Outcomes of Post-Operative Treatment with Concurrent Chemoradiotherapy (CRT) in High-Risk Resected Oral Cavity Squamous Cell Carcinoma (OCSCC): A Multi-Institutional Collaboration
Abstract
:1. Introduction
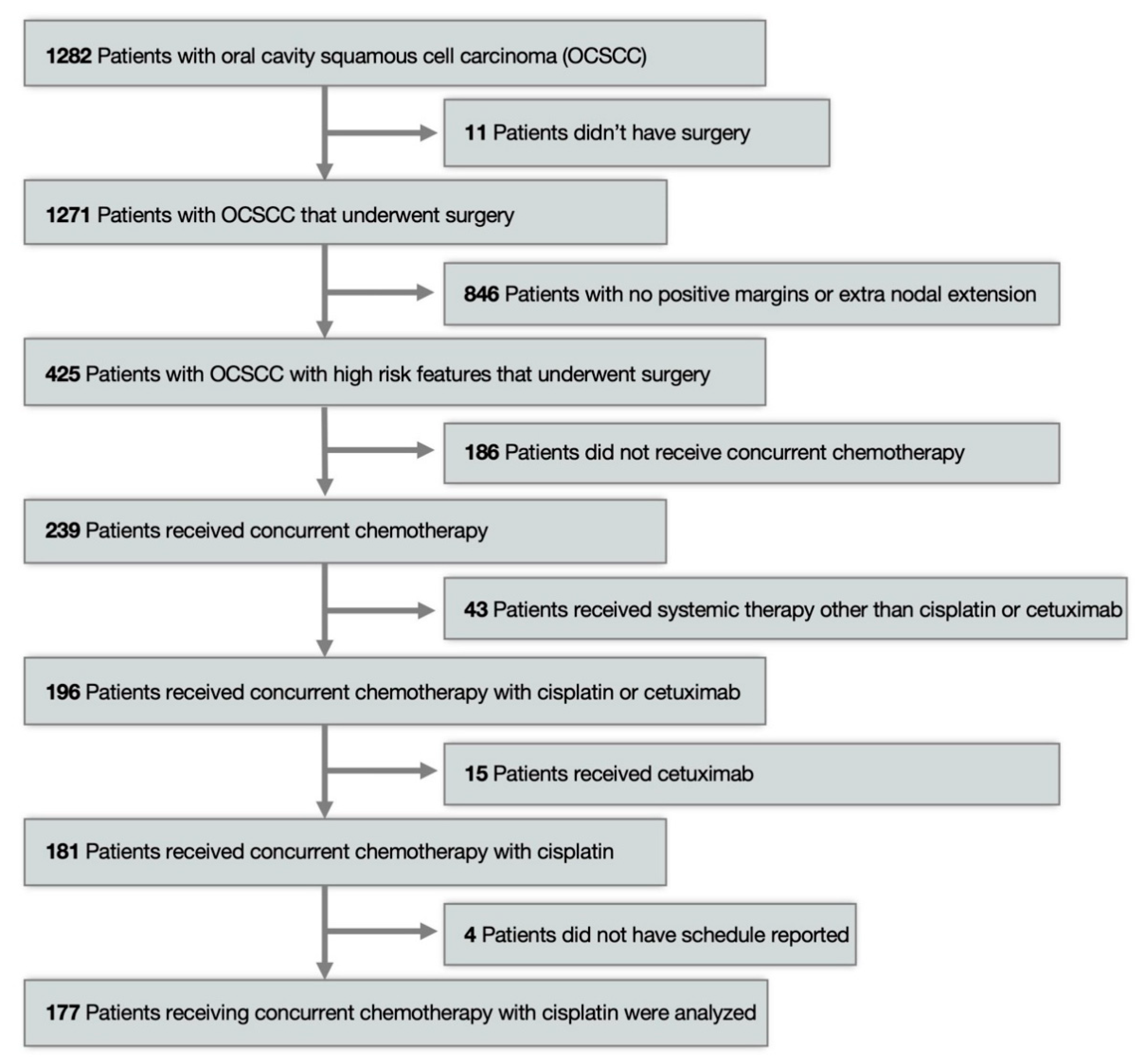
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.S.; Zhang, Q.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: Postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers, Version 2. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 4 August 2019).
