Cancer, Clinical Trials, and Canada: Our Contribution to Worldwide Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Search Strategy
2.2. Data Abstraction and Classification
2.3. Outcomes and Statistical Analysis
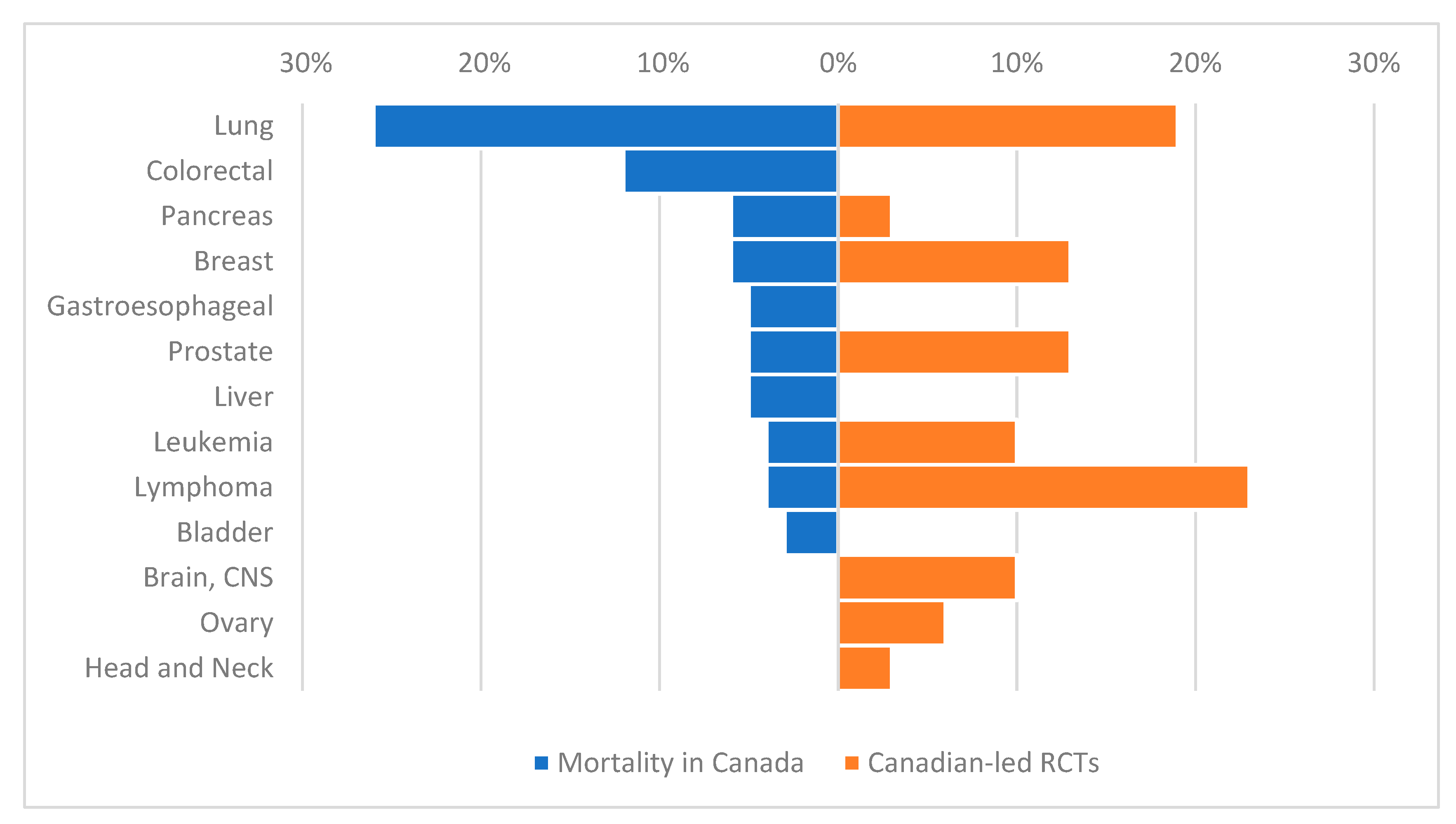
3. Results
3.1. Results of the Search Strategy
3.2. Design Characteristics of Canadian RCTs
3.3. Results of Canadian RCTs
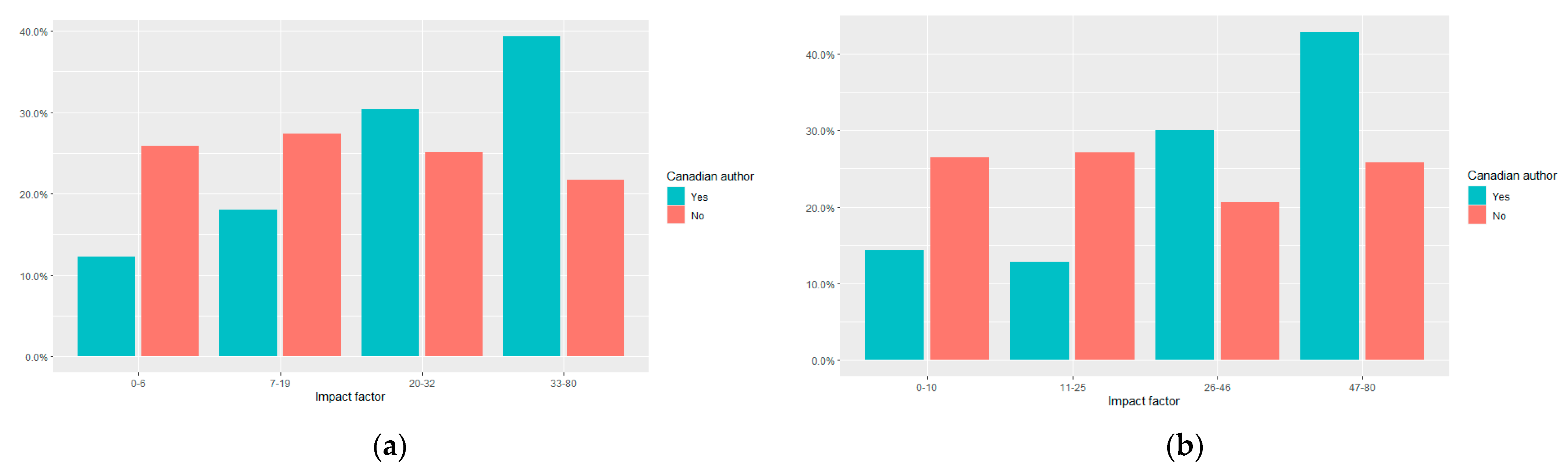
3.4. Journal Impact Factor of Canadian RCTs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tannock, I.F.; Amir, E.; Booth, C.M.; Niraula, S.; Ocana, A.; Seruga, B.; Templeton, A.J.; Vera-Badillo, F. Relevance of randomised controlled trials in oncology. Lancet Oncol. 2016, 17, e560–e567. [Google Scholar] [CrossRef]
- Booth, C.M.; Cescon, D.W.; Wang, L.; Tannock, I.F.; Krzyzanowska, M.K. Evolution of the randomized controlled trial in oncology over three decades. J. Clin. Oncol. 2008, 26, 5458–5464. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.A.; Pond, G.R.; Welch, S.; Chen, E.X. Factors associated with publication of randomized phase iii cancer trials in journals with a high impact factor. Curr. Oncol. 2014, 21, e564–e572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Canadian Cancer Research Alliance. Report on the State of Cancer Clinical Trials in Canada; Canadian Cancer Research Alliance: Toronto, ON, Canada, 2011. [Google Scholar]
- Chen, E.Y.; Joshi, S.K.; Tran, A.; Prasad, V. Estimation of study time reduction using surrogate end points rather than overall survival in oncology clinical trials. JAMA Intern. Med. 2019, 179, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Syrimi, E.; Lewison, G.; Sullivan, R.; Kearns, P. Analysis of global pediatric cancer research and publications. JCO Global Oncol. 2020, 6, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Eckhouse, S.; Lewison, G.; Sullivan, R. Trends in the global funding and activity of cancer research. Mol. Oncol. 