Abstract
Patients with advanced pancreatic cancer (APC) experience many disease-related symptoms. ESAS-r measures the severity of 9 symptom domains and has been validated for use in the ambulatory oncology setting. We aimed to describe symptom burden at baseline for patients with APC treated with modern chemotherapy (CT), and to determine whether symptom burden at baseline is prognostic. Patients diagnosed with APC between 2012–2016, treated with ≥1 cycle of CT, who completed ≥1 ESAS-r were identified. Descriptive statistics were used to report symptom burden and common moderate-to-severe symptoms. A joint model was used to describe the trajectory of ESAS-r during follow-up while controlling for death. Multivariable Cox regression was used to identify independent predictors of death. Of 123 patients identified, the median age was 65 and 61% had metastatic disease. The median baseline ESAS-r total symptom distress score (TSDS) was 24. A total of 86% of patients had at least one symptom score of ≥4 at baseline, with the most common being: fatigue, nausea, anxiety, and shortness of breath. Median overall survival was 10.2 months. Baseline TSDS was not predictive for worse survival in the era of modern CT. Patients with APC have a high burden of cancer-associated symptoms and a high prevalence of moderate-to-severe symptoms. Early intervention has the potential to improve quality of life in this group of patients and should be investigated.
1. Introduction
Pancreatic cancer is the third leading cause of cancer death in Canada [1]. Most cases are diagnosed when disease is advanced and incurable [2]. Patients with advanced pancreatic cancer (APC) experience many disease-related symptoms, including pain, anorexia, weight loss, fatigue, nausea, diarrhea, depression, and anxiety [3,4,5,6]. Symptom burden increases closer to death [7,8].
The revised Edmonton Symptom Assessment System (ESAS-r) [9] is a patient-reported outcome (PRO) tool which assesses the intensity of nine symptoms, including pain, tiredness, drowsiness, nausea, appetite, dyspnea, anxiety, depression, and overall wellbeing. Each symptom is given a score on a scale of 0–10, with 0 defining the absence of a symptom and 10 defining the worst possible severity. A rating scale of 1–3 represents mild severity, 4–6 moderate severity, and 7–10 high severity [10,11]. The total symptom distress score (TSDS) is the sum of the 9 individual symptom scores with 90 being the highest possible total [12]. ESAS-r has been shown to be a reliable and valid tool in patients with advanced cancer in an outpatient oncology setting [9,13,14,15,16]. Since 2011–2012, ESAS-r has been completed by patients at each ambulatory visit at our provincial cancer center. Patients complete a paper copy, which clinical staff subsequently input into the electronic medical record.
Over the last decade, combination chemotherapy regimens such as 5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) and nab-paclitaxel and gemcitabine (NG) have become the standard of care for APC, as they have been shown to improve survival, without a deterioration on quality of life (QOL), compared to single-agent gemcitabine [17,18,19]. The symptom burden soon after diagnosis in patients with APC who receive chemotherapy (CT) is not clearly defined. It is unknown whether higher baseline symptom burden is a poor prognostic factor for overall survival (OS) in patients with APC receiving modern CT. The aims of this study were:
- 1
- To describe the symptom burden of patients with APC at baseline, using the ESAS-r.
- 2
- To examine whether baseline ESAS-r is a prognostic marker for OS.
2. Materials and Methods
Patients ≥ 18 years of age and diagnosed with locally advanced unresectable or metastatic pancreatic cancer between 2012 to 2016 and treated with at least one cycle of CT in Manitoba were identified using the Manitoba Cancer Registry. Patients were included if at least one ESAS-r entry was present in the electronic medical record. All ESAS-r scores completed from baseline to death were extracted from the electronic medical record. The baseline ESAS-r measurement was completed within the 30 days preceding the start of CT. ESAS-r assessments with missing data were excluded.
Baseline patient and treatment characteristics, including age, sex, date of diagnosis, clinical stage, Eastern Cooperative Oncology Group (ECOG) performance status, CT regimen received, date of progression, and date of death were extracted via the Manitoba Cancer Registry and retrospective chart review.
The TSDS was calculated by adding up the sum of the scores for each of the 9 symptom domains. Box-percentile plots were used to demonstrate distributions. Symptoms were also categorized into a physical subset score (sum of pain, tiredness, drowsiness, nausea, lack of appetite and shortness of breath) and a psychological subset score (sum of depression and anxiety) for descriptive purposes. The correlations between TSDS and the physical and psychological subsets were assessed using Spearman correlation.