- Zuur, C.L.; Simis, Y.J.; Verkaik, R.S.; Schornagel, J.H.; Balm, A.J.; Dreschler, W.A.; Rasch, C.R. Hearing loss due to concurrent daily low-dose cisplatin chemoradiation for locally advanced head and neck cancer. Radiother. Oncol. 2008, 89, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Szturz, P.; Wouters, K.; Kiyota, N.; Tahara, M.; Prabhash, K.; Noronha, V.; Adelstein, D.; Van Gestel, D.; Vermorken, J.B. Low-Dose vs. High-Dose Cisplatin: Lessons Learned From 59 Chemoradiotherapy Trials in Head and Neck Cancer. Front. Oncol. 2019, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Espeli, V.; Zucca, E.; Ghielmini, M.; Giannini, O.; Salatino, A.; Martucci, F.; Richetti, A. Weekly and 3-weekly cisplatin concurrent with intensity-modulated radiotherapy in locally advanced head and neck squamous cell cancer. Oral Oncol. 2012, 48, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Homma, A.; Inamura, N.; Oridate, N.; Suzuki, S.; Hatakeyama, H.; Mizumachi, T.; Kano, S.; Sakashita, T.; Onimaru, R.; Yasuda, K.; et al. Concomitant weekly cisplatin and radiotherapy for head and neck cancer. Jpn. J. Clin. Oncol. 2011, 41, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Otty, Z.; Skinner, M.B.; Dass, J.; Collins, M.; Mooi, J.; Thuraisingam, K.; Sabesan, S. Efficacy and tolerability of weekly low-dose cisplatin concurrent with radiotherapy in head and neck cancer patients. Asia Pac. J. Clin. Oncol. 2011, 7, 287–292. [Google Scholar] [CrossRef]
- Sharma, A.; Mohanti, B.K.; Thakar, A.; Bahadur, S.; Bhasker, S. Concomitant chemoradiation versus radical radiotherapy in advanced squamous cell carcinoma of oropharynx and nasopharynx using weekly cisplatin: A phase II randomized trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 2272–2277. [Google Scholar] [CrossRef]
- Rampino, M.; Ricardi, U.; Munoz, F.; Reali, A.; Barone, C.; Musu, A.R.; Balcet, V.; Franco, P.; Grillo, R.; Bustreo, S.; et al. Concomitant adjuvant chemoradiotherapy with weekly low-dose cisplatin for high-risk squamous cell carcinoma of the head and neck: A phase II prospective trial. Clin. Oncol. (R. Coll. Radiol.) 2011, 23, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Bachaud, J.M.; Cohen-Jonathan, E.; Alzieu, C.; David, J.M.; Serrano, E.; Daly-Schveitzer, N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: Final report of a randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 999–1004. [Google Scholar] [CrossRef]
