HER2 Positive and HER2 Negative Classical Type Invasive Lobular Carcinomas: Comparison of Clinicopathologic Features
Abstract
1. Introduction
2. Materials and Methods
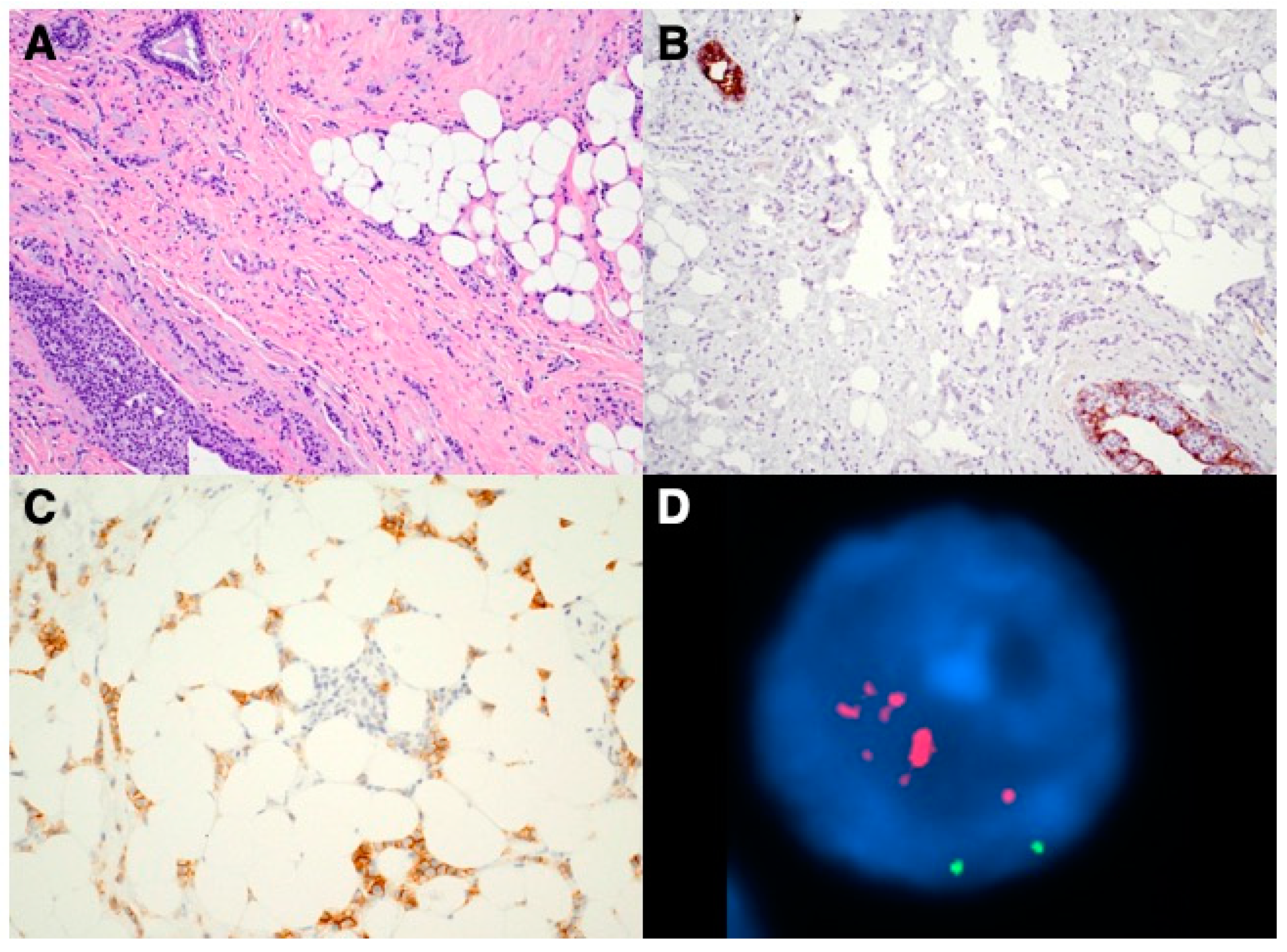
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiCostanzo, D.; Rosen, P.P.; Gareen, I.; Franklin, S.; Lesser, M. Prognosis in Infiltrating Lobular Carcinoma. An Analysis of “Classical” and Variant Tumors. Am. J. Surg. Pathol. 1990, 14, 12–23. [Google Scholar] [CrossRef]
- Henson, D.; Tarone, R. A study of lobular carcinoma of the breast based on the Third National Cancer Survey in The United States of America. Tumori J. 1979, 65, 133–142. [Google Scholar] [CrossRef]
- Ladekarl, M.; Sørensen, F.B. Prognostic, Quantitative Histopathologic Variables in Lobular Carcinoma of the Breast. Cancer 1993, 72, 2602–2611. [Google Scholar] [CrossRef]
- Li, C.I.; Anderson, B.O.; Daling, J.R.; Moe, R.E. Trends in Incidence Rates of Invasive Lobular and Ductal Breast Carcinoma. JAMA 2003, 289, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Azzopardi, J.G. Invasive Lobular Carcinoma of the Breast: Incidence and Variants. Histopathology 1979, 3, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Newman, W. Lobular Carcinoma of the Female Breast. Report of 73 Cases. Ann. Surg. 1996, 164, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Page, D.L.; Kidd, T.E.; Dupont, W.D.; Simpson, J.F.; Rogers, L.W. Lobular Neoplasia of the Breast: Higher Risk for Subsequent Invasive Cancer Predicted by More Extensive Disease. Hum. Pathol. 1991, 22, 1232–1239. [Google Scholar] [CrossRef]
- Richter, G.O.; Dockerty, M.B.; Clagett, O.T. Diffuse Infiltrating Scirrhous Carcinoma of the Breast. Special Consideration of the Single-Filing Phenomenon. Cancer 1967, 20, 363–370. [Google Scholar] [CrossRef]
- Rosen, P.P.; Lesser, M.L.; Senie, R.T.; Duthie, K. Epidemiology of Breast Carcinoma Iv: Age and Histologic Tumor Type. J. Surg. Oncol. 1982, 19, 44–51. [Google Scholar] [CrossRef]
- Foote, F.W.; Stewart, F.W. Lobular Carcinoma in Situ: A Rare Form of Mammary Cancer. Am. J. Pathol. 1941, 17, 491–496.3. [Google Scholar] [CrossRef]
- Foote, F.W.; Stewart, F.W. A Histologic Classification of Carcinoma of the Breast. Surgery 1946, 19, 74–99. [Google Scholar]
- Acs, G.; Lawton, T.J.; Rebbeck, T.R.; Livolsi, V.A.; Zhang, P.J. Differential Expression of E-Cadherin in Lobular and Ductal Neoplasms of the Breast and Its Biologic and Diagnostic Implications. Am. J. Clin. Pathol. 2001, 115, 85–98. [Google Scholar] [CrossRef]
- De Leeuw, W.J.; Berx, G.; Vos, C.B.; Peterse, J.L.; Van de Vijver, M.J.; Litvinov, S.; Van Roy, F.; Cornelisse, C.J.; Cleton-Jansen, A.M. Simultaneous Loss of E-Cadherin and Catenins in Invasive Lobular Breast Cancer and Lobular Carcinoma in Situ. J. Pathol. 1997, 183, 404–411. [Google Scholar] [CrossRef]
- Moll, R.; Mitze, M.; Frixen, U.H.; Birchmeier, W. Differential Loss of E-Cadherin Expression in Infiltrating Ductal and Lobular Breast Carcinomas. Am. J. Pathol. 1993, 143, 1731–1742. [Google Scholar]
