lung cancer. Cancer 2020, 126, 978–985. [Google Scholar] [CrossRef]
- Takigawa, N.; Ochi, N.; Nakagawa, N.; Nagasaki, Y.; Taoka, M.; Ichiyama, N.; Mimura, A.; Nakanishi, H.; Kohara, H.; Yamane, H. Do elderly lung cancer patients aged ≥75 years benefit from immune checkpoint inhibitors? Cancer 2020, 12, 1995. [Google Scholar] [CrossRef]
- Kanesvaran, R.; Cordoba, R. Immunotherapy in Older Adults with Advanced Cancers: Implications for Clinical Decision-Making and Future Research. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 400–414. [Google Scholar] [CrossRef] [PubMed]
- van Holstein, Y.; Kapiteijn, E. Efficacy and Adverse Events of Immunotherapy with Checkpoint Inhibitors in Older Patients with Cancer. Drugs Aging 2019, 36, 927–938. [Google Scholar] [CrossRef]
- Sattar, J.; Kartolo, A. The efficacy and toxicity of immune checkpoint inhibitors in a real-world older patient population. J. Geriatr. Oncol. 2019, 10, 411–414. [Google Scholar] [CrossRef]
- Betof, A.S.; Nipp, R.D.; Giobbie-Hurder, A.; Johnpulle, R.A.N.; Rubin, K.; Rubinstein, S.M.; Flaherty, K.T.; Lawrence, D.P.; Johnson, D.B.; Sullivan, R.J. Impact of Age on Outcomes with Immunotherapy for Patients with Melanoma. Oncologist 2017, 22, 963–971. [Google Scholar] [CrossRef]
- Fulop, T.; Dupuis, G.; Baehl, S.; Le Page, A.; Bourgade, K.; Frost, E.; Witkowski, J.M.; Pawelec, G.; Larbi, A.; Cunnane, S. From inflamm-aging to immune-paralysis: A slippery slope during aging for immune-adaptation. Biogerontology 2016, 17, 147–157. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Brüünsgaard, H.; Pedersen, B.K. Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. 2003, 23, 15–39. [Google Scholar] [CrossRef]
- Spano, J.; Chaïbi, P.; Vignot, S.; Thery, J.C.; Rouge, T.D.L.M.; Gil-Delgado, M.; Khayat, D.; Mouawad, R. Age-related changes in plasma levels of inflammatory and angiogenic cytokines in patients with cancer. J. Clin. Oncol. 2011, 29, e19699. [Google Scholar] [CrossRef]
- Cunha, L.L.; Perazzio, S.F. Remodeling of the Immune Response with Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response. Front. Immunol. 2020, 11, 1748. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi (Version 1.6) [Computer Software]. 2021. Available online: https://www.jamovi.org (accessed on 9 January 2021).
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Samani, A.; Spiers, L.; Tippu, Z.; Mohamed, A.; Merrick, S.; Van Hemelrijck, M.; Payne, M.; Faust, G.; Papa, S.; et al. The impact of patient age on toxicity and efficacy of immunotherapy agents. J. Clin. Oncol. 2018, 36, e15116. [Google Scholar] [CrossRef]