- Wolff, H.A.; Overbeck, T.; Roedel, R.M.; Hermann, R.M.; Herrmann, M.K.; Kertesz, T.; Vorwerk, H.; Hille, A.; Matthias, C.; Hess, C.F.; et al. Toxicity of daily low dose cisplatin in radiochemotherapy for locally advanced head and neck cancer. J. Cancer Res. Clin. Oncol. 2009, 135, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) American Joint Committee on Cancer (AJCC). In AJCC Cancer Staging Manual, 7th ed.; Springer International Publishing: Cham, Switzerland, 2010; Volume 17, pp. 1471–1474. [Google Scholar]
- Bauml, J.M.; Vinnakota, R.; Anna Park, Y.H.; Bates, S.E.; Fojo, T.; Aggarwal, C.; Limaye, S.; Damjanov, N.; Di Stefano, J.; Ciunci, C.; et al. Cisplatin Every 3 Weeks Versus Weekly With Definitive Concurrent Radiotherapy for Squamous Cell Carcinoma of the Head and Neck. JNCI J. Natl. Cancer Inst. 2019, 111, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; Twardy, B.; Zordok, M.A.; Ashraf, K.; Alkhoder, A.; Schrapp, K.; Steuer, C.; Chen, Z.; Pakkala, S.; Pillai, R.; et al. Concurrent chemoradiotherapy with weekly versus triweekly cisplatin in locally advanced squamous cell carcinoma of the head and neck: Comparative analysis. Head Neck 2019, 41, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Kunieda, F.; Kiyota, N.; Tahara, M.; Kodaira, T.; Hayashi, R.; Ishikura, S.; Mizusawa, J.; Nakamura, K.; Fukuda, H.; Fujii, M.; et al. Randomized Phase II/III Trial of Post-operative Chemoradiotherapy Comparing 3-Weekly Cisplatin with Weekly Cisplatin in High-risk Patients with Squamous Cell Carcinoma of Head and Neck: Japan Clinical Oncology Group Study (JCOG1008). Jpn. J. Clin. Oncol. 2014, 44, 770–774. [Google Scholar] [CrossRef] [Green Version]
- Noronha, V.; Joshi, A.; Patil, V.M.; Agarwal, J.; Ghosh-Laskar, S.; Budrukkar, A.; Murthy, V.; Gupta, T.; D’Cruz, A.K.; Banavali, S.; et al. Once-a-Week Versus Once-Every-3-Weeks Cisplatin Chemoradiation for Locally Advanced Head and Neck Cancer: A Phase III Randomized Noninferiority Trial. J. Clin. Oncol. 2018, 36, 1064–1072. [Google Scholar] [CrossRef]
- Geiger, J.L.; Lazim, A.F.; Walsh, F.J.; Foote, R.L.; Moore, E.J.; Okuno, S.H.; Olsen, K.D.; Kasperbauer, J.L.; Price, D.L.; Garces, Y.I.; et al. Adjuvant chemoradiation therapy with high-dose versus weekly cisplatin for resected, locally-advanced HPV/p16-positive and negative head and neck squamous cell carcinoma. Oral Oncol. 2014, 50, 311–318. [Google Scholar] [CrossRef]
