Efficacy of Oral Cryotherapy in the Prevention of Oral Mucositis Associated with Cancer Chemotherapy: Systematic Review with Meta-Analysis and Trial Sequential Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Data Resources and Search Strategy
2.3. Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Data Synthesis
3. Results
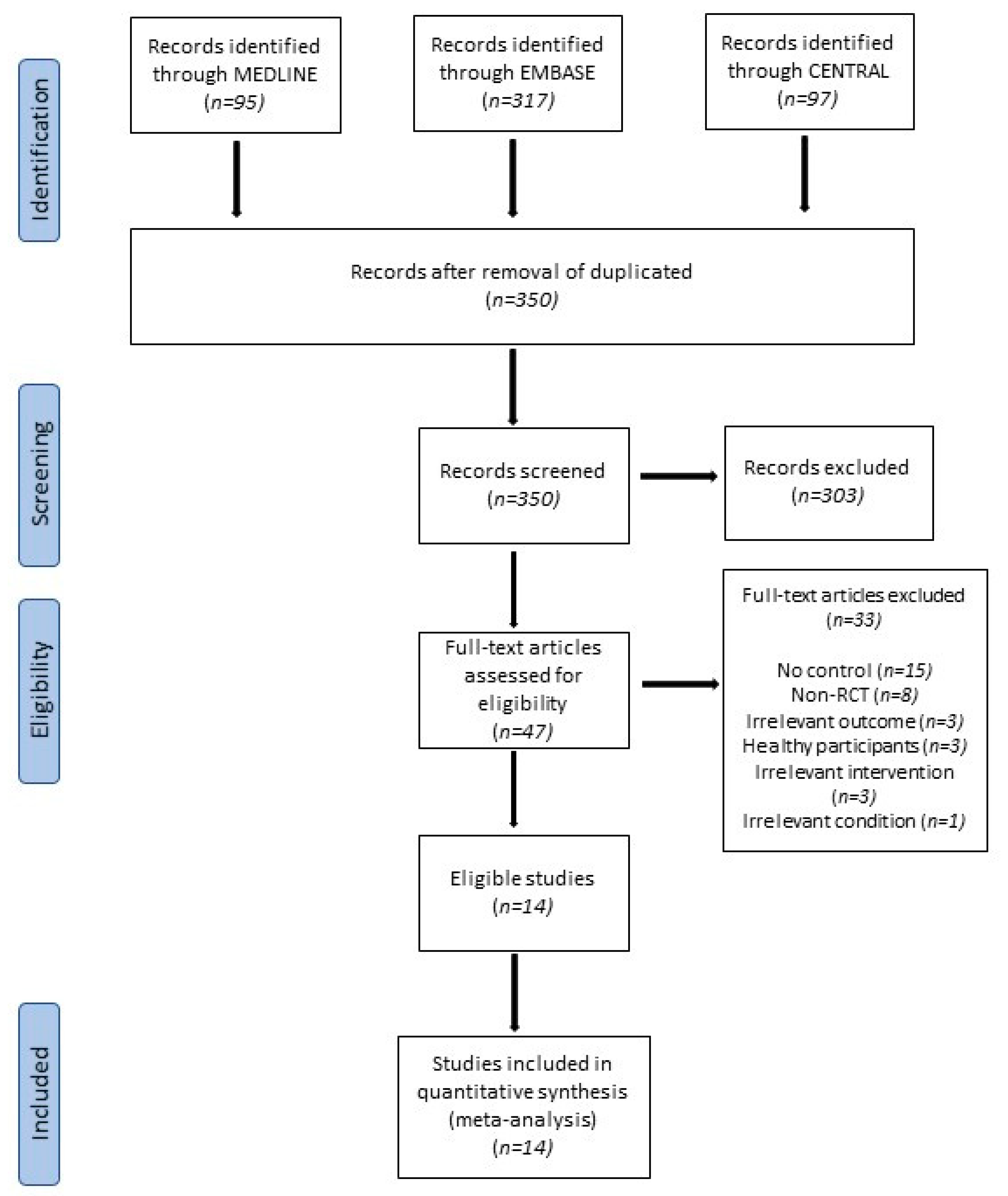
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Risk of Bias Analysis
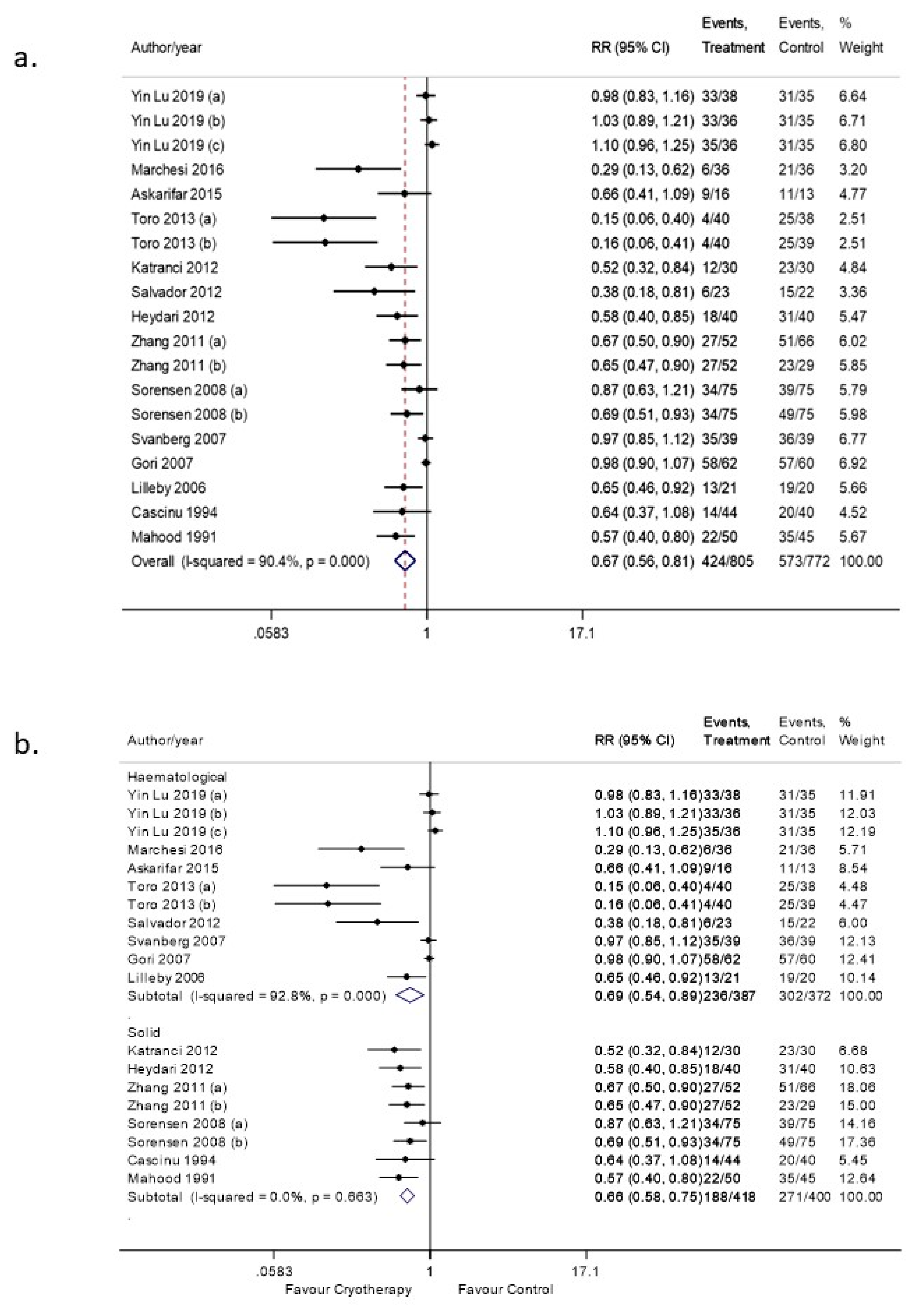
3.4. OC Efficacy in Preventing OM (Any Grade)
3.4.1. Sensitivity Analysis
3.4.2. Subgroup Analyses
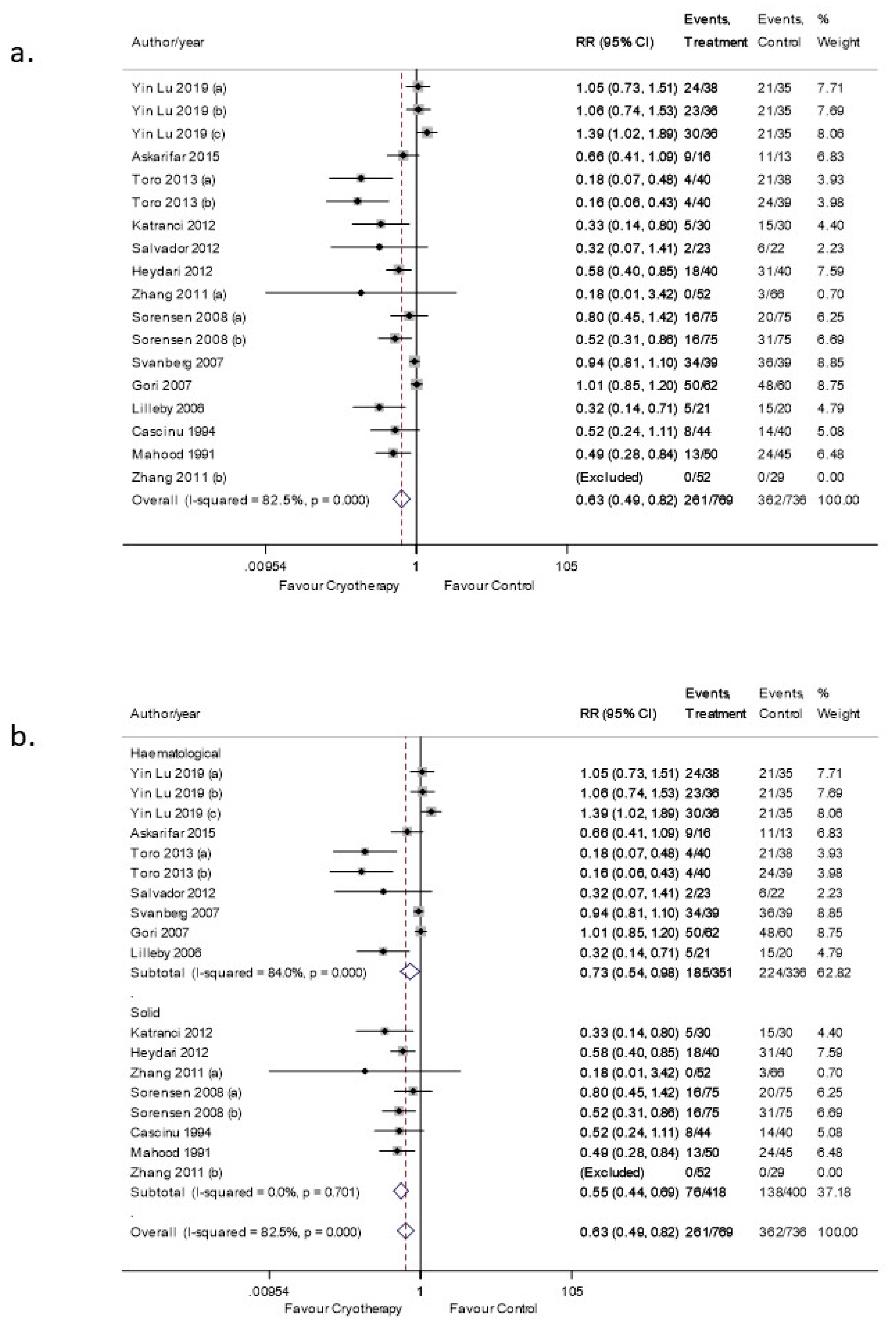
3.5. OC Efficacy in Preventing OM (Moderate-Severe Grade)
3.5.1. Sensitivity Analysis
3.5.2. Subgroup Analysis
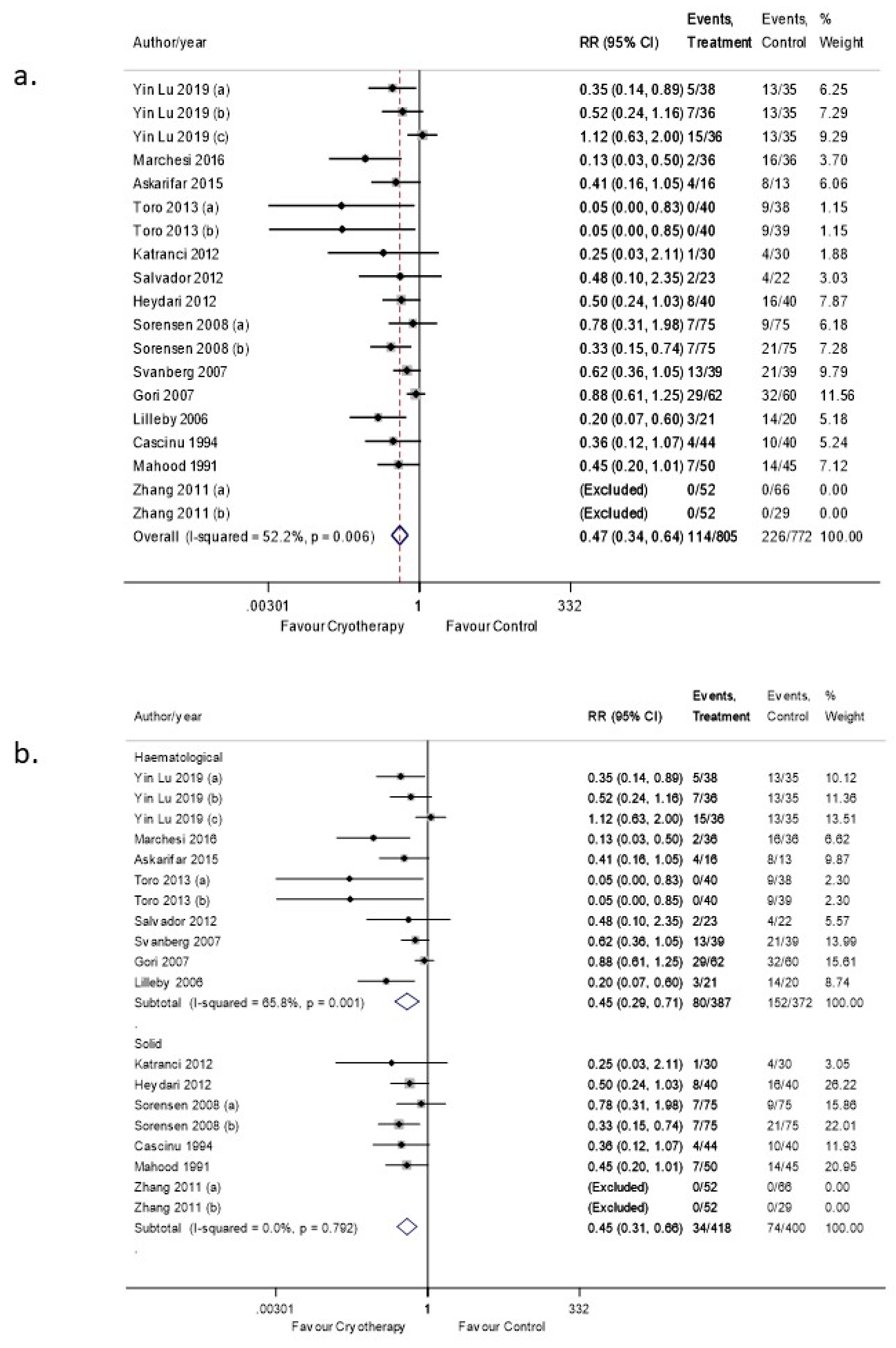
3.6. OC Efficacy in Preventing OM (Severe Grade)
3.6.1. Sensitivity Analysis
3.6.2. Subgroup Analyses
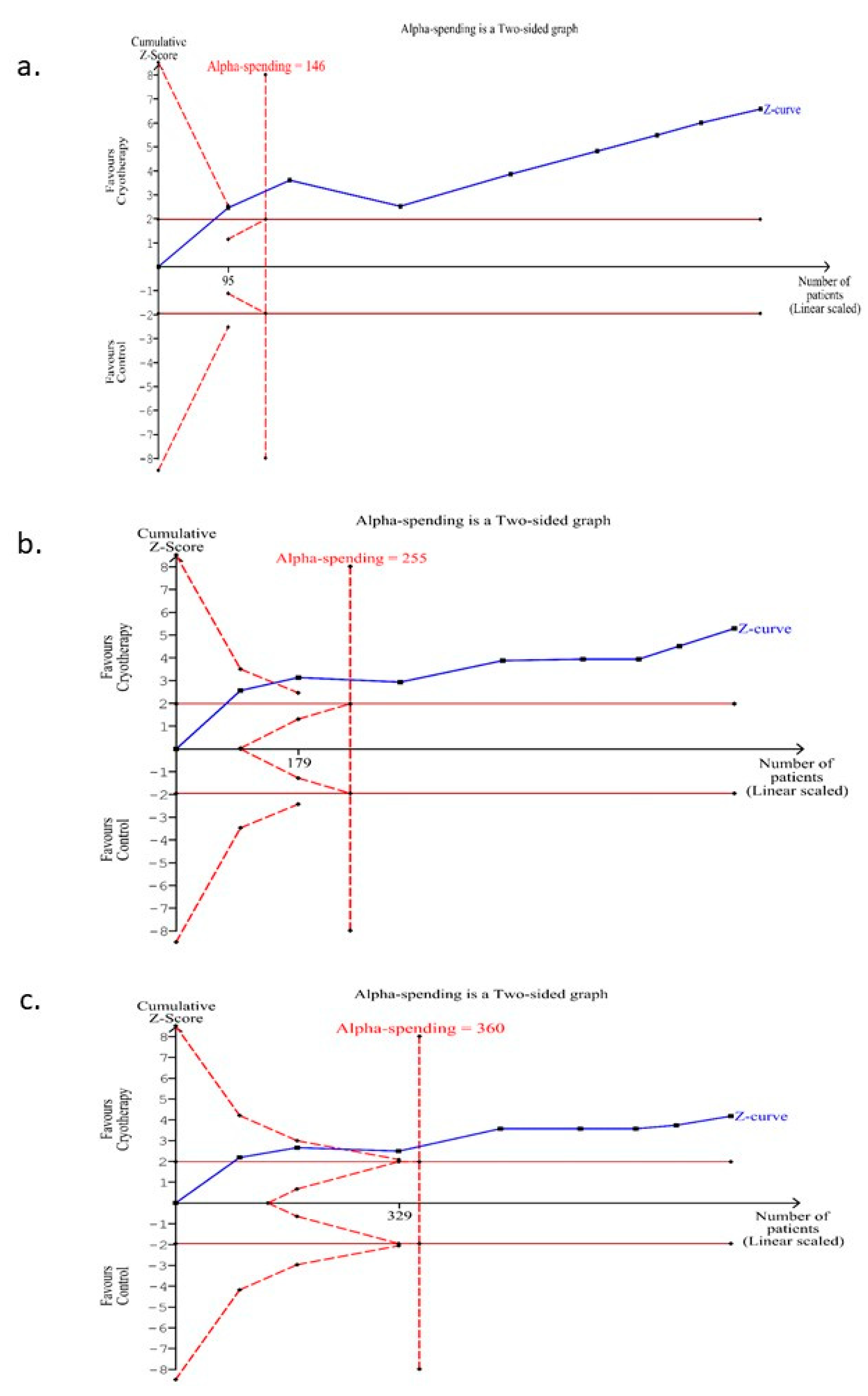
3.7. Trial Sequential Analysis for the Incidence of OM (Any Grade) for Those Trials Conducted on the Subgroup of Patients with Solid Malignancies Receiving Conventional Chemotherapy