- Qureshi, H.S.; Linden, M.D.; Divine, G.; Raju, U.B. E-Cadherin Status in Breast Cancer Correlates with Histologic Type but Does Not Correlate with Established Prognostic Parameters. Am. J. Clin. Pathol. 2006, 125, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Schechter, A.L.; Stern, D.F.; Vaidyanathan, L.; Decker, S.J.; Drebin, J.A.; Greene, M.I.; Weinberg, R.A. The Neu Oncogene: An Erb-B-Related Gene Encoding a 185,000-Mr Tumour Antigen. Nature 1984, 312, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.; Padhy, L.C.; Murray, M.; Weinberg, R.A. Transforming Genes of Carcinomas and Neuroblastomas Introduced into Mouse Fibroblasts. Nature 1981, 290, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the Her-2/Neu Oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the Her-2/Neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Ross, J.S.; Fletcher, J.A. Her-2/Neu (C-Erb-B2) Gene and Protein in Breast Cancer. Am. J. Clin. Pathol. 1999, 112, S53–S67. [Google Scholar] [PubMed]
- Shepard, H.M.; Shepard, H.M.; Jin, P.; Jin, P.; Slamon, D.J.; Slamon, D.J.; Pirot, Z.; Pirot, Z.; Maneval, D.C.; Maneval, D.C. Herceptin. In Therapeutic Antibodies; Chernajovsky, Y., Nissim, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 183–219. [Google Scholar]
- Browne, B.C.; O’Brien, N.; Duffy, M.J.; Crown, J.; O’Donovan, N. Her-2 Signaling and Inhibition in Breast Cancer. Curr. Cancer Drug Targets 2009, 9, 419–438. [Google Scholar] [CrossRef]
- Patel, A.; Unni, N.; Peng, Y. The Changing Paradigm for the Treatment of Her2-Positive Breast Cancer. Cancers 2020, 12, 2081. [Google Scholar] [CrossRef]
- Bhargava, R.; Striebel, J.; Beriwal, S.; Flickinger, J.C.; Onisko, A.; Ahrendt, G.; Dabbs, D.J. Prevalence, Morphologic Features and Proliferation Indices of Breast Carcinoma Molecular Classes Using Immunohistochemical Surrogate Markers. Int. J. Clin. Exp. Pathol. 2009, 2, 444–455. [Google Scholar] [PubMed]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Reed, A.E.M.; Kutasovic, J.R.; Lakhani, S.R.; Simpson, P.T. Invasive Lobular Carcinoma of the Breast: Morphology, Biomarkers and ‘Omics. Breast Cancer Res. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Weigelt, B.; Geyer, F.C.; Natrajan, R.; Lopez-Garcia, M.A.; Ahmad, A.S.; Savage, K.; Kreike, B.; Reis-Filho, J.S. The Molecular Underpinning of Lobular Histological Growth Pattern: A Genome-Wide Transcriptomic Analysis of Invasive Lobular Carcinomas and Grade- and Molecular Subtype-Matched Invasive Ductal Carcinomas of No Special Type. J. Pathol. 2010, 220, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Langerød, A.; Ji, Y.; Nowels, K.W.; Nesland, J.M.; Tibshirani, R.; Bukholm, I.K.; Kåresen, R.; Botstein, D.; Børresen-Dale, A.