- Rades, D.; Seidl, D.; Janssen, S.; Bajrovic, A.; Karner, K.; Strojan, P. Schild SE.Comparison of weekly administration of cisplatin versus three courses of cisplatin 100 mg/m2 for definitive radiochemotherapy of locally advanced head-and-neck cancers. BMC Cancer 2016, 16, 437. [Google Scholar] [CrossRef] [Green Version]
- Helfenstein, S.; Riesterer, O.; Meier, U.R.; Papachristofilou, A.; Kasenda, B.; Pless, M.; Rothschild, S.I. 3-weekly or weekly cisplatin concurrently with radiotherapy for patients with squamous cell carcinoma of the head and neck—A multicentre, retrospective analysis. Radiat. Oncol. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, K.; Zhang, Q.; Wheeler, R.H.; Rosenthal, D.I.; Nguyen-Tan, F.; Lu, C.; Kim, H.; Axelrod, R.S.; Silverman, C.I.; Weber, R.S. A phase III trial (RTOG 0129) of two radiation-cisplatin regimens for head and neck carcinomas (HNC): Impact of radiation and cisplatin intensity on outcome. J. Clin. Oncol. 2010, 28, 5507. [Google Scholar] [CrossRef]
- Pignon, J.-P.; le Maître, A.; Maillard, E.; Bourhis, J. MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Vermorken, J.B.; Beitler, J.J.; Saba, N.F.; Haigentz, M., Jr.; Bossi, P.; Worden, F.P.; Langendijk, J.A.; Eisbruch, A.; Mendenhall, W.M.; et al. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: A systematic review. Head Neck 2016, 38, E2151–E2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spreafico, A.; Huang, S.H.; Xu, W.; Granata, R.; Liu, C.S.; Waldron, J.N.; Chen, E.; Ringash, J.; Bayley, A.; Chan, K.K.; et al. Impact of cisplatin dose intensity on human papillomavirus-related and -unrelated locally advanced head and neck squamous cell carcinoma. Eur. J. Cancer 2016, 67, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.B.; Spector, M.E.; Bellile, E.L.; McHugh, J.B.; Gernon, T.J.; Bradford, C.R.; Wolf, G.T.; Eisbruch, A.; Chepeha, D.B. Impact of perineural invasion in the pathologically N0 neck in oral cavity squamous cell carcinoma. Otolaryngol. Head Neck Surg. 2013, 149, 893–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagan, J.J.; Collins, B.; Barnes, L.; D’Amico, F.; Myers, E.N.; Johnson, J.T. Perineural invasion in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 637–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soo, K.C.; Carter, R.L.; O’Brien, C.J.; Barr, L.; Bliss, J.M.; Shaw, H.J. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope 1986, 96, 1145–1148. [Google Scholar] [CrossRef]
| Characteristic | n | % |
|---|---|---|
| Sex | ||
| Female | 72 | 36.7 |
| Male | 124 | 63.3 |
| Race | ||
| Black | 14 | 7.1 |
| White | 159 | 81.1 |