- Friedman, C.F.; Horvat, T.Z.; Minehart, J.; Panageas, K.; Callahan, M.K.; Chapman, P.B.; Momtaz, P.; Postow, M.A.; Shoushtari, A.N.; Wolchok, J.D.; et al. Efficacy and safety of checkpoint blockade for treatment of advanced melanoma (mel) in patients (pts) age 80 and older (80+). J. Clin. Oncol. 2016, 34, 10009. [Google Scholar] [CrossRef]
- Rai, R.; McQuade, J.L.; Wang, D.; Park, J.; Nahar, K.; Sosman, J.; Beckermann, K.; Haydu, L.; Lo, S.; Rubinstein, S.; et al. Safety and efficacy of anti-PD-1 antibodies in elderly patients with metastatic melanoma. Ann. Oncol. 2016, 27, vi381. [Google Scholar] [CrossRef][Green Version]
- Chiarion-Sileni, V.; Pigozzo, J.; A Ascierto, P.; Simeone, E.; Maio, M.; Calabro’, L.; Marchetti, P.; De Galitiis, F.; Testori, A.; Ferrucci, P.F.; et al. Ipilimumab retreatment in patients with pretreated advanced melanoma: The expanded access program in Italy. Br. J. Cancer 2014, 110, 1721–1726. [Google Scholar] [CrossRef]
- Daste, A.; Domblides, C.; Gross-Goupil, M.; Chakiba, C.; Quivy, A.; Cochin, V.; de Mones, E.; Larmonier, N.; Soubeyran, P.-L.; Ravaud, A. Immune checkpoint inhibitors and elderly people: A review. Eur. J. Cancer 2017, 82, 155–166. [Google Scholar] [CrossRef]
- Bauer, M.E.; Fuente, M.D. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech. Ageing Dev. 2016, 158, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Gruver, A.L.; Hudson, L.L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2007, 211, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Denkinger, M.D.; Leins, H. Aging and Senescent Immune Remodeling. Trends Immunol. 2015, 36, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yan, W. T-cell immunometabolism against cancer. Cancer Lett. 2016, 382, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Bettonville, M.; D’Aria, S.; Braun, M.Y. Metabolic programming in chronically stimulated T cells: Lessons from cancer and viral infections. Eur. J. Immunol. 2016, 46, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Samani, A.; Zhang, S.; Spiers, L.; Mohamed, A.A.; Merrick, S.; Tippu, Z.; Payne, M.; Faust, G.; Papa, S.; Fields, P.; et al. Impact of age on the toxicity of immune checkpoint inhibition. J. Immunother. Cancer 2020, 8, e000871. [Google Scholar] [CrossRef]