-L.; et al. Different Gene Expression Patterns in Invasive Lobular and Ductal Carcinomas of the Breast. Mol. Biol. Cell 2004, 15, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Hoff, E.R.; Raymond, R.T.; Jonathan, L.M.; Gary, W.P. Her2/Neu Amplification in Breast Cancer: Stratification by Tumor Type and Grade. Am. J. Clin. Pathol. 2002, 117, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Lal, P.; Lee, K.T.; Chen, B. Correlation of Her-2 Status with Estrogen and Progesterone Receptors and Histologic Features in 3,655 Invasive Breast Carcinomas. Am. J. Clin. Pathol. 2005, 123, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.-C.; Chen, Y.-L.; Juang, Y.-L.; Jeng, Y.-M. Frequent Alterations of Her2 through Mutation, Amplification, or Overexpression in Pleomorphic Lobular Carcinoma of the Breast. Breast Cancer Res. Treat. 2015, 150, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Porter, P.L.; Garcia, R.; Moe, R.; Gown, A.M.; Corwin, D.J. C-Erbb-2 Oncogene Protein in in Situ and Invasive Lobular Breast Neoplasia. Cancer 1991, 68, 331–334. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Nakagomi, H.; Nakada, H.; Furuya, K.; Ikegame, K.; Watanabe, H.; Omata, M.; Oyama, T. Specific Sites of Metastases in Invasive Lobular Carcinoma: A Retrospective Cohort Study of Metastatic Breast Cancer. Breast Cancer 2017, 24, 667–672. [Google Scholar] [CrossRef]
- Rosenthal, S.I.; Depowski, P.L.; Sheehan, C.E.; Ross, J.S. Comparison of Her-2/Neu Oncogene Amplification Detected by Fluorescence in Situ Hybridization in Lobular and Ductal Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2002, 10, 40–46. [Google Scholar] [CrossRef]
- Jing, Y.; David, J.D.; Shuai, Y.; Leo, A.N.; Bhargava, R. Classical-Type Invasive Lobular Carcinoma with Her2 Overexpression: Clinical, Histologic, and Hormone Receptor Characteristics. Am. J. Clin. Pathol. 2011, 136, 88–97. [Google Scholar]
- Zhang, H.; Moisini, I.; Ajabnoor, R.M.; Turner, B.M.; D’Aguiar, M.; Cai, X.; Gao, S.; Yang, Q.; Wang, X.; Schiffhauer, L.; et al. Frequency, Clinicopathologic Characteristics, and Follow-up of Her2-Positive Nonpleomorphic Invasive Lobular Carcinoma of the Breast. Am. J. Clin. Pathol. 2020, 153, 583–592. [Google Scholar] [CrossRef]
- Rilke, F.; Colnaghi, M.I.; Cascinelli, N.; Andreola, S.; Baldini, M.T.; Bufalino, R.; Della Porta, G.; Ménard, S.; Pierotti, M.A.; Testori, A. Prognostic Significance of Her-2/Neu Expression in Breast Cancer and Its Relationship to Other Prognostic Factors. Int. J. Cancer 1991, 49, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Hirohashi, S.; Shimosato, Y.; Hirota, T.; Tsugane, S.; Watanabe, S.; Terada, M.; Yamamoto, H. Correlation between Histologic Grade of Malignancy and Copy Number of C-Erbb-2 Gene in Breast Carcinoma. A Retrospective Analysis of 176 Cases. Cancer 1991, 65, 1794–1800. [Google Scholar] [CrossRef]
- Yu, J.; Bhargava, R.; Dabbs, D.J. Invasive Lobular Carcinoma with Extracellular Mucin Production and Her-2 Overexpression: A Case Report and Further Case Studies. Diagn. Pathol. 2010, 5, 36. [Google Scholar] [CrossRef]