Descriptive statistics were used to report baseline characteristics, symptoms at baseline, and the TSDS. TSDS according to primary tumor location and according to younger (<65) and older (≥65) age was explored. A joint model was used to describe the trajectory of ESAS-r during follow-up while controlling for death [20]. This is a model that joins a mixed model (predicting ESAS-r) and a survival model (predicting death). Splines were used to account for non-linear relationships and an interaction term between baseline TSDS and follow-up time was also included. To evaluate the impact of the time-varying status of TSDS during follow-up on death, a joint model was again used, which creates an endogenous variable through the mixed model to be applied in the survival model. R version 4.0.3 [21] was used to perform analyses using the R packages of JM [22] and rms [23]. OS was estimated using the Kaplan-Meier method. Multivariable Cox regression was used to identify independent predictors of death, including baseline TSDS.
3. Results
3.1. Patient Characteristics
A total of 123 patients diagnosed with APC from 2012–2016 who received at least one cycle of CT and completed at least one ESAS-r assessment were identified. Baseline characteristics can be seen in Table 1. The median age was 65 years (range 42 to 88), 52.8% were male, and 61% had metastatic disease. Most patients (82.1%) had an ECOG performance status of 0–1. The most common CT regimen received was 5-Fluorouracil, Irinotecan and Oxaliplatin (FOLFIRINOX) (69.1%), followed by nab-Paclitaxel plus Gemcitabine (NG) (22%). Only 11 (8.9%) patients received single-agent gemcitabine. Among the 123 patients, there were 1608 unique ESAS-r assessments. A total of 107 (87.0%) of patients completed ≥2 assessments, while 22.8% of patients had >21 completed assessments. In the cohort, 9.2% had at least 1 ESAS-r assessment with missing data, which were removed from analysis.

Table 1.
Baseline characteristics of patients with APC who received chemotherapy (n = 123).
3.2. Symptom Burden at Baseline
At baseline, the median TSDS of the whole cohort was 24. ESAS-r TSDS in the 10th percentile and 90th percentile was 6.2 and 53, respectively. The median symptom scores at baseline for pain, tiredness, drowsiness, nausea, lack of appetite, shortness of breath, depression, anxiety, and wellbeing were 1, 4, 1, 2, 1, 3, 1, 2 and 2 respectively (Figure 1).
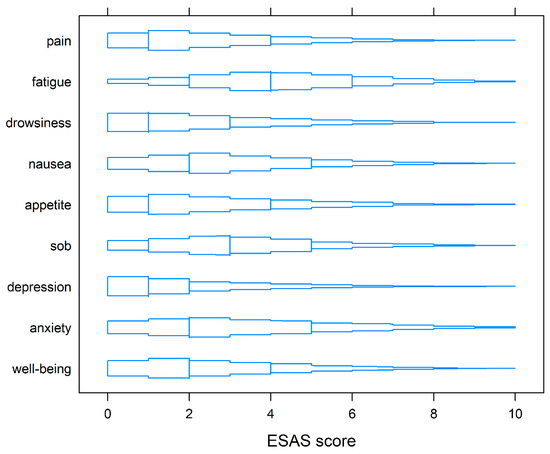
Figure 1.
Box-percentile plots of symptoms on ESAS-r at baseline. Similar to boxplots, the median, 25th, and 75th percentiles are indicated with line segments across the box. Up to the median, the width indicates the percentile of that height. Beyond the median, the width indicates 100% minus the percentile.
Younger patients (under 65 years old) had a median TSDS of 29, whereas older patients (65 years and older) had a median TSDS of 21. At baseline, the median TSDS according to tumor location was 21.5 for a primary in the head/neck of the pancreas, 28 for the body of the pancreas, and 24.5 for the tail of the pancreas.
At baseline, 106 (86.2%) patients reported at least one moderate-to-severe symptom (score ≥ 4). Ninety-five (77.2%) had at least one physical symptom scored at ≥4, while sixty-five (52.9%) had at least one psychological symptom scored at ≥4 at baseline. The most common symptoms with a reported score of ≥4 at baseline were tiredness (56.9%), anxiety (50.4%), shortness of breath (48.8%) and nausea (9.8%) (Table 2).

Table 2.
Most common moderate-to-severe symptoms at baseline.
Spearman correlations demonstrated high associations between baseline TSDS and physical symptom scores (rho = 0.94; p < 0.001) and psychological symptom scores (rho = 0.83; p < 0.001). Point-biserial correlations between TSDS and the subsets indicated moderate correlation. However, the results from multivariable models indicated little change when the subsets were included/excluded.
3.3. Symptom Burden over Time
ESAS-r over time (0 to 18 months) is represented in Figure 2. Using a joint model (to predict TSDS values during follow-up while adjusting for death during follow-up), baseline TSDS was related to TSDS during follow-up. As seen graphically in Figure 2A, TSDS decreases for the first few months after initiating CT, but after 6 months, TSDS starts to increase. TSDS at 15 to 18 months is similar to TSDS at baseline. When disease progression was included as a time-varying predictor, it was not related to worsening TSDS (p = 0.750). When tumor location was included as a predictor of ESAS-r over time, patients with tumors in the head/neck and tail had higher ESAS-r scores than patients with tumors in the body of the pancreas (p = 0.032).
