Distinct Outcomes of Oropharyngeal Squamous Cell Carcinoma Patients after Distant Failure According to p16 Status: Implication in Therapeutic Options
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
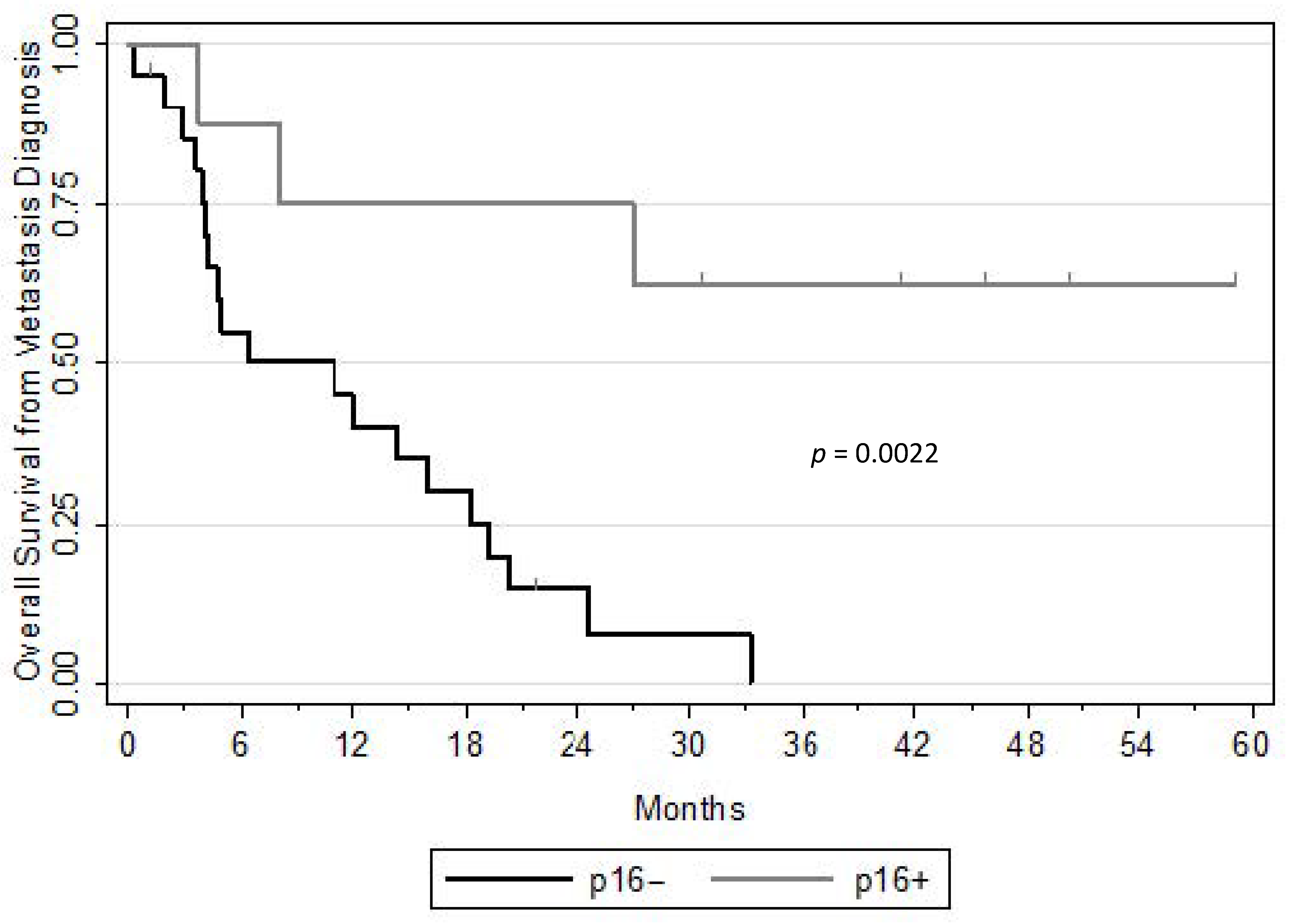
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braakhuis, B.J.; Visser, O.; Leemans, C.R. Oral and oropharyngeal cancer in The Netherlands between 1989 and 2006: Increasing incidence, but not in young adults. Oral Oncol. 2009, 45, e85–e89. [Google Scholar] [CrossRef]
- Blomberg, M.; Nielsen, A.; Munk, C.; Kjaer, S.K. Trends in head and neck cancer incidence in Denmark, 1978–2007: Focus on human papillomavirus associated sites. Int. J. Cancer 2010, 129, 733–741. [Google Scholar] [CrossRef]
- Rischin, D. Oropharyngeal Cancer, Human Papilloma Virus, and Clinical Trials. J. Clin. Oncol. 2010, 28, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.G.; Cohen, E.E. Current Treatment Options for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3305–3313. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Conibear, J.; Chia, B.; Ngai, Y.; Bates, A.T.; Counsell, N.; Patel, R.; Eaton, D.; Faivre-Finn, C.; Fenwick, J.; Forster, M.; et al. Study protocol for the SARON trial: A multicentre, randomised controlled phase III trial comparing the addition of stereotactic ablative radiotherapy and radical radiotherapy with standard chemotherapy alone for oligometastatic non-small cell lung cancer. BMJ Open 2018, 8, e020690. [Google Scholar] [CrossRef]
- Moyer, C.L.; Phillips, R.; Deek, M.P.; Radwan, N.; Ross, A.E.; Antonarakis, E.S.; Reyes, D.; Wright, J.; Terezakis, S.A.; Song, D.Y.; et al. Stereotactic ablative radiation therapy for oligometastatic prostate cancer delays time-to-next systemic treatment. World J. Urol. 2019, 37, 2623–2629. [Google Scholar] [CrossRef]
- Sutera, P.; Clump, D.A.; Kalash, R.; D’Ambrosio, D.; Mihai, A.; Wang, H.; Petro, D.P.; Burton, S.A.; Heron, D.E. Initial Results of a Multicenter Phase 2 Trial of Stereotactic Ablative Radiation Therapy for Oligometastatic Cancer. Int. J. Radiat. Oncol. 2019, 103, 116–122. [Google Scholar] [CrossRef]
- Modesto, A.; Galissier, T.; Lusque, A.; Delord, J.-P.; Uro-Coste, E.; Sarini, J.; Mouchet, F.; Lopez, R.; Laprie, A.; Graff, P.; et al. Definitive radiochemotherapy or initial surgery for oropharyngeal cancer. Strahlenther. Onkol. 2019, 195, 496–503. [Google Scholar] [CrossRef]