3.8. Trial Sequential Analysis for the Incidence of OM (Moderate-Severe Grade) for Those Trials Conducted on the Subgroup of Patients with Solid Malignancies Receiving Conventional Chemotherapy
3.9. Trial Sequential Analysis for the Incidence of OM (Severe Grade) for Those Trials Conducted on the Subgroup of Patients with Solid Malignancies Receiving Conventional Chemotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villa, A.; Sonis, S.T. Pharmacotherapy for the Management of Cancer Regimen-Related Oral Mucositis. Expert Opin. Pharm. 2016, 17, 1801–1807. [Google Scholar] [CrossRef]
- Shankar, A.; Roy, S.; Bhandari, M.; Rath, G.K.; Biswas, A.S.; Kanodia, R.; Adhikari, N.; Sachan, R. Current Trends in Management of Oral Mucositis in Cancer Treatment. Asian. Pac. J. Cancer Prev. 2017, 18, 2019–2026. [Google Scholar] [CrossRef]
- Sonis, S.T. The Pathobiology of Mucositis. Nat. Rev. Cancer 2004, 4, 277. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Mucositis Study Section of the Multinational Association for Supportive Care in Cancer, International Society for Oral Oncology. Perspectives on Cancer Therapy-Induced Mucosal Injury: Pathogenesis, Measurement, Epidemiology, and Consequences for Patients. Cancer 2004, 100 (Suppl. 