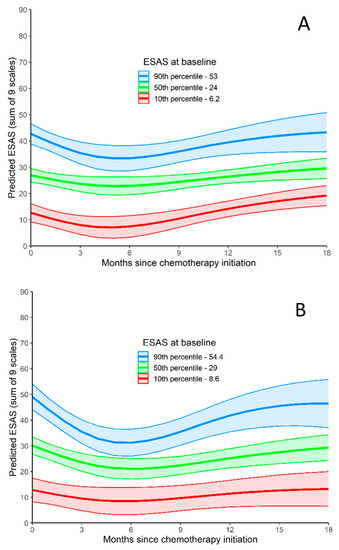
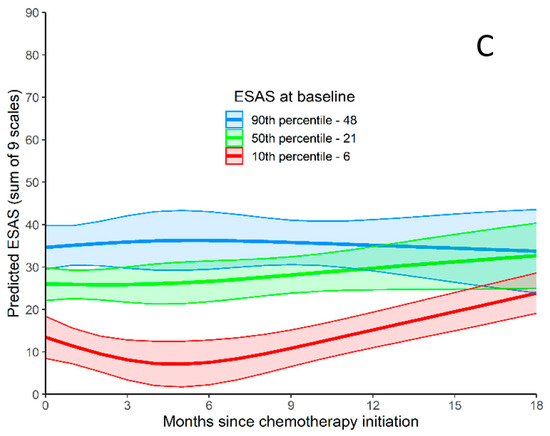
Figure 2.
ESAS-r Total Symptom Distress Score. (A) ESAS-r Total Symptom Distress Score relative to months since chemotherapy initiation. Predicted outcome values are produced for three baseline ESAS-r values (values at the 10th, 50th, and 90th). (B) ESAS-r Total Symptom Distress Score for patients <65 relative to months since chemotherapy initiation. Predicted outcome values are produced for three baseline ESAS-r values (values at the 10th, 50th, and 90th). (C) ESAS-r Total Symptom Distress Score for patients >65 relative to months since chemotherapy initiation. Predicted outcome values are produced for three baseline ESAS-r values (values at the 10th, 50th, and 90th).
3.4. Survival Outcomes
The median progression free survival of the cohort was 6.7 months, and the median OS was 10.2 months. Multivariable Cox regression was used to identify independent predictors of death. The absence of metastatic disease and receipt of combination chemotherapy were associated with improved OS. TSDS at baseline and the presence of a physical or psychological symptom ≥ 4 were not prognostic for worse survival (Table 3).

Table 3.
Cox regression predicting survival (baseline measures of TSDS, Physical, and Psychological Scales).
In a subgroup analysis of younger (<65) and older (≥65) patients, TSDS at baseline and the presence of a physical or psychological symptom ≥ 4 were not prognostic for worse survival.
However, a joint model that included time-varying TSDS indicated that higher TSDS during follow-up was related to a higher risk of death (Table 4).

Table 4.
Cox regression from joint model predicting survival (baseline measures of physical and psychological scales plus time-varying TSDS).
4. Discussion
Our study adds new information to a growing body of literature describing the symptoms of patients with cancer using a validated and widely used PRO tool. Previous studies have demonstrated that patients with a variety of cancer types experience moderate-to-severe symptoms in the period after cancer diagnosis [24,25,26], and while undergoing cancer treatment [27]. Because pancreatic cancer is a relatively rare diagnosis, it is often grouped together with other gastrointestinal cancers in large data sets. However, pancreatic cancer is particularly aggressive and fatal, and as such, understanding the constellation and severity of symptoms of this specific group of patients is beneficial. Although studies describing symptoms in APC using PROs are limited, the existing data are consistent with what is seen in our population, with the presence of moderate-to-severe symptoms at the time of diagnosis [28,29], while receiving treatment [6], and at the time of hospice enrolment [30].