| Other | 23 | 11.7 |
| Tobacco use (1 unknown) | ||
| Yes | 139 | 71.3 |
| No | 56 | 28.7 |
| Tumor site | ||
| Tongue | 103 | 52.6 |
| Floor of mouth | 34 | 17.3 |
| Gingiva | 19 | 9.7 |
| Retromolar trigone (RMT) | 11 | 5.6 |
| Buccal | 17 | 8.7 |
| Other | 12 | 6.1 |
| Margin status | ||
| Positive | 70 | 35.7 |
| Negative | 126 | 64.3 |
| Extranodal extension (ENE) (3 unknown) | ||
| Yes | 162 | 83.9 |
| No | 31 | 16.1 |
| Perineural invasion (PNI) (1 unknown) | ||
| Yes | 128 | 65.6 |
| No | 67 | 34.4 |
| Lymphovascular space invasion (LVSI) (3 unknown) | ||
| Yes | 96 | 49.7 |
| No | 97 | 50.3 |
| Grade | ||
| Well differentiated | 8 | 4.1 |
| Moderately differentiated | 127 | 64.8 |
| Poorly differentiated | 61 | 31.1 |
| AJCC 7 pathologic T | ||
| T1 | 28 | 14.3 |
| T2 | 65 | 33.2 |
| T3 | 19 | 9.7 |
| T4a/T4b | 84 | 42.8 |
| AJCC 7 pathologic N | ||
| N0/no nodal dissection | 15 | 7.7 |
| N1/N2a | 37 | 18.8 |
| N2b | 115 | 58.7 |
| N2c | 28 | 14.3 |
| N3 | 1 | 0.5 |
| Systemic therapy | ||
| Cisplatin | 181 | 92.3 |
| Schedule: | ||
| Q 3 week | 122 | 67.4 |
| Q week | 55 | 30.4 |
| Unknown | 4 | 2.2 |
| Non-cisplatin-based chemotherapy (cetuximab) | 15 | 7.7 |
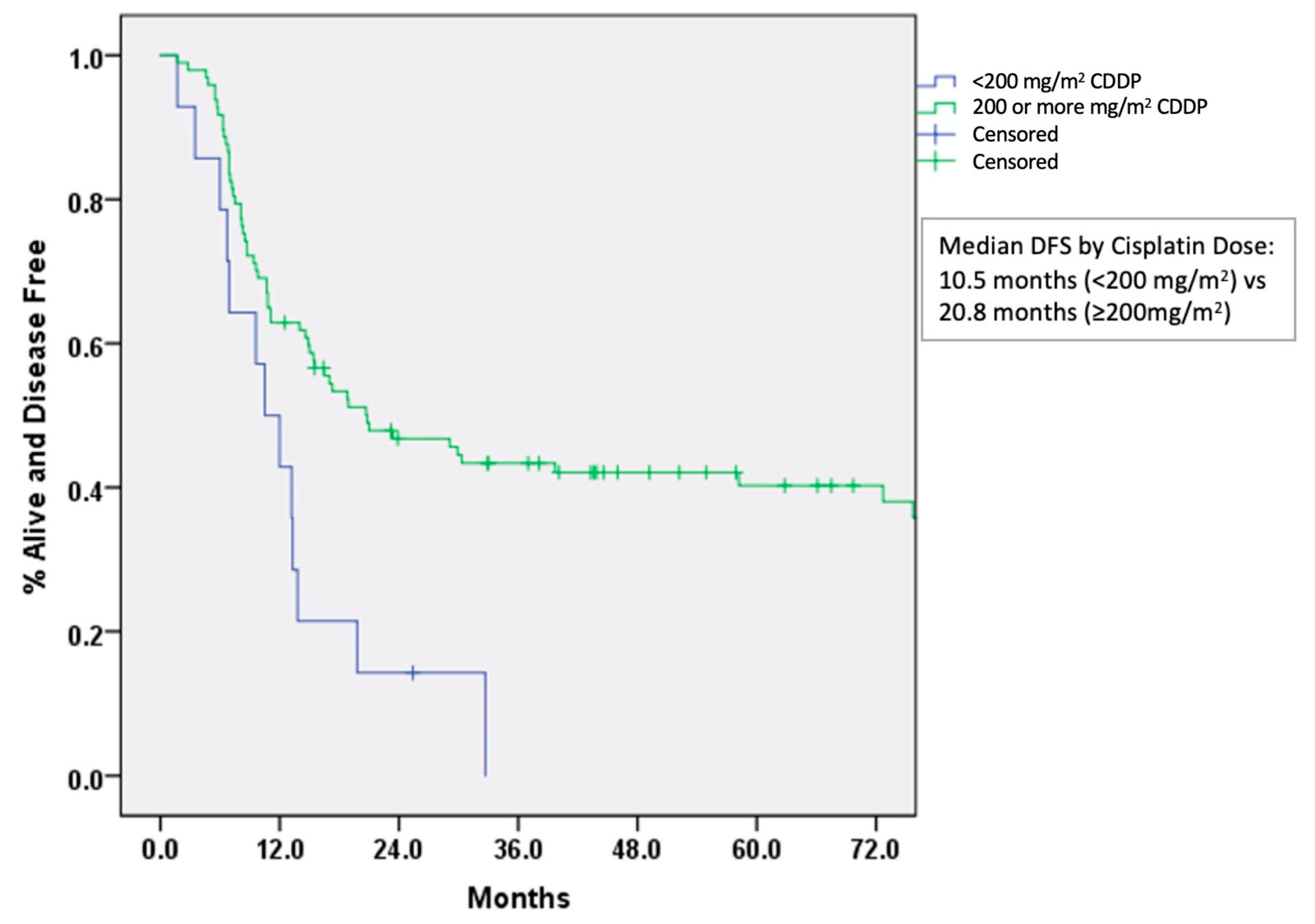
| Cisplatin dose received: Median: 200 mg/m2 (range 80–300) | ||
| ≥200 mg/m2 | 158 | 87.4 |
| <200 mg/m2 | 23 | 12.6 |
| Radiation dose received: Median 66 Gy (range 10–76) | ||
| Treatment Characteristics | Hazard Ratio (HR) | 95% Confidence Interval (CI) | p-Value |
|---|---|---|---|
| Cisplatin (CDDP) dose received (per 100 mg/m2) | 0.951 | 0.914–0.990 | 0.007 |
| Perineural invasion (PNI) | 3.077 | 1.706–5.525 | <0.001 |
| Study | Therapy Intent | Study Arms | Number of Patients | Oral Cavity | Median or Cumulative Cisplatin Dose (mg/m2) | Outcomes: Weekly vs. 3-Weekly | Conclusions | Cumulative Dose Outcomes for OS |
|---|---|---|---|---|---|---|---|---|
| Espeli et al. 2012 [8] | Adjuvant (44.7%) Definitive (52.3%) | Weekly (40 mg/m2) 3-weekly (100 mg/m2) | Total: 94 Weekly: 40 (42.6%) 3-weekly: 54 (57.4%) | Total: 33 (35%) Weekly: 15 (37.5%) 3-weekly: 18 (33.3%) | Weekly: 186 mg/m2 3-weekly: 232 mg/m2 (p = 0.0002) | Median OS at 2.8 years: 1.9 years vs. 4.3 years (p = 0.041) Median PFS: 1.5 years vs. 2.1 years (p = 0.47) | Improved OS with 3-weekly cisplatin Increased chronic renal toxicity with 3-weekly cisplatin (p = 0.04) | >240 mg/m2 cisplatin associated with better OS |