9), 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Al-Dasooqi, N.; Bossi, P.; Wardill, H.; Van Sebille, Y.; Al-Azri, A.; Bateman, E.; Correa, M.E.; Raber-Durlacher, J.; Kandwal, A.; et al. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). The Pathogenesis of Mucositis: Updated Perspectives and Emerging Targets. Support. Care Cancer 2019, 27, 4023–4033. [Google Scholar] [CrossRef] [Green Version]
- Al-Rudayni, A.H.M.; Gopinath, D.; Maharajan, M.K.; Menon, R.K. Impact of Oral Mucositis on Quality of Life in Patients Undergoing Oncological Treatment: A Systematic Review. Transl. Cancer Res. 2020, 9. [Google Scholar] [CrossRef]
- Valeh, M.; Kargar, M.; Mansouri, A.; Kamranzadeh, H.; Gholami, K.; Heidari, K.; Hajibabaei, M. Factors Affecting the Incidence and Severity of Oral Mucositis Following Hematopoietic Stem Cell Transplantation. Int. J. Hematol. Oncol. Stem Cell Res. 2018, 12, 142–152. [Google Scholar]
- Bowen, J.M.; Wardill, H.R. Advances in the Understanding and Management of Mucositis during Stem Cell Transplantation. Curr. Opin. Support. Palliat. Care 2017, 11, 341–346. [Google Scholar] [CrossRef]
- Chaudhry, H.M.; Bruce, A.J.; Wolf, R.C.; Litzow, M.R.; Hogan, W.J.; Patnaik, M.S.; Kremers, W.K.; Phillips, G.L.; Hashmi, S.K. The Incidence and Severity of Oral Mucositis among Allogeneic Hematopoietic Stem Cell Transplantation Patients: A Systematic Review. Biol. Blood Marrow Transpl. 2016, 22, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Roila, F.; Molassiotis, A.; Herrstedt, J.; Aapro, M.; Gralla, R.J.; Bruera, E.; Clark-Snow, R.A.; Dupuis, L.L.; Einhorn, L.H.; Feyer, P.; et al. MASCC and ESMO Guideline Update for the Prevention of Chemotherapy-and Radiotherapy-Induced Nausea and Vomiting and of Nausea and Vomiting in Advanced Cancer Patients. Ann. Oncol. 2016, 27, v119–v133. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Walladbegi, J.; Smith, S.A.; Grayson, A.K.; Murdoch, C.; Jontell, M.; Colley, H.E. Cooling of the Oral Mucosa to Prevent Adverse Effects of Chemotherapeutic Agents: An In Vitro Study. J. Oral. Pathol. Med. 2018, 47, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, J.-E.; Bratel, J.; Hardling, M.; Heikki, L.; Mellqvist, U.-H.; Hasséus, B. Cryotherapy as Prophylaxis against Oral Mucositis after High-Dose Melphalan and Autologous Stem Cell Transplantation for Myeloma: A Randomised, Open-Label, Phase 3, Non-Inferiority Trial. Bone Marrow Transpl. 2019, 54, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.E.; Boers-Doets, C.B.; Bensadoun, R.J.; Herrstedt, J. ESMO Guidelines Committee. Management of Oral and Gastrointestinal Mucosal Injury: ESMO Clinical Practice Guidelines for Diagnosis, Treatment, and Follow-up. Ann. Oncol. 2015, 6, V139–V151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brok, J.; Thorlund, K.; Gluud, C.; Wetterslev, J. Trial Sequential Analysis Reveals Insufficient Information Size and Potentially False Positive Results in Many Meta-Analyses. J. Clin. Epidemiol. 2008, 61, 763–769. [Google Scholar] [CrossRef]
- Cochrane. Cochrane Handbook for Systematic Reviews of Intervention. Available online: Https://Community.Cochrane.Org/Book_pdf/764 (accessed on 14 January 2021).