The median baseline TSDS of our population was 24, which is similar to what has been reported in another group of patients with APC receiving CT [29], and higher than what has been described in many other cancer types [31,32]. For example, in a report of patients with metastatic renal cell carcinoma receiving systemic therapy, the median baseline TSDS was 16 [32], and in a cohort of patients diagnosed with a variety of cancer types, including breast, colorectal, lung, prostate, and hematologic cancers, the mean TSDS at baseline was 22.9 [31]. Most patients (86%) in our cohort had at least one moderate-to-severe physical symptom, and over half of patients had at least one moderate-to-severe psychological symptom at baseline. Comparatively, in metastatic lung, colorectal, prostate, and breast cancers, 42.3%, 26.7%, 24.5%, and 22.6%, of patients respectively reported moderate-to-severe physical symptoms, while 33.4%, 24,3%, 19.5% and 26.1% respectively reported moderate-to-severe psychological symptoms [25]. Similar to what has been reported in other cancer populations [6,24,26,28,29,30,33,34], fatigue/tiredness was a major symptom in our cohort. Other common moderate-to-severe symptoms in our population included anxiety, shortness of breath, and nausea.
There is limited data assessing the symptom burden of patients with APC receiving active CT treatment. In a phase 1 study of patients with locally advanced pancreatic cancer, receiving chemoradiation with capecitabine and bevacizumab, the most common moderate-to-severe symptoms using the MD Anderson Symptom Inventory were fatigue, loss of appetite, pain, distress, and drowsiness. Prior to starting treatment, 42% reported at least one moderate-to-severe symptom, and the presence of comorbid medical conditions was associated with symptom severity [28]. In a population-based retrospective review of ESAS-r records of patients with APC, receiving CT, moderate-to-severe symptoms included fatigue, lack of appetite and lack of wellbeing [29]. In a study prospectively describing symptoms of patients with APC, fatigue, loss of appetite, and impaired wellbeing were also prominent at baseline; however, only 6 of 51 included patients received CT [35]. In an integrative review describing symptoms of patients with APC, assessed with 9 different PROs, including ESAS-r, major symptoms included fatigue, lack of appetite, pain, gastrointestinal symptoms, anxiety, and depression. However, it is unclear how many patients included in this review received systemic therapy [6].
The lack of moderate-to-severe pain in our study may reflect the unique nature of our cohort, which was patients with APC who were functionally well enough to receive palliative intent modern CT. However, even in a group of patients with APC requiring hospice care [30], 28% of patients did not report pain. Others have hypothesized that tumor location and proximity to the celiac plexus may be responsible for differing reports of pain [29].
In an assessment of the symptom trajectory in our cohort, symptoms improved after starting CT, but then started to deteriorate after 5 to 6 months. In a study of patients with APC receiving chemoradiation, there was an improvement in pain but worsening fatigue, sleep, and nausea during treatment, and a significant improvement in overall moderate-to-severe symptoms after treatment [28]. Interestingly, the 5-to-6-month range in which the symptoms of our cohort began to deteriorate corresponds with the median progression free survival with modern CT regimens [18,19]. This is an intriguing observation; however, a detailed investigation of symptom trajectory is outside of the scope of this study.
In our cohort, both baseline TSDS and the presence of a moderate-to-severe symptom were not associated with shorter survival. In a study of patients with advanced renal cell carcinoma receiving palliative intent therapy, baseline ESAS-r was prognostic [32]. In another study of patients with advanced cancer that did not include APC, both TSDS and the presence of a moderate-to-severe physical symptom, were associated with inferior survival [25]. In a population-based study of patients with APC receiving CT, patients who had a higher TSDS had a worse OS [29]. However, it should be noted that 54% of patients in this study received single-agent gemcitabine as first-line chemotherapy, while less than 40% received FOLFIRINOX, and only 6% received NG.
The difference seen in our study may be more reflective of the impact of a modern-day CT approach, with agents which are known to improve not only OS, but also disease-associated symptoms and QOL [36,37]. It is plausible that in patients who receive combination CT, baseline TSDS is no longer prognostic because patients experience both symptom and survival improvement with CT. Our study shows that TSDS later in the disease trajectory is more telling in patients with APC receiving modern CT. Other studies have also shown that in patients with advanced cancer, symptoms worsen before death [7], and that higher TSDS is associated with a shorter time to death [8].
The impact of novel therapies on symptom burden in APC is also of interest. Clinical trials thus far have shown minimal impact of immunotherapy on outcomes in APC [38,39,40]. Studies assessing the role of immunotherapy in combination with chemotherapy have shown some promise [41,42,43]; however, biomarker analyses and results of prospective phase III trials are awaited. Immunotherapy has been shown to be beneficial in the treatment of mismatch repair deficient (dMMR) or microsatellite high (MSI-H) disease, which occurs rarely, in 1 to 2% of patients with pancreatic cancer [44]. In the phase II KEYNOTE-158 study of patients with non-colorectal dMMR or MSI-H cancers, among 22 patients with pancreatic cancer, 4 experienced a radiologic response to the anti-PD-1 monoclonal antibody, Pembrolizumab [45]. In an assessment of tumor mutational burden (TMB) in KEYNOTE-158 [46], patients with a high TMB also experienced an increased response to immunotherapy. Assessing symptom burden in the subset patients with APC treated with immunotherapy will be informative in future studies.