- Argiris, A.; Li, S.; Ghebremichael, M.; Egloff, A.M.; Wang, L.; Forastiere, A.A.; Burtness, B.; Mehra, R. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: An analysis of Eastern Cooperative Oncology Group trials. Ann. Oncol. 2014, 25, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Perez-Ordonez, B.; Weinreb, I.; Hope, A.; Massey, C.; Waldron, J.N.; Kim, J.; Bayley, A.J.; Cummings, B.; Cho, B.J.; et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013, 49, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Al-Khudari, S.; Guo, S.; Chen, Y.; Nwizu, T.; Greskovich, J.F.; Lorenz, R.; Burkey, B.B.; Adelstein, D.J.; Koyfman, S.A. Solitary dural metastasis at presentation in a patient with untreated human papillomavirus-associated squamous cell carcinoma of the oropharynx. Head Neck 2013, 36, E103–E105. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Thorstad, W.; Nussenbaum, B.; Haughey, B.; Adkins, D.; Kallogjeri, D.; Lewis, J.S., Jr. Distant metastasis in p16-positive oropharyngeal squamous cell carcinoma: A critical analysis of patterns and outcomes. Oral Oncol. 2014, 50, 45–51. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Fakhry, C.; Zhang, Q.; Nguyen-Tan, P.F.; Rosenthal, D.; El-Naggar, A.; Garden, A.S.; Soulieres, D.; Trotti, A.; Avizonis, V.; Ridge, J.A.; et al. Human Papillomavirus and Overall Survival After Progression of Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2014, 32, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Trosman, S.J.; Koyfman, S.A.; Ward, M.C.; Al-Khudari, S.; Nwizu, T.; Greskovich, J.F.; Lamarre, E.D.; Scharpf, J.; Khan, M.J.; Lorenz, R.R.; et al. Effect of Human Papillomavirus on Patterns of Distant Metastatic Failure in Oropharyngeal Squamous Cell Carcinoma Treated with Chemoradiotherapy. JAMA Otolaryngol. Neck Surg. 2015, 141, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, A.W.; Galloway, T.J.; Bhayani, M.K.; Liu, J.C. Effect of HPV Status on Survival of Oropharynx Cancer with Distant Metastasis. Otolaryngol. Neck Surg. 2020, 163, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Albergotti, W.G.; Ms, S.A.; Mathews, F.; Ferris, R.L.; Johnson, J.T.; Duvvuri, U.; Kim, S. Oligometastatic status as predictor of survival in metastatic human papillomavirus-positive oropharyngeal carcinoma. Head Neck 2018, 40, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Taberna, M.; Mena, M.; Pavón, M.A.; Alemany, L.; Gillison, M.L.; Mesía, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Lo, S.S.; Redmond, K.J.; Poon, I.; Foote, M.C.; Lohr, F.; Ricardi, U.; Sahgal, A. A multi-national report on stereotactic body radiotherapy for oligometastases: Patient selection and follow-up*. Acta Oncol. 2016, 55, 633–637. [Google Scholar] [CrossRef]