- McCoy, C.E. Understanding the Intention-to-Treat Principle in Randomized Controlled Trials. West. J. Emerg. Med. 2017, 18, 1075–1078. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane. 2019. Available online: www.Training.Cochrane.Org/Handbook (accessed on 15 January 2021).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Thorlund, K.; Devereaux, P.J.; Wetterslev, J.; Guyatt, G.; Ioannidis, J.P.A.; Thabane, L.; Gluud, L.-L.; Als-Nielsen, B.; Gluud, C. Can Trial Sequential Monitoring Boundaries Reduce Spurious Inferences from Meta-Analyses? Int. J. Epidemiol. 2009, 38, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H. GRADE Working Group A GRADE Working Group Approach for Rating the Quality of Treatment Effect Estimates from Network Meta-Analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhu, X.; Ma, Q.; Wang, J.; Jiang, P.; Teng, S.; Zhou, L.; Wu, D.; Wang, H. Oral Cryotherapy for Oral Mucositis Management in Patients Receiving Allogeneic Hematopoietic Stem Cell Transplantation: A Prospective Randomized Study. Support. Care Cancer 2020, 28, 1747–1754. [Google Scholar] [CrossRef]
- Marchesi, F.; Tendas, A.; Giannarelli, D.; Viggiani, C.; Gumenyuk, S.; Renzi, D.; Franceschini, L.; Caffarella, G.; Rizzo, M.; Palombi, F.; et al. Cryotherapy Reduces Oral Mucositis and Febrile Episodes in Myeloma Patients Treated with High-Dose Melphalan and Autologous Stem Cell Transplant: A Prospective, Randomized Study. Bone Marrow Transpl. 2017, 52, 154–156. [Google Scholar] [CrossRef]
- Askarifar, M.; Lakdizaji, S.; Ramzi, M.; Rahmani, A.; Jabbarzadeh, F. The Effects of Oral Cryotherapy on Chemotherapy-Induced Oral Mucositis in Patients Undergoing Autologous Transplantation of Blood Stem Cells: A Clinical Trial. Iran. Red. Crescent Med. J. 2016, 18, e24775. [Google Scholar] [CrossRef] [Green Version]
- Toro, J.; Schneider, D.; Alonzo, R.; Hasan, A.; Lee, S.; Gushiken, F. A randomized trial of oral cryotherapy, saline solution and Caphosol for the prevention of high-dose melphalan-induced oral mucositis followed by autologous hematopoietic stem cell transplantation. Support. Care Cancer 2013, 21, S138. [Google Scholar]
- Katrancı, N.; Ovayolu, N.; Ovayolu, O.; Sevinc, A. Evaluation of the Effect of Cryotherapy in Preventing Oral Mucositis Associated with Chemotherapy—A Randomized Controlled Trial. Eur. J. Oncol. Nurs. 2012, 16, 339–344. [Google Scholar] [CrossRef]
- Salvador, P.; Azusano, C.; Wang, L.; Howell, D. A Pilot Randomized Controlled Trial of an Oral Care Intervention to Reduce Mucositis Severity in Stem Cell Transplant Patients. J. Pain Symptom. Manag. 2012, 44, 64–73. [Google Scholar] [CrossRef]
- Heydari, A.; Sharifi, H.; Salek, R. Effect of Oral Cryotherapy on Combination Chemotherapy-induced Oral Mucositis: A Randomized Clinical Trial. Middle East J. Cancer 2012, 3, 55–64. [Google Scholar]
- Zhang, W.; Li, L.S.; Lu, Y.W.; Zhen, J.C.; Zhang, X.L. Intervention research on preventing oral mucositis afer using high dose methotrexate chemotherapy in osteosarcoma by gargling with calcium folinic. Chin. Pharm. J. (China) 2011, 46, 1126–1128. [Google Scholar]
- Sorensen, J.B.; Skovsgaard, T.; Bork, E.; Damstrup, L.; Ingeberg, S. Double-Blind, Placebo-Controlled, Randomized Study of Chlorhexidine Prophylaxis for 5-Fluorouracil-Based Chemotherapy-Induced Oral Mucositis with Nonblinded Randomized Comparison to Oral Cooling (Cryotherapy) in Gastrointestinal Malignancies. Cancer 2008, 112, 1600–1606. [Google Scholar] [CrossRef]
- Svanberg, A.; Birgegård, G.; Ohrn, K. Oral Cryotherapy Reduces Mucositis and Opioid Use after Myeloablative Therapy—A Randomized Controlled Trial. Support. Care Cancer 2007, 15, 1155–1161. [Google Scholar] [CrossRef]