There are important limitations to this study. Many patients with APC are not candidates for CT for a variety of reasons, including age, comorbidities, and poor functional status. It is possible that patients who are not candidates for CT have a different symptom profile. As such, our results may not be representative of all patients with APC. Due to the retrospective nature of our study, we could not control for all known prognostic variables, but included clinically relevant variables in our analysis, including clinical stage, ECOG, age and CT type received. The retrospective design also limits the ability to state why patients received the treatment that they did. This is particularly pertinent to the 9 patients who received single-agent gemcitabine; however, during the beginning of the time period of our study, gemcitabine would have still been a relatively common CT regimen for APC, and this regimen can still be considered in patients who wish for less toxicity, patients who are elderly, or those with a poor functional status. Our sample size is small, as it was limited to those who received CT and completed ESAS-r. Inconsistent results by subgroups such as age and tumor location may be due to limited power in the subgroup analyses. ESAS-r completion is voluntary at all outpatient visits at our institution. It is possible that some patients declined to complete the ESAS-r, and symptoms of patients admitted to hospital would not have been captured. ESAS-r began to be routinely collected at our institution in 2012. It is completed by patients on paper and then transcribed by clinical staff into the electronic medical record. It is possible that some PROs were missed in the transcription process. These factors limit the generalizability of our results to a broader population of patients with APC.
Our study confirms that in a select group of patients with APC, there is a high burden of symptoms at baseline, a high prevalence of moderate-to-severe symptoms, along with a poor prognosis. Although ESAS-r is a tool that measures symptom burden and not QOL, our findings suggest that investigating QOL in patients with APC is important. Poor QOL has been reported in pancreatic cancer [47,48,49], and studies have reported an association between poor QOL and shorter survival [47,50,51,52]. As APC is a lethal disease, with a high symptom burden, it is critical to explore how QOL can be improved for this group of patients. Early interventions, such as pain and symptom management or automatic palliative care referral, could be considered [9,53,54,55,56]. Using baseline symptoms and symptom trajectory as opportunities for intervention may result in optimization of patient-centered care in the outpatient setting.
Author Contributions
Conceptualization, C.A.K. and P.J.L.; methodology, C.A.K. and P.J.L.; data collection J.W. and S.L.; writing—original draft preparation, S.L.; writing—review and editing, J.W., P.J.L. and C.A.K.; supervision, C.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Institutional Review Board of UNIVERSITY OF MANITOBA (HS20710, H2017:126).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical/privacy concerns.
Conflicts of Interest
C.A.K. has a research grant from Celgene Inc. The other authors declare no conflict of interest.
References
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected Estimates of Cancer in Canada in 2020. CMAJ 2020, 192, e199–e205. [Google Scholar] [CrossRef] [Green Version]
- SEER. Cancer Stat Facts: Pancreatic Cancer. 2020. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 12 November 2020).
- Johnson, A.M.; Wolf, S.P.; Xuan, M.; Samsa, G.; Kamal, A.; Fisher, D.A. Patient-reported symptom burden in pancreatic cancer across a multi-site palliative care collaborative. Gastroenterology 2019, 156, s318. [Google Scholar] [CrossRef]
- Moffat, G.T.; Epstein, A.S.; O’Reilly, E.M. Pancreatic Cancer—A Disease in Need: Optimizing and Integrating Supportive Care. Cancer 2019, 125, 3927–3935. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, S.; Wiebe, L.A. Supportive Care of the Patient with Advanced Pancreatic Cancer. Oncol 2013, 27, 183–190. [Google Scholar]