- Hong, J.C.; Salama, J.K. The expanding role of stereotactic body radiation therapy in oligometastatic solid tumors: What do we know and where are we going? Cancer Treat. Rev. 2017, 52, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Tree, A.C.; Khoo, V.S.; Eeles, R.A.; Ahmed, M.; Dearnaley, D.P.; A Hawkins, M.; Huddart, R.A.; Nutting, C.M.; Ostler, P.J.; van As, N.J. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013, 14, e28–e37. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Tomlinson, J.S.; Jarnagin, W.R.; DeMatteo, R.P.; Fong, Y.; Kornprat, P.; Gonen, M.; Kemeny, N.; Brennan, M.F.; Blumgart, L.H.; D’Angelica, M. Actual 10-Year Survival After Resection of Colorectal Liver Metastases Defines Cure. J. Clin. Oncol. 2007, 25, 4575–4580. [Google Scholar] [CrossRef]
- Rieber, J.; Streblow, J.; Uhlmann, L.; Flentje, M.; Duma, M.; Ernst, I.; Blanck, O.; Wittig, A.; Boda-Heggemann, J.; Krempien, R.; et al. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases—A pooled analysis of the German working group “stereotactic radiotherapy”. Lung Cancer 2016, 97, 51–58. [Google Scholar] [CrossRef]
- Pannone, G.; Rodolico, V.; Santoro, A.; Lo Muzio, L.; Franco, R.; Botti, G.; Aquino, G.; Pedicillo, M.C.; Cagiano, S.; Campisi, G.; et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, Consensus PCR HPV-DNA, and In Situ Hybridization. Infect. Agents Cancer 2012, 7, 4. [Google Scholar] [CrossRef]
- Jin, K.; Wang, K.; Zhang, H.; Pan, Y.; Cao, D.; Wang, M.; Chen, J.; Wu, D.; Chen, B.; Xie, X. Solitary Pulmonary Lesion in Patients with History of Malignancy: Primary Lung Cancer or Metastatic Cancer? Ann. Surg. Oncol. 2018, 25, 1237–1244. [Google Scholar] [CrossRef]
| Characteristics | Overall Cohort (N = 29) | p16− (N = 21) | p16+ (N = 8) | p-Value |
|---|---|---|---|---|
| Age, median (range) | 55 (42–79) | 55 (42–79) | 55 (49–73) | 0.23 |
| Gender M/F (%) | 21 (72)/8 (28) | 15 (71)/6 (29) | 6 (75)/2 (25) | 1.00 |
| Performance status 0/1–3 (%) | 11 (38)/18 (62) | 5 (24)/16 (76) | 6 (75)/2 (25) | 0.028 |