- Gori, E.; Arpinati, M.; Bonifazi, F.; Errico, A.; Mega, A.; Alberani, F.; Sabbi, V.; Costazza, G.; Leanza, S.; Borrelli, C.; et al. Cryotherapy in the Prevention of Oral Mucositis in Patients Receiving Low-Dose Methotrexate Following Myeloablative Allogeneic Stem Cell Transplantation: A Prospective Randomized Study of the Gruppo Italiano Trapianto Di Midollo Osseo Nurses Group. Bone Marrow Transpl. 2007, 39, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilleby, K.; Garcia, P.; Gooley, T.; McDonnnell, P.; Taber, R.; Holmberg, L.; Maloney, D.G.; Press, O.W.; Bensinger, W. A Prospective, Randomized Study of Cryotherapy during Administration of High-Dose Melphalan to Decrease the Severity and Duration of Oral Mucositis in Patients with Multiple Myeloma Undergoing Autologous Peripheral Blood Stem Cell Transplantation. Bone Marrow Transpl. 2006, 37, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Cascinu, S.; Fedeli, A.; Fedeli, S.L.; Catalano, G. Oral Cooling (Cryotherapy), an Effective Treatment for the Prevention of 5-Fluorouracil-Induced Stomatitis. Eur. J. Cancer B Oral. Oncol. 1994, 30, 234–236. [Google Scholar] [CrossRef]
- Mahood, D.J.; Dose, A.M.; Loprinzi, C.L.; Veeder, M.H.; Athmann, L.M.; Therneau, T.M.; Sorensen, J.M.; Gainey, D.K.; Mailliard, J.A.; Gusa, N.L. Inhibition of Fluorouracil-Induced Stomatitis by Oral Cryotherapy. J. Clin. Oncol. 1991, 9, 449–452. [Google Scholar] [CrossRef]
- MASCC 2013 Abstracts. Support. Care Cancer 2013, 21, 1–301. [CrossRef] [Green Version]
- Lalla, R.V.; Saunders, D.P.; Peterson, D.E. Chemotherapy or Radiation-Induced Oral Mucositis. Dent. Clin. N. Am. 2014, 58, 341–349. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.; Chambers, M.; Cantor, S.B.; Manzullo, E.; Rubenstein, E.B. The Burdens of Cancer Therapy. Clinical and Economic Outcomes of Chemotherapy-Induced Mucositis. Cancer 2003, 98, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E.B.; Peterson, D.E.; Schubert, M.; Keefe, D.; McGuire, D.; Epstein, J.; Elting, L.S.; Fox, P.C.; Cooksley, C.; Sonis, S.T. Clinical Practice Guidelines for the Prevention and Treatment of Cancer Therapy-Induced Oral and Gastrointestinal Mucositis. Cancer 2004, 100, 2026–2046. [Google Scholar] [CrossRef]
- Clarke, M. Standardising Outcomes for Clinical Trials and Systematic Reviews. Trials 2007, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Dwan, K.; Altman, D.G.; Arnaiz, J.A.; Bloom, J.; Chan, A.W.; Cronin, E.; Decullier, E.; Easterbrook, P.J.; Von Elm, E.; Gamble, C.; et al. Systematic Review of the Empirical Evidence of Study Publication Bias and Outcome Reporting Bias. PLoS ONE 2008, 3, 3081. [Google Scholar] [CrossRef] [Green Version]
- Mucositis Study Group of the Multinational Association of Supportive Care in Cancer; International Society of Oral Oncology; McGuire, D.B.; Fulton, J.S.; Park, J.; Brown, C.G.; Correa, M.E.P.; Eilers, J.; Elad, S.; Gibson, F.; et al. Systematic Review of Basic Oral Care for the Management of Oral Mucositis in Cancer Patients. Support. Care Cancer 2013, 21, 3165–3177. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Gu, Z.; Zhai, R.; Zhao, S.; Luo, L.; Li, D.; Zhao, X.; Wei, H.; Pang, Z.; Wang, L.; et al. Efficacy of Oral Cryotherapy on Oral Mucositis Prevention in Patients with Hematological Malignancies Undergoing Hematopoietic Stem Cell Transplantation: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0128763. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.; Glenny, A.-M.; Worthington, H.V.; Littlewood, A.; Clarkson, J.E.; McCabe, M.G. Interventions for Preventing Oral Mucositis in Patients with Cancer Receiving Treatment: Oral Cryotherapy. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.K.; Sborov, D.W.; Lamprecht, M. Associations of High-Dose Melphalan Pharmacokinetics and Outcomes in the Setting of a Randomized Cryotherapy Trial. Clin. Pharm. Ther. 2017, 102, 511–519. [Google Scholar] [CrossRef]
- Sung, L.; Robinson, P.; Treister, N.; Baggott, T.; Gibson, P.; Tissing, W.; Wiernikowski, J.; Brinklow, J.; Dupuis, L.L. Guideline for the Prevention of Oral and Oropharyngeal Mucositis in Children Receiving Treatment for Cancer or Undergoing Haematopoietic Stem Cell Transplantation. BMJ Support. Palliat. Care 2017, 7, 7–16. [Google Scholar] [CrossRef]