- Tang, C.C.; Von Ah, D.; Fulton, J.S. The Symptom Experience of Patients with Advanced Pancreatic Cancer: An Integrative Review. Cancer Nurs. 2018, 41, 33–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammad, A.; Davis, L.E.; Mahar, A.L.; Bubis, L.D.; Zhao, H.; Earle, C.C.; Barbera, L.; Hallet, J.; Coburn, N.G. Symptom Trajectories and Predictors of Severe Symptoms in Pancreatic Adenocarcinoma at the End-of-Life: A Population Based Analysis of 2538 Patients. HPB 2019, 30, 1. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Barmala, N.; Zarinehbaf, S.; Rodin, G.; Le, L.W.; Zimmermann, C. The Association of Physical and Psychological Symptom Burden with Time to Death Among Palliative Cancer Outpatients. J. Pain Symptom Manag. 2009, 37, 297–304. [Google Scholar] [CrossRef]
- Watanabe, S.M.; Nekolaichuk, C.L.; Beaumont, C. The Edmonton Symptom Assessment System, a Proposed Tool for Distress Screening in Cancer Patients: Development and Refinement. Psychooncology 2012, 21, 977–985. [Google Scholar] [CrossRef]
- Oldenmenger, W.H.; De Raaf, P.J.; De Klerk, C.; Van Der Rijt, C.C.D. Cut Points on 0–10 Numeric Rating Scales for Symptoms Included in the Edmonton Symptom Assessment Scale in Cancer Patients: A Systematic Review. J. Pain Symptom Manag. 2013, 45, 1083–1093. [Google Scholar] [CrossRef] [Green Version]
- Selby, D.; Cascella, A.; Gardiner, K.; Do, R.; Moravan, V.; Myers, J.; Chow, E. A Single Set of Numerical Cutpoints to Define Moderate and Severe Symptoms for the Edmonton Symptom Assessment System. J. Pain Symptom Manag. 2010, 39, 241–249. [Google Scholar] [CrossRef]
- Bruera, E.; Kuehn, N.; Miller, M.J.; Selmser, P.; Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A Simple Method for the Assessment of Palliative Care Patients. J. Palliat. Care 1991, 7, 6–9. [Google Scholar] [CrossRef]
- Nekolaichuk, C.; Watanabe, S.; Beaumont, C. The Edmonton Symptom Assessment System: A 15-Year Retrospective Review of Validation Studies (1991–2006). Palliat. Med. 2008, 22, 111–222. [Google Scholar] [CrossRef]
- Richardson, L.A.; Jones, G.W. A Review of the Reliability and Validity of the Edmonton Symptom Assessment System. Curr. Oncol. 2009, 16, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Hannon, B.; Dyck, M.; Pope, A.; Swami, N.; Banerjee, S.; Mak, E.; Bryson, J.; Rodin, G.; Ridley, J.; Lo, C.; et al. Modified Edmonton Symptom Assessment System Including Constipation and Sleep: Validation in Outpatients with Cancer. J. Pain Symptom Manag. 2015, 49, 945–952. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present, and Future Developments. J. Pain Symptom Manag. 2017, 53, 630–643. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, S.; Dane, F.; Oksuzoglu, B.; Ozdemir, N.Y.; Isikdogan, A.; Ozkan, M.; Demirag, G.G.; Coskun, H.S.; Karabulut, B.; Evrensel, T.; et al. Quality of Life Study of Patients with Unresectable Locally Advanced or Metastatic Pancreatic Adenocarcinoma Treated with Gemcitabine+nab-Paclitaxel versus Gemcitabine Alone: AX-PANC-SY001, a Randomized Phase-2 Study. BMC Cancer 2020, 20, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizopoulos, D. JM: An R Package for the Joint Modelling of Longitudinal and Time-To-Event Data. J. Stat. Softw. 2010, 35, 1–33. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 26 January 2021).
- Rizopoulos, D. JM: Joint Modeling of Longitudinal and Survival Data. R Package Version 1.4-8. Available online: https://CRAN.R-project.org/package=JM (accessed on 26 January 2021).
- Harrell, F.E.J. RMS: Regression Modeling Strategies. R Package Version 6.1-0. Available online: https://CRAN.R-project.org/package=rms (accessed on 26 January 2021).
- Bubis, L.D.; Davis, L.; Mahar, A.; Barbera, L.; Li, Q.; Moody, L.; Karanicolas, P.; Sutradhar, R.; Coburn, N.G. Symptom Burden in the First Year after Cancer Diagnosis: An Analysis of Patient-Reported Outcomes. J. Clin. Oncol. 2018, 36, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Yang, L.; Boyne, D.J.; Harper, A.; Cheung, W.Y.; Cuthbert, C.A. Associations between Baseline Symptom Burden as Assessed by Patient-Reported Outcomes and Overall Survival of Patients with Metastatic Cancer. Support. Care Cancer 2020, 29, 1423–1431. [Google Scholar] [CrossRef]
- McGee, S.F.; Zhang, T.; Jonker, H.; Laurie, S.A.; Goss, G.; Nicholas, G.; Albaimani, K.; Wheatley-Price, P. The Impact of Baseline Edmonton Symptom Assessment Scale Scores on Treatment and Survival in Patients With Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, e91–e99. [Google Scholar] [CrossRef]