- Singh, H.; Kim, G.; Maher, V.E.; Beaver, J.A.; Pai-Scherf, L.H.; Balasubramaniam, S.; Theoret, M.R.; Blumenthal, G.M.; Pazdur, R. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers. J. Clin. Oncol. 2016, 34, 10010. [Google Scholar] [CrossRef]
- Berger, T.G.; Shive, M.; Harper, G.M. Pruritus in the older patient: A clinical review. JAMA 2013, 310, 2443–2450. [Google Scholar] [CrossRef]
- Poropatich, K.; Fontanarosa, J. Cancer Immunotherapies: Are They as Effective in the Elderly? Drugs Aging 2017, 34, 567–581. [Google Scholar] [CrossRef]
- Nishijima, T.F.; Muss, H.B. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer Treat. Rev. 2016, 45, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lavacchi, D.; Pellegrini, E.; Palmieri, V.E.; Doni, L.; Mela, M.M.; Di Maida, F.; Amedei, A.; Pillozzi, S.; Carini, M.; Antonuzzo, L. Immune Checkpoint Inhibitors in the Treatment of Renal Cancer: Current State and Future Perspective. Int. J. Mol. Sci. 2020, 21, 4691. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
| Patients’ Characteristics | Overall n = 146 (%) | <70 n = 57 (%) | ≥70 n = 89 (%) | p (<70 vs. ≥70) |
|---|---|---|---|---|
| Sex | ||||
| Male | 98 (67.1) | 36 (63.2) | 62 (69.7) | p = 0.414 |
| Female | 48 (32.9) | 21 (36.8) | 27 (30.3) | |
| Age, years | ||||
| Average | 67.5 | 55.2 | 75.4 | |
| Median | 70 | 57 | 75 | |
| Min–Max | 27–91 | 27–69 | 70–91 | |
| Smoker | ||||
| Yes | 99 (67.8) | 38 (66.6) | 61 (68.5) | p = 0.813 |
| No | 47 (32.2) | 19 (33.4) | 28 (31.4) | |
| PS at the time of diagnosis | ||||
| 0 | 100 (68.5) | 44 (77.1) | 56 (62.9) | p = 0.190 |
| 1 | 38 (26.0) | 11 (19.2) | 27 (30.3) | |
| 2 | 8 (5.5) | 2 (3.5) | 6 (6.7) | |
| Primary site | ||||
| NSCLC | 67 (45.9) | 27 (47.4) | 40 (44.9) | p = 0.937 |
| Melanoma | 46 (31.5) | 17 (29.8) | 29 (32.6) | |
| RCC | 33 (22.6) | 13 (22.8) | 20 (22.5) | |
| Therapy line | ||||
| First | 63 (43.2) | 25 (43.8) | 38 (42.7) | p = 0.822 |
| Second | 70 (47.9) | 26 (45.6) | 44 (49.4) | |
| Third | 13 (8.9) | 6 (10.5) | 7 (7.9) | |
| Outcome | ||||
| CR | 9 (6.2) | 7 (12.3) | 2 (2.3) | p = 0.045 |
| PR | 19 (13.0) | 5 (8.8) | 14 (15.7) | |
| SD | 43 (29.4) | 19 (33.3) | 24 (27.0) | |
| PD | 75 (51.4) | 26 (45.6) | 49 (55.0) |
| Overall (%) n = 146 | <70 (%) n = 57 | ≥70 (%) n = 89 | p (<70 vs. ≥70) | |
|---|---|---|---|---|
| Patients who experience toxicity | ||||
| yes | 77 (52.7) | 37 (64.9) | 40 (44.9) | p = 0.018 |
| no | 69 (47.3) | 20 (35.1) | 49 (55.1) | |
| number of irAEs /patients | ||||