- Correa, M.; Cheng, K.; Chiang, K.; Kandwal, A.; Loprinzi, C.L.; Mori, T.; Potting, C.; Rouleau, T.; Toro, J.J.; Ranna, V.; et al. Systematic Review of Oral Cryotherapy for the Management of Oral Mucositis in Cancer Patients and Clinical Practice Guidelines. Support. Care Cancer 2020, 28, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Lee, C.M.; Saunders, D.P.; Curtis, A.; Dunlap, N.; Nangia, C.; Lee, A.S.; Gordon, S.M.; Kovoor, P.; Arevalo-Araujo, R.; et al. Phase IIb, Randomized, Double-Blind Trial of GC4419 Versus Placebo to Reduce Severe Oral Mucositis Due to Concurrent Radiotherapy and Cisplatin for Head and Neck Cancer. J. Clin. Oncol. 2019, 37, 3256–3265. [Google Scholar] [CrossRef] [PubMed]
| Author | Country | Design | Age Groups | No. | Cooling Method | Control | Malignancy Type | Antineoplastic Therapy | OC Duration |
|---|---|---|---|---|---|---|---|---|---|
| Lu 2019 [23] | China | Parallel RCT, 4 arms | Adults | 160 | Ice cubes | CHX | Hematological | BMT, BUCY | Entire session |
| Marchesi 2017 [24] | Italy | Parallel RCT, 2 arms | Adults | 72 | Ice chips | PLACEBO | Hematological | BMT, MELPH | 15−30 min |
| Askarifar 2016 [25] | Iran | Parallel RCT, 2 arms | Adults | 33 | Nylon ice packs | NS | Hematological | BMT, MELPH | 15−30 min |
| Toro 2013 [26] | US | Parallel RCT, 3 arms | Adults | 78 | Crushed ice | NS | Hematological | BMT, MELPH | 105 min |
| Katrancı 2012 [27] | Turkey | Parallel RCT, 2 arms | N/A | 60 | Ice chips | BOC | Solid | CT, 5FU | 30 min |
| Salvador 2012 [28] | Canada | Parallel RCT, 2 arms | Adults | 46 | Ice chips | BOC | Hematological | BMT, HD-MELPH | 60 min |
| Heydari 2012 [29] | Iran | Parallel RCT, 2 arms | Adults | 80 | Ice chips | BOC | Solid | CT, MAYO, CAF, CMF | Whole session |
| Zhang 2011 [30] | China | Parallel RCT, 4 arms | Mixed | 147 | Iced water | PLACEBO | Solid | CT, MTX | N/A |
| Sorensen 2008 [31] | Denmark | Parallel RCT, 3 arms | Adults | 225 | Crushed ice | LV | Solid | CT, 5FU | 45 min |
| Svanberg 2007 [32] | Sweden | Parallel RCT, 2 arms | Adults | 78 | Ice chips | NS/CHX | Hematological | BMT, HD-MELPH | Entire session |
| Gori 2007 [33] | Italy | Parallel RCT, 2 arms | Adults | 130 | Ice chips/ Popsicles | BOC | Hematological | BMT, CT/RT | 60 min |
| Lilleby 2006 [34] | US | Parallel RCT, 2 arms | Adults | 41 | Ice chips | PLACEBO | Hematological | BMT, HD-MELPH | 6 h |
| Cascinu 1994 [35] | Italy | Parallel RCT, 2 arms | Adults | 84 | Ice chips | PLACEBO | Solid | CT, 5FU | 35 min |
| Mahood 1991 [36] | US | Crossover RCT, 2 arms | Adults | 95 | Ice chips | PLACEBO | Solid | CT, 5FU | 35 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rudayni, A.H.M.; Gopinath, D.; Maharajan, M.K.; Veettil, S.K.; Menon, R.K. Efficacy of Oral Cryotherapy in the Prevention of Oral Mucositis Associated with Cancer Chemotherapy: Systematic Review with Meta-Analysis and Trial Sequential Analysis. Curr. Oncol. 2021, 28, 2852-2867. https://doi.org/10.3390/curroncol28040250
Al-Rudayni AHM, Gopinath D, Maharajan MK, Veettil SK, Menon RK. Efficacy of Oral Cryotherapy in the Prevention of Oral Mucositis Associated with Cancer Chemotherapy: Systematic Review with Meta-Analysis and Trial Sequential Analysis. Current Oncology. 2021; 28(4):2852-2867. https://doi.org/10.3390/curroncol28040250
Chicago/Turabian StyleAl-Rudayni, Ali Hatem Manfi, Divya Gopinath, Mari Kannan Maharajan, Sajesh Kalkandi Veettil, and Rohit Kunnath Menon. 2021. "Efficacy of Oral Cryotherapy in the Prevention of Oral Mucositis Associated with Cancer Chemotherapy: Systematic Review with Meta-Analysis and Trial Sequential Analysis" Current Oncology 28, no. 4: 2852-2867. https://doi.org/10.3390/curroncol28040250
APA StyleAl-Rudayni, A. H. M., Gopinath, D., Maharajan, M. K., Veettil, S. K., & Menon, R. K. (2021). Efficacy of Oral Cryotherapy in the Prevention of Oral Mucositis Associated with Cancer Chemotherapy: Systematic Review with Meta-Analysis and Trial Sequential Analysis. Current Oncology, 28(4), 2852-2867. https://doi.org/10.3390/curroncol28040250