- Reilly, C.M.; Bruner, D.W.; Mitchell, S.A.; Minasian, L.M.; Basch, E.; Dueck, A.C.; Cella, D.; Reeve, B.B. A Literature Synthesis of Symptom Prevalence and Severity in Persons Receiving Active Cancer Treatment. Support. Care Cancer 2013, 21, 1525–1550. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Gibby, C.C.; Chan, W.; Abbruzzese, J.L.; Xiong, H.Q.; Ho, L.; Evans, D.B.; Varadhachary, G.; Bhat, S.; Wolff, R.A.; Crane, C. Patterns of Self-Reported Symptoms in Pancreatic Cancer Patients Receiving Chemoradiation. J. Pain Symptom Manag. 2007, 34, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.F.; Beca, J.; Guo, H.; Isaranawatchai, W.; Schwartz, D.; Naipaul, R.; Arias, J.; Qiao, Y.; Gavura, S.; Redmond-Misner, R.; et al. Are Population-Based Patient-Reported Outcomes Associated with Overall Survival in Patients with Advanced Pancreatic Cancer? Cancer Med. 2020, 9, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, D.W.; Mcmillan, S.C. The Physical Health of Patients with Advanced Pancreatic Cancer and the Psychological Health of Their Family Caregivers When Newly Enrolled in Hospice. J. Hosp. Palliat. Nurs. 2015, 17, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, C.A.; Boyne, D.J.; Yuan, X.; Hemmelgarn, B.R.; Cheung, W.Y. Patient-Reported Symptom Burden and Supportive Care Needs at Cancer Diagnosis: A Retrospective Cohort Study. Support. Care Cancer 2020, 28, 5889–5899. [Google Scholar] [CrossRef]
- Graham, J.; Gingerich, J.; Lambert, P.; Alamri, A.; Czaykowski, P. Baseline Edmonton Symptom Assessment System and Survival in Metastatic Renal Cell Carcinoma. Curr. Oncol. 2018, 25, e319–e323. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Kim, H.S.; Lee, S.W.; Park, Y.R.; Kim, E.H.; Lee, B.; Hu, Y.J.; Kim, K.A.; Kim, D.A.; Cho, H.Y.; et al. Pre-Screening of Patient-Reported Symptoms Using the Edmonton Symptom Assessment System in Outpatient Palliative Cancer Care. Eur. J. Cancer Care 2020, 29, e13305. [Google Scholar] [CrossRef] [PubMed]
- Barbera, L.; Seow, H.; Howell, D.; Sutradhar, R.; Earle, C.; Liu, Y.; Stitt, A.; Husain, A.; Sussman, J.; Dudgeon, D. Symptom Burden and Performance Status in a Population-Based Cohort of Ambulatory Cancer Patients. Cancer 2010, 116, 5767–5776. [Google Scholar] [CrossRef]
- Mendoza, T.R.; Kehl, K.L.; Bamidele, O.; Williams, L.A.; Shi, Q.; Cleeland, C.S.; Simon, G. Assessment of Baseline Symptom Burden in Treatment-Naïve Patients with Lung Cancer: An Observational Study. Support. Care Cancer 2019, 27, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Gourgou-Bourgade, S.; Bascoul-Mollevi, C.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Boige, V.; et al. Impact of FOLFIRINOX Compared with Gemcitabine on Quality of Life in Patients with Metastatic Pancreatic Cancer: Results from the PRODIGE 4/ACCORD 11 Randomized Trial. J. Clin. Oncol. 2013, 31, 23–29. [Google Scholar] [CrossRef]
- Schönherr, C.; Götze, T.O.; zur Hausen, G.; Reichart, A.; Pauligk, C.; Schlag, R.; Siegler, G.M.; Dörfel, S.; Aldaoud, A.; Hahn, L.; et al. Quality of Life (QoL) in Patients with Metastatic Pancreatic Cancer Receiving First-Line Nab-Paclitaxel/Gemcitabine Chemotherapy: Results of the Large QoL Study AIO-QoliXane/PARAGON. Ann. Oncol. 2018, 29, 252. [Google Scholar] [CrossRef]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.C.; Vlahovic, G.; et al. Durvalumab with or Without Tremelimumab for Patients with Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5. [Google Scholar] [CrossRef]
- AstraZeneca. Study of Tremelimumab in Patients With Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/results/NCT02527434 (accessed on 17 July 2021).
- Weiss, G.J.; Blaydorn, L.; Beck, J.; Bornemann-Kolatzki, K.; Urnovitz, H.; Schütz, E.; Khemka, V. Phase Ib/II Study of Gemcitabine, Nab-Paclitaxel, and Pembrolizumab in Metastatic Pancreatic Adenocarcinoma. Investig. New Drugs 2018, 36, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Renouf, D.J.; Knox, J.J.; Kavan, P.; Jonker, D.; Welch, S.; Couture, S.; Lemay, F.; Tehfe, M.; Harb, M.; Aucoin, N.; et al. LBA65—The Canadian Cancer Trials Group PA.7 trial: Results of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) vs GEM, nab-P, durvalumab (D) and tremelimumab (T) as first line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). In proceedings of the 2020 ESMO Conference. Ann. Oncol. 2020, 31 (Suppl. 4), s1142–s1215. [Google Scholar]
- Nct. A Study of Nivolumab by Itself or Nivolumab Combined With Ipilimumab in Patients With Advanced or Metastatic Solid Tumors. Available online: https://clinicaltrials.gov/show/NCT01928394 (accessed on 17 July 2021).