| Geiger et al. 2014 [20] | Adjuvant | Weekly (30 mg/m2) 3-weekly (100 mg/m2) | Total: 104 Weekly: 53 (50.9%) 3-weekly: 51 (49%) | Total: 26 (25%) Weekly: 16 (30%) 3-weekly: 10 (20%) | Weekly: 150 mg/m2 3-weekly: 200 mg/m2 (p = 0.01) | 3-year OS: 75% vs. 84% (p = 0.30) 3-year RFS: 74% vs. 71% (p = 0.95) | Trend towards improved survival with high-dose cisplatin in HPV/p16-positive oropharynx cancer | NR |
| Rades et al. 2016 [21] | Definitive | Weekly (30–40 mg/m2) 3-weekly (100 mg/m2) | Total: 133 Weekly: 75 (56.3%) 3-weekly: 58 (43.7%) | Total: 15 (11%) Weekly: 8 (11%) 3-weekly: 7 (12%) | NR | Improved LRC [HR] 1.57; p = 0.008) and OS in three-weekly (HR 1.33; p = 0.023). | Improved OS and LRC with 3-weekly cisplatin Increased hematotoxicity, renal failure, and pneumonia/sepsis with 3-weekly cisplatin | NR |
| Helfenstein et al. 2019 [22] | Adjuvant Definitive | Weekly (40–50 mg/m2) 3-weekly (100 mg/m2) | Total: 314 Weekly: 187 (60.0%) 3-weekly: 127 (40.4%) | Total: 57 (18.3%) Weekly: 27 (14.5%) 3-weekly: 30 (23.8%) | Weekly: 160 mg/m2 3-weekly: 200 mg/m2 (p = 0.001) | No difference in survival outcomes. | Higher number of patients received cumulative dose >200 mg/m2, 75.6% vs. 47.1% (p < 0.001) Higher acute renal toxicity with 3-weekly cisplatin | No difference in OS seen with a cumulative dose of >200 mg/m2 |
| Bauml et al. 2019 [16] | Definitive | Weekly (40 mg/m2) 3-weekly (100 mg/m2) | Total: 2901 Weekly: 701 (24.1%) 3-weekly: 2200 (75.9%) | Total: 183 (6.3%) Weekly: 55 (30%) 3-weekly: 128 (70%) | Weekly: 145 mg/m2 3-weekly: 215 mg/m2 | No difference in survival outcomes. | Higher acute renal toxicity, neutropenia, dehydration/electrolyte imbalance, and hearing loss with 3-weekly cisplatin | NR |
| Mohamed et al. 2019 [17] 39 studies included in the comparative analysis. | Definitive | Weekly (40 mg/m2) 3-weekly (100 mg/m2) | Total: 3668 Weekly: 1186 (32%) 3-weekly: 2482 (67%) | NR | Weekly: 200 mg/m2 3-weekly: 300 mg/m2 | Similar OS at 2 years: 74% vs. 67% (p = 0.67). Similar LRC: 58% vs. 61% (p = 0.7) Similar 2-year PFS: 69% vs. 62% (p = 0.9) | Weekly cisplatin comparable in efficacy and safety to 3-weekly cisplatin | NR |
| Noronha et al. 2017 [19] | Adjuvant (93%) Definitive (7%) | Weekly (30 mg/m2) 3-weekly (100 mg/m2) | Total: 300 Weekly: 150 3-weekly: 150 | Oral cavity: 262 (87%) Weekly: 136 3-weekly: 126 | Weekly: 180–200 mg/m2 3-weekly: 300 mg/m2 | Trend towards better OS in 3-weekly. Median OS 39.5 months in weekly. Median OS not reached in 3-weekly. HR (1.14 [95% CI, 0.79 to 1.65]; p = 0.48). LRC better in 3-weekly vs. weekly: 73.1% vs. 58.5%, (p = 0.014) | Better LRC in 3-weekly vs. weekly Higher grade-3 toxicities in 3-weekly vs. weekly, 84.6% vs. 71.6% (p = 0.006) | NR |