| Tobacco consumption > 10 pack-year (%) | 21 (81) 3 missing | 18 (100) 3 missing | 3 (38) | 0.001 |
| Alcohol abuse (%) | 20 (69) | 18 (86) | 2 (25) | 0.004 |
| Primary tumour stage T1–2/T3–4 (%) | 11 (38)/18 (62) | 6 (29)/15 (71) | 5 (62.5)/3 (37.5) | 0.20 |
| Primary nodal stage N0–N1/N2–N3 (%) | 12 (41)/17 (59) | 10 (48)/11 (52) | 2 (25)/6 (75) | 0.41 |
| Lung synchronous metastasis (%) | 2 (7) | 1 (5) | 1 (12.5) | |
| Locations (%) -Tonsil -Glossotonsillar sulcus -Base of tongue | 14 (48) 12 (42) 3 (10) | 10 (48) 8 (38) 3 (14) | 4 (50) 4 (50) 0 (0) | |
| -Initial primary treatment (%) -Definitive RCT -Surgery +/− adjuvant RCT -Induction chemotherapy (%) -Concurrent systemic therapy (%) -Cisplatin (%), n = 14 -Cetuximab (%), n = 7 | 19 (65.5) 10 (34.5) 7 (24) 21 (72) 14 (67) 7 (33) | 15 (71) 6 (29) 7 (33) 16 (76) 9 (56) 7 (44) | 4 (50) 4 (50) 0 (0) 5 (62) 5 (100) 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modesto, A.; Siegfried, A.; Lusque, A.; Vergez, S.; Sarini, J.; Brouchet, L.; Uro-Coste, E.; Graff-Cailleaud, P.; Delord, J.P. Distinct Outcomes of Oropharyngeal Squamous Cell Carcinoma Patients after Distant Failure According to p16 Status: Implication in Therapeutic Options. Curr. Oncol. 2021, 28, 1673-1680. https://doi.org/10.3390/curroncol28030156
Modesto A, Siegfried A, Lusque A, Vergez S, Sarini J, Brouchet L, Uro-Coste E, Graff-Cailleaud P, Delord JP. Distinct Outcomes of Oropharyngeal Squamous Cell Carcinoma Patients after Distant Failure According to p16 Status: Implication in Therapeutic Options. Current Oncology. 2021; 28(3):1673-1680. https://doi.org/10.3390/curroncol28030156
Chicago/Turabian StyleModesto, Anouchka, Aurore Siegfried, Amelie Lusque, Sébastien Vergez, Jerome Sarini, Laurent Brouchet, Emmanuelle Uro-Coste, Pierre Graff-Cailleaud, and Jean Pierre Delord. 2021. "Distinct Outcomes of Oropharyngeal Squamous Cell Carcinoma Patients after Distant Failure According to p16 Status: Implication in Therapeutic Options" Current Oncology 28, no. 3: 1673-1680. https://doi.org/10.3390/curroncol28030156
APA StyleModesto, A., Siegfried, A., Lusque, A., Vergez, S., Sarini, J., Brouchet, L., Uro-Coste, E., Graff-Cailleaud, P., & Delord, J. P. (2021). Distinct Outcomes of Oropharyngeal Squamous Cell Carcinoma Patients after Distant Failure According to p16 Status: Implication in Therapeutic Options. Current Oncology, 28(3), 1673-1680. https://doi.org/10.3390/curroncol28030156