- Luchini, C.; Brosens, L.A.A.; Wood, L.D.; Chatterjee, D.; Shin, J., II; Sciammarella, C.; Fiadone, G.; Malleo, G.; Salvia, R.; Kryklyva, V.; et al. Comprehensive Characterisation of Pancreatic Ductal Adenocarcinoma with Microsatellite Instability: Histology, Molecular Pathology and Clinical Implications. Gut 2021, 70, 148–156. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/ Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.G.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.; et al. Association of tumour mutational burden with outcomes in patients with select advanced solid tumours treated with pembrolizumab in KEYNOTE-158. Ann. Oncol. 2019, 30, v477–v478. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hofheinz, R.D.; Reichart, A.; Pauligk, C.; Schönherr, C.; Schlag, R.; Siegler, G.; Dörfel, S.; Koenigsmann, M.; Zahn, M.O.; et al. Quality of Life and Outcome of Patients with Metastatic Pancreatic Cancer Receiving First-Line Chemotherapy with Nab-Paclitaxel and Gemcitabine: Real-Life Results from the Prospective QOLIXANE Trial of the Platform for Outcome, Quality of Life and Translational Research on Pancreatic Cancer Registry. Int. J. Cancer 2021, 148, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Gooden, H.M.; White, K.J. Pancreatic Cancer and Supportive Care—Pancreatic Exocrine Insufficiency Negatively Impacts on Quality of Life. Support. Care Cancer 2013, 21, 1835–1841. [Google Scholar] [CrossRef]
- Steindorf, K.; Clauss, D.; Tjaden, C.; Hackert, T.; Herbolsheimer, F.; Bruckner, T.; Schneider, L.; Ulrich, C.M.; Wiskemann, J. Quality of Life, Fatigue, and Sleep Problems in Pancreatic Cancer Patients—a Randomized Trial on the Effects of Exercise. Dtsch. Arztebl. Int. 2019, 116, 471–478. [Google Scholar] [CrossRef]
- Sloan, J.A.; Zhao, X.; Novotny, P.J.; Wampfler, J.; Garces, Y.; Clark, M.M.; Yang, P. Relationship between Deficits in Overall Quality of Life and Non-Small-Cell Lung Cancer Survival. J. Clin. Oncol. 2012, 30, 1498–1504. [Google Scholar] [CrossRef]
- Gotay, C.C.; Kawamoto, C.T.; Bottomley, A.; Efficace, F. The Prognostic Significance of Patient-Reported Outcomes in Cancer Clinical Trials. J. Clin. Oncol. 2008, 26, 1355–1363. [Google Scholar] [CrossRef]
- Quinten, C.; Coens, C.; Mauer, M.; Comte, S.; Sprangers, M.A.; Cleeland, C.; Osoba, D.; Bjordal, K.; Bottomley, A. Baseline Quality of Life as a Prognostic Indicator of Survival: A Meta-Analysis of Individual Patient Data from EORTC Clinical Trials. Lancet Oncol. 2009, 10, 865–871. [Google Scholar] [CrossRef]
- Nguyen, J.; Di Giovanni, J.; Zhang, L.; Popovic, M.; Zeng, L.; Jamani, R.; Cramarossa, G.; Culleton, S.; Jon, F.; Chow, E. Projected Referral to Other Healthcare Services in an Outpatient Palliative Radiotherapy Clinic. Expert Rev. Pharmacoecon. Outcomes Res. 2012, 12, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Mori, M.; Watanabe, S.M.; Caraceni, A.; Strasser, F.; Saarto, T.; Cherny, N.; Glare, P.; Kaasa, S.; Bruera, E. Referral Criteria for Outpatient Specialty Palliative Cancer Care: An International Consensus. Lancet Oncol. 2016, 17, e552–e559. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, C.; Hannon, B.; Krzyzanowska, M.K.; Li, M.; Rodin, G.; Pope, A.; Swami, N.; Giruparajah, M.; Howell, D.; Oza, A.M.; et al. Phase 2 Trial of Symptom Screening with Targeted Early Palliative Care (STEP) for Patients with Advanced Cancer. J. Clin. Oncol. 2019, 37, s11604. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Philip, P.A.; O’Reilly, E.M.; Uronis, H.E.; Ramanathan, R.K.; Crane, C.H.; Engebretson, A.; et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2784–2796. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).