| Kunieda et al. 2014 [18] Phase II/III trial (JCOG1008) | Adjuvant | Weekly (40 mg/m2) 3-weekly (100 mg/m2) | Total: 261 Weekly: 129 (49.5%) 3-weekly: 132 (50.5%) | NR | Weekly: 239 mg/m2 3-weekly: 280 mg/m2 | 3-year OS in 3-weekly vs. weekly, 59.1% vs. 71.5% [HR, 0.69 (99.1% CI, 0.374–1.273 [<1.32] p for non-inferiority = 0.00272 [<0.00433] | Weekly cisplatin is non-inferior to 3-weekly cisplatin. Higher kidney injury, neutropenia, and mucositis in 3-weekly arm | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babar, A.; Woody, N.M.; Ghanem, A.I.; Tsai, J.; Dunlap, N.E.; Schymick, M.; Liu, H.Y.; Burkey, B.B.; Lamarre, E.D.; Ku, J.A.; et al. Outcomes of Post-Operative Treatment with Concurrent Chemoradiotherapy (CRT) in High-Risk Resected Oral Cavity Squamous Cell Carcinoma (OCSCC): A Multi-Institutional Collaboration. Curr. Oncol. 2021, 28, 2409-2419. https://doi.org/10.3390/curroncol28040221
Babar A, Woody NM, Ghanem AI, Tsai J, Dunlap NE, Schymick M, Liu HY, Burkey BB, Lamarre ED, Ku JA, et al. Outcomes of Post-Operative Treatment with Concurrent Chemoradiotherapy (CRT) in High-Risk Resected Oral Cavity Squamous Cell Carcinoma (OCSCC): A Multi-Institutional Collaboration. Current Oncology. 2021; 28(4):2409-2419. https://doi.org/10.3390/curroncol28040221
Chicago/Turabian StyleBabar, Arslan, Neil M. Woody, Ahmed I. Ghanem, Jillian Tsai, Neal E. Dunlap, Matthew Schymick, Howard Y. Liu, Brian B. Burkey, Eric D. Lamarre, Jamie A. Ku, and et al. 2021. "Outcomes of Post-Operative Treatment with Concurrent Chemoradiotherapy (CRT) in High-Risk Resected Oral Cavity Squamous Cell Carcinoma (OCSCC): A Multi-Institutional Collaboration" Current Oncology 28, no. 4: 2409-2419. https://doi.org/10.3390/curroncol28040221
APA StyleBabar, A., Woody, N. M., Ghanem, A. I., Tsai, J., Dunlap, N. E., Schymick, M., Liu, H. Y., Burkey, B. B., Lamarre, E. D., Ku, J. A., Scharpf, J., Prendes, B. L., Joshi, N. P., Caudell, J. J., Siddiqui, F., Porceddu, S. V., Lee, N., Schwartzman, L., Koyfman, S. A., ... Geiger, J. L. (2021). Outcomes of Post-Operative Treatment with Concurrent Chemoradiotherapy (CRT) in High-Risk Resected Oral Cavity Squamous Cell Carcinoma (OCSCC): A Multi-Institutional Collaboration. Current Oncology, 28(4), 2409-2419. https://doi.org/10.3390/curroncol28040221