- Kaptain, S.; Tan, L.K.; Chen, B. Her-2/Neu and Breast Cancer. Diagn. Mol. Pathol. 2011, 10, 139–152. [Google Scholar] [CrossRef]
- Gupta, D.; Croitoru, C.M.; Ayala, A.G.; Sahin, A.A.; Middleton, L.P. E-Cadherin Immunohistochemical Analysis of Histiocytoid Carcinoma of the Breast. Ann. Diagn. Pathol. 2002, 6, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hood, C.I.; Font, R.L.; Zimmerman, L.E. Metastatic Mammary Carcinoma in the Eyelid with Histiocytoid Appearance. Cancer 1973, 31, 793–800. [Google Scholar] [CrossRef]
- Shimizu, S.; Kitamura, H.; Ito, T.; Nakamura, T.; Fujisawa, J.; Matsukawa, H. Histiocytoid Breast Carcinoma: Histological, Immunohistochemical, Ultrastructural, Cytological and Clinicopathological Studies. Pathol. Int. 1998, 48, 549–556. [Google Scholar] [CrossRef]
- Eusebi, V.; Betts, C.; Haagensen, D.E.; Gugliotta, P.; Bussolati, G.; Azzopardi, J.G. Apocrine Differentiation in Lobular Carcinoma of the Breast: A Morphologic, Immunologic, and Ultrastructural Study. Hum. Pathol. 1984, 15, 134–140. [Google Scholar] [CrossRef]
- Raju, U.; Ma, C.K.; Shaw, A. Signet Ring Variant of Lobular Carcinoma of the Breast: A Clinicopathologic and Immunohistochemical Study. Mod. Pathol. 1993, 6, 516–520. [Google Scholar] [PubMed]
- Weidner, N.; Semple, J.P. Pleomorphic Variant of Invasive Lobular Carcinoma of the Breast. Hum. Pathol. 1992, 23, 1167–1171. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Fulford, L.G.; Freeman, A.; Lakhani, S.R. Pathologic Quiz Case: A 93-Year-Old Woman with an Enlarged and Tender Left Breast. Histiocytoid Variant of Lobular Breast Carcinoma. Arch. Pathol. Lab. Med. 2003, 127, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Sutton, L.M.; Cao, D.; Molberg, K.H.; Torgbe, K.; Haley, B.; Sarode, V.; Peng, Y. Decreased Androgen Receptor Expression Is Associated with Distant Metastases in Patients with Androgen Receptor-Expressing Triple-Negative Breast Carcinoma. Am. J. Clin. Pathol. 2012, 138, 511–516. [Google Scholar] [CrossRef]
- Gahlaut, R.; Bennett, A.; Fatayer, H.; Dall, B.J.; Sharma, N.; Velikova, G.; Perren, T.; Dodwell, D.; Lansdown, M.; Shaaban, A.M. Effect of Neoadjuvant Chemotherapy on Breast Cancer Phenotype, Er/Pr and Her2 Expression - Implications for the Practising Oncologist. Eur. J. Cancer 2016, 60, 40–48. [Google Scholar] [CrossRef]
- Hirata, T.; Shimizu, C.; Yonemori, K.; Hirakawa, A.; Kouno, T.; Tamura, K.; Ando, M.; Katsumata, N.; Fujiwara, Y. Change in the Hormone Receptor Status Following Administration of Neoadjuvant Chemotherapy and Its Impact on the Long-Term Outcome in Patients with Primary Breast Cancer. Br. J. Cancer 2009, 101, 1529–1536. [Google Scholar] [CrossRef]