2008, 2, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Bentley, C.; Sundquist, S.; Dancey, J.; Peacock, S. Barriers to conducting cancer trials in Canada: An analysis of key informant interviews. Curr. Oncol. 2020, 27, e307–e312. [Google Scholar] [CrossRef] [PubMed]
- Patafio, F.M.; Brooks, S.C.; Wei, X.; Peng, Y.; Biagi, J.; Booth, C.M. Research output and the public health burden of cancer: Is there any relationship? Curr. Oncol. 2016, 23, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Coronado, A.C.; Finley, C.; Badovinac, K.; Han, J.; Niu, J.; Rahal, R. Discrepancies between Canadian cancer research funding and site-specific cancer burden: A spotlight on ten disease sites. Curr. Oncol. 2018, 25, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Lewison, G.; Idir, S.; Peters, M.; Aldige, C.; Boerckel, W.; Boyle, P.; Trimble, E.L.; Roe, P.; Sethi, T.; et al. The state of lung cancer research: A global analysis. J. Thorac. Oncol. 2016, 11, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Branton, P.E. Does Canadian research investment relate to cancer burden? Lancet Oncol. 2008, 9, 82–83. [Google Scholar] [CrossRef]
- Carter, A.J.R.; Nguyen, C.N. A comparison of cancer burden and research spending reveals discrepancies in the distribution of research funding. BMC Public Health 2012, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Sharma, S.; Del Paggio, J.C.; Hopman, W.M.; Gyawali, B.; Mukherji, D.; Hammad, N.; Pramesh, C.S.; Aggarwal, A.; Sullivan, R.; et al. An analysis of contemporary oncology randomized clinical trials from low/middle-income vs high-income countries. JAMA Oncol. 2021, 7, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Clarivate Analytics. Journal Citation Reports: Impact Factor. Available online: https://jcr.clarivate.com.proxy.queensu.ca/JCRJournalHomeAction.action? (accessed on 10 July 2020).