| 1 | 47 (32.2) | 20 (54.0) | 27 (67.5) | p = 0.227 |
| ≥2 | 30 (20.5) | 17 (46.0) | 13 (32.5) | |
| max grade (CTCAE) of toxicity/patients | ||||
| grade 1–2 | 50 (34.2) | 23 (62.2) | 27 (67.5) | p = 0.624 |
| grade 3–4 | 27 (18.5) | 14 (37.8) | 13 (32.5) | |
| discontinuation due to an irAE | ||||
| yes | 7 (4.8) | 3 (5.3) | 4 (4.5) | p = 0.832 |
| no | 7 (95.2) | 54 (94.7) | 85 (95.5) |
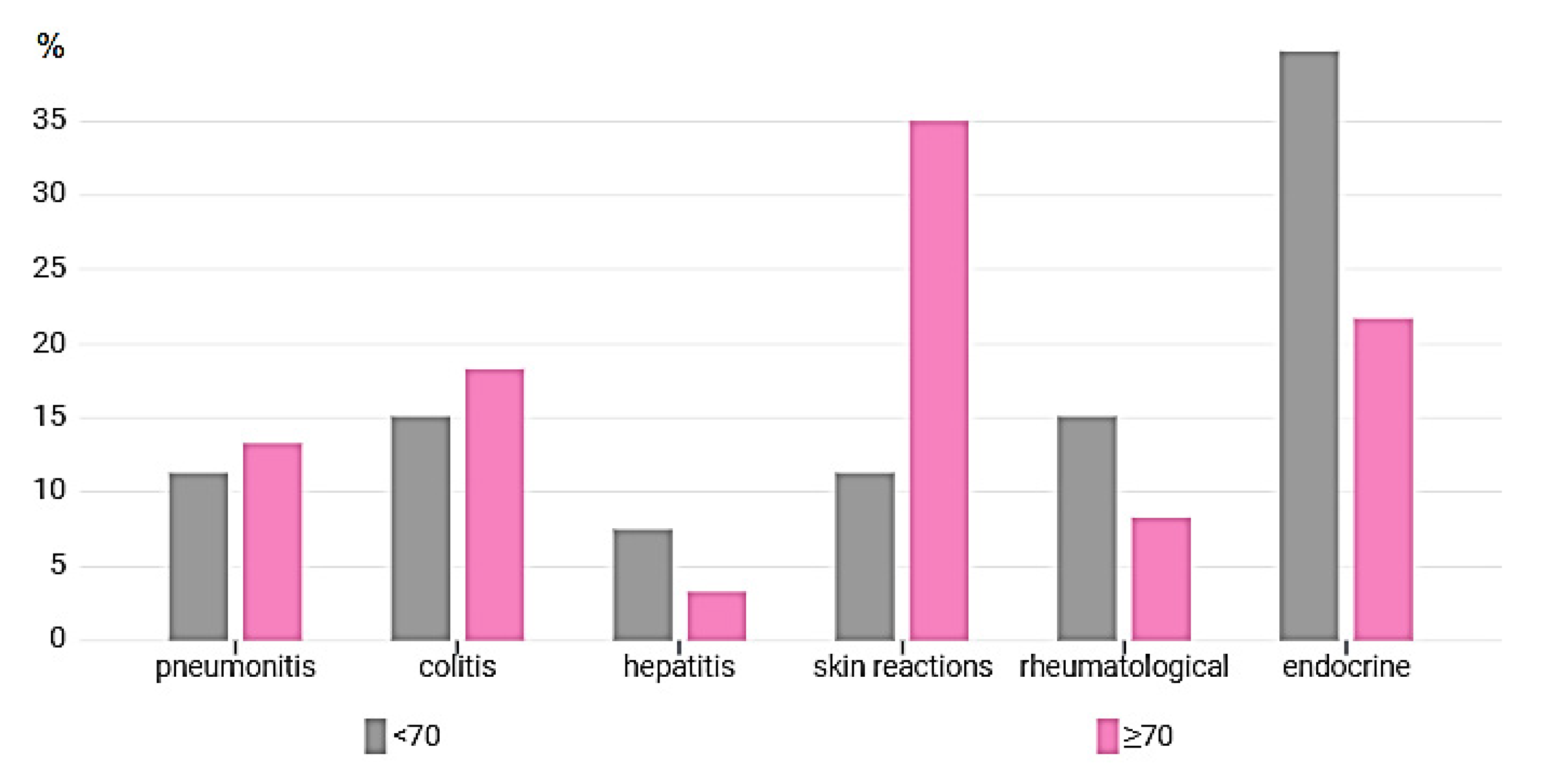
| Type of irAE | All Toxicity n = 113 (%) | <70 n = 53 (%) | ≥70 n = 60 (%) | p (<70 vs. ≥70) |
|---|---|---|---|---|
| Pneumonitis | 14 (9.6) | 6 (11.3) | 8 (13.3) | p = 0.758 |
| Colitis | 19 (13) | 8 (15.1) | 11 (18.3) | p = 0.769 |
| Hepatitis | 6 (4.1) | 4 (7.5) | 2 (3.3) | p = 0.157 |
| Skin reactions | 27 (18.5) | 6 (11.3) | 21 (35.0) | p = 0.047 |
| Rheumatological | 13 (8.9) | 8 (15.1) | 5 (8.3) | p = 0.081 |
| Endocrine related | 34 (23.3) | 21 (39.7) | 13 (21.7) | p = 0.002 |
| Use of CCS | Overall (%) n = 41 | p (<70 vs. ≥70) | |
|---|---|---|---|
| <70 (%) n = 15 | ≥70 (%) n = 26 | p = 0.704 | |
| Duration | |||
| <2weeks | 9 (60.0) | 12 (46.1) | p = 0.393 |
| ≥2weeks | 6 (40.0) | 14 (53,9) | |
| Dose | |||
| <1mg/kg | 8 (53.3) | 17 (65.4) | p = 0.446 |
| ≥1mg/kg | 7 (46.7) | 9 (34.6) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paderi, A.; Fancelli, S.; Caliman, E.; Pillozzi, S.; Gambale, E.; Mela, M.M.; Doni, L.; Mazzoni, F.; Antonuzzo, L. Safety of Immune Checkpoint Inhibitors in Elderly Patients: An Observational Study. Curr. Oncol. 2021, 28, 3259-3267. https://doi.org/10.3390/curroncol28050283
Paderi A, Fancelli S, Caliman E, Pillozzi S, Gambale E, Mela MM, Doni L, Mazzoni F, Antonuzzo L. Safety of Immune Checkpoint Inhibitors in Elderly Patients: An Observational Study. Current Oncology. 2021; 28(5):3259-3267. https://doi.org/10.3390/curroncol28050283
Chicago/Turabian StylePaderi, Agnese, Sara Fancelli, Enrico Caliman, Serena Pillozzi, Elisabetta Gambale, Marinella Micol Mela, Laura Doni, Francesca Mazzoni, and Lorenzo Antonuzzo. 2021. "Safety of Immune Checkpoint Inhibitors in Elderly Patients: An Observational Study" Current Oncology 28, no. 5: 3259-3267. https://doi.org/10.3390/curroncol28050283
APA StylePaderi, A., Fancelli, S., Caliman, E., Pillozzi, S., Gambale, E., Mela, M. M., Doni, L., Mazzoni, F., & Antonuzzo, L. (2021). Safety of Immune Checkpoint Inhibitors in Elderly Patients: An Observational Study. Current Oncology, 28(5), 3259-3267. https://doi.org/10.3390/curroncol28050283