- Ignatov, T.; Gorbunow, F.; Eggemann, H.; Ortmann, O.; Ignatov, A. Loss of Her2 after Her2-Targeted Treatment. Breast Cancer Res. Treat. 2019, 175, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Niikura, N.; Tomotaki, A.; Miyata, H.; Iwamoto, T.; Kawai, M.; Anan, K.; Hayashi, N.; Aogi, K.; Ishida, T.; Masuoka, H.; et al. Changes in Tumor Expression of Her2 and Hormone Receptors Status after Neoadjuvant Chemotherapy in 21,755 Patients from the Japanese Breast Cancer Registry. Ann. Oncol. 2016, 27, 480–487. [Google Scholar] [CrossRef]
- Nassar, A.; Radhakrishnan, A.; Cabrero, I.A.; Cotsonis, G.A.; Cohen, C. Intratumoral Heterogeneity of Immunohistochemical Marker Expression in Breast Carcinoma: A Tissue Microarray-Based Study. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 433–441. [Google Scholar] [CrossRef]
- Christgen, M.; Steinemann, D.; Kühnle, E.; Länger, F.; Gluz, O.; Harbeck, N.; Kreipe, H. Lobular Breast Cancer: Clinical, Molecular and Morphological Characteristics. Pathol. Res. Pract. 2016, 212, 583–597. [Google Scholar] [CrossRef]
- Sørlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated Observation of Breast Tumor Subtypes in Independent Gene Expression Data Sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef]
- Horne, H.N.; Oh, H.; Sherman, M.E.; Palakal, M.; Hewitt, S.M.; Schmidt, M.K.; Milne, R.L.; Hardisson, D.; Benitez, J.; Blomqvist, C.; et al. E-Cadherin Breast Tumor Expression, Risk Factors and Survival: Pooled Analysis of 5,933 Cases from 12 Studies in the Breast Cancer Association Consortium. Sci. Rep. 2018, 8, 6574. [Google Scholar] [CrossRef]
- Bertucci, F.; Orsetti, B.; Nègre, V.; Finetti, P.; Rougé, C.; Ahomadegbe, J.C.; Bibeau, F.; Mathieu, M.C.; Treilleux, I.; Jacquemier, J.; et al. Lobular and Ductal Carcinomas of the Breast Have Distinct Genomic and Expression Profiles. Oncogene 2008, 27, 5359–5372. [Google Scholar] [CrossRef]
- Mamtani, A.; Tari, A.K. Lobular Breast Cancer: Different Disease, Different Algorithms? Surg. Oncol. Clin. N. Am. 2018, 27, 81–94. [Google Scholar] [CrossRef]
- Ciriello, G.; Gatza, M.L.; Beck, A.H.; Wilkerson, M.D.; Rhie, S.K.; Pastore, A.; Zhang, H.; McLellan, M.; Yau, C.; Kandoth, C.; et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 2015, 163, 506–519. [Google Scholar] [CrossRef]
- Lau, M.-T.; Klausen, C.; Leung, P.C.K. E-Cadherin Inhibits Tumor Cell Growth by Suppressing Pi3k/Akt Signaling Via Β-Catenin-Egr1-Mediated Pten Expression. Oncogene 2011, 30, 2753–2766. [Google Scholar] [CrossRef]
- Liu, X.; Su, L.; Liu, X. Loss of Cdh1 up-Regulates Epidermal Growth Factor Receptor Via Phosphorylation of Ybx1 in Non-Small Cell Lung Cancer Cells. FEBS Lett. 2013, 587, 3995–4000. [Google Scholar] [CrossRef]
- Desmedt, C.; Zoppoli, G.; Gundem, G.G.; Pruneri, G.; Larsimont, D.; Fornili, M.M.; Fumagalli, D.; Brown, D.N.; Rothé, F.; Vincent, D.; et al. Genomic Characterization of Primary Invasive Lobular Breast Cancer. J. Clin. Oncol. 2016, 34, 1872–1881. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Sheehan, C.E.; Boguniewicz, A.B.; Otto, G.; Downing, S.R.; Sun, J.; He, J.; Curran, J.A.; Ali, S.; et al. Relapsed Classic E-Cadherin (Cdh1)-Mutated Invasive Lobular Breast Cancer Shows a High Frequency of Her2 (Erbb2) Gene Mutations. Clin. Cancer Res. 2013, 19, 2668–2676. [Google Scholar] [CrossRef]