- Cherny, N.I.; Dafni, U.; Bogaerts, J.; Latino, N.J.; Pentheroudakis, G.; Douillard, J.Y.; Tabernero, J.; Zielinski, C.; Piccart, M.J.; de Vries, E.G.E. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann. Oncol. 2017, 28, 2340–2366. [Google Scholar] [CrossRef] [PubMed]
- Canadian Partnership Against Cancer. Canadian Strategy for Cancer Control 2019–2029; Canadian Partnership Against Cancer: Toronto, ON, Canada, 2019. [Google Scholar]
- Statistics Canada. Table 13-10-0392-01: Deaths and Age-Specific Mortality Rates, by Selected Grouped Causes; Statistics Canada: Ottawa, ON, Canada, 2019.
- Ellison, L.F. Progress in net cancer survival in Canada over 20 years. Health Rep. 2018, 29, 10–18. [Google Scholar] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Bertagnolli, M.M.; Blanke, C.D.; Curran, W.J.; Hawkins, D.S.; Mannel, R.S.; O’Dwyer, P.J.; Schnall, M.D.; Wolmark, N. What happened to the US cancer cooperative groups? A status update ten years after the Institute of Medicine report. Cancer 2020, 126, 5022–5029. [Google Scholar] [CrossRef] [PubMed]
- Crump, M.; Kuruvilla, J.; Couban, S.; MacDonald, D.A.; Kukreti, V.; Kouroukis, C.T.; Rubinger, M.; Buckstein, R.; Imrie, K.R.; Federico, M.; et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J. Clin. Oncol. 2014, 32, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Schilsky, R.L. The National Clinical Trials Network and the cooperative groups: The road not taken. Cancer 2020, 126, 5008–5013. [Google Scholar] [CrossRef] [PubMed]
| All HIC RCTs | Author Involvement | |||
|---|---|---|---|---|
| n = 636 | Canada (n = 155) | Other HIC (n = 481) | p-Value | |
| n (%) | n (%) | |||
| Disease site | ||||
| Breast | 115 (18%) | 23 (15%) | 92 (19%) | <0.001 |
| Lung | 84 (13%) | 19 (12%) | 65 (14%) | |
| GI | 116 (18%) | 11 (7%) | 105 (22%) | |
| Head and Neck | 24 (4%) | 5 (3%) | 19 (4%) | |
| Heme | 118 (19%) | 32 (21%) | 86 (18%) | |
| GU | 64 (10%) | 26 (17%) | 38 (8%) | |
| Gyne | 35 (6%) | 9 (6%) | 26 (5%) | |
| Skin | 32 (5%) | 12 (8%) | 20 (4%) | |
| CNS/Brain | 20 (3%) | 10 (6%) | 10 (2%) | |
| Other | 28 (4%) | 8 (5%) | 20 (4%) | |
| Treatment intent 1 | ||||
| Palliative | 411 (65%) | 112 (72%) | 299 (62%) | 0.066 |
| Curative | 61 (10%) | 13 (8%) | 48 (10%) | |
| Neoadjuvant/adjuvant | 164 (26%) | 30 (19%) | 134 (28%) | |
| Experimental arm | ||||
| Systemic | 556 (87%) | 137 (88%) | 419 (87%) | 0.671 |
| Radiation | 34 (5%) | 5 (3%) | 29 (6%) | |
| Surgery | 15 (2%) | 4 (3%) | 11 (2%) | |
| Combination 2 | 26 (4%) | 8 (5%) | 18 (4%) | |
| Other 3 | 5 (1%) | 1 (1%) | 4 (1%) | |
| Control arm | ||||
| Active therapy | 525 (83%) | 123 (79%) | 402 (84%) | <0.001 |
| Placebo | 63 (10%) | 28 (18%) | 35 (7%) | |
| Observation/BSC | 48 (8%) | 4 (3%) | 44 (9%) | |
| Primary endpoint | ||||
| OS | 198 (31%) | 61 (39%) | 137 (29%) | 0.057 |
| DFS/EFS/RFS | 142 (22%) | 28 (18%) | 114 (24%) | |
| PFS/TTF | 213 (34%) | 54 (35%) | 159 (33%) | |
| QOL/toxicity | 20 (3%) | 3 (2%) | 17 (4%) | |
| RR | 35 (5%) | 5 (3%) | 30 (6%) | |
| Other | 28 (4%) | 4 (3%) | 24 (5%) | |
| Industry funding | ||||
| Yes | 464 (73%) | 132 (85%) | 332 (69%) | <0.001 |
| No | 149 (23%) | 21 (14%) | 128 (27%) | |
| Unstated | 23 (4%) | 2 (1%) | 21 (4%) | |
| All HIC RCTs | Author Involvement | p-Value | ||
|---|---|---|---|---|
| n = 636 | Canada n = 155 | Other HIC n = 481 | ||
| Sample size | ||||
| Median (IQR) | 474 (262–743) | 637 (410–991) | 419 (237–687) | <0.001 |
| p < 0.05 for primary endpoint 