- Wasif, N.; Maggard, M.A.; Ko, C.Y.; Giuliano, A.E. Invasive Lobular Vs. Ductal Breast Cancer: A Stage-Matched Comparison of Outcomes. Ann. Surg. Oncol. 2011, 17, 1862–1869. [Google Scholar] [CrossRef]
- Li, C.I.; Uribe, D.J.; Daling, J.R. Clinical Characteristics of Different Histologic Types of Breast Cancer. Br. J. Cancer 2005, 93, 1046–1052. [Google Scholar] [CrossRef]
- Jing, L.; Guo, H.; Treekitkarnmongkol, W.; Li, P.; Zhang, J.; Shi, B.; Ling, C.; Zhou, X.; Chen, T.; Chiao, P.J.; et al. 14–3-3zeta Cooperates with Erbb2 to Promote Ductal Carcinoma in Situ Progression to Invasive Breast Cancer by Inducing Epithelial-Mesenchymal Transition. Cancer Cell 2009, 16, 195–207. [Google Scholar]
- Harper, K.; Sosa, M.S.; Entenberg, D.; Hosseini, H.; Cheung, J.; Nobre, R.; Avivar-Valderas, A.; Nagi, C.; Girnius, N.; Davis, R.; et al. Mechanism of Early Dissemination and Metastasis in Her2+ Mammary Cancer. Nature 2016, 540, 588–592. [Google Scholar] [CrossRef]
- Sawyers, C.L. Herceptin: A First Assault on Oncogenes That Launched a Revolution. Cell 2019, 179, 8–12. [Google Scholar] [CrossRef]
| Clinicopathologic Features | HER2(+) cILC | HER2(−) cILC |
|---|---|---|
| Number of cases | 9 | 20 |
| Age at diagnosis | 59.5 ± 3.1 | 68.1 ± 2.8 |
| Follow-up time (month) | 38.2 ± 8.2 (4–85) | 38.4 ± 2.6 (24–74) |
| Laterality (left breast) | 8 (87.5%) | 11 (55.0%) |
| Tumor size (cm) | 3.4 ± 1.0 | 3.7 ± 0.6 |
| Nottingham grade | 1.8 ± 0.1 | 1.6 ± 0.1 |
| Nodal/distant metastasis | 5 (55.6%) | 5 (21.1%) |
| ER expression | 75.8 ± 14.6% | 99.2 ± 0.4% |
| PR expression | 34.1 ± 14.0% | 43.8 ± 10.1% |
| HER2 FISH ratio/gene copy # | 3.5 ± 0.7 / 7.1 ± 1.3 | Not performed |
| Ki-67 expression | 23.7 ± 6.8% | 9.3 ± 1.6% |
| Chemotherapy | 100% (+ Herceptin) | 35% |
| Hormone therapy | 88.9% | 100% |
| Radiation therapy | 55.6% | 50.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Araj, E.; Peng, Y. HER2 Positive and HER2 Negative Classical Type Invasive Lobular Carcinomas: Comparison of Clinicopathologic Features. Curr. Oncol. 2021, 28, 1608-1617. https://doi.org/10.3390/curroncol28030150
He L, Araj E, Peng Y. HER2 Positive and HER2 Negative Classical Type Invasive Lobular Carcinomas: Comparison of Clinicopathologic Features. Current Oncology. 2021; 28(3):1608-1617. https://doi.org/10.3390/curroncol28030150
Chicago/Turabian StyleHe, Lin, Ellen Araj, and Yan Peng. 2021. "HER2 Positive and HER2 Negative Classical Type Invasive Lobular Carcinomas: Comparison of Clinicopathologic Features" Current Oncology 28, no. 3: 1608-1617. https://doi.org/10.3390/curroncol28030150
APA StyleHe, L., Araj, E., & Peng, Y. (2021). HER2 Positive and HER2 Negative Classical Type Invasive Lobular Carcinomas: Comparison of Clinicopathologic Features. Current Oncology, 28(3), 1608-1617. https://doi.org/10.3390/curroncol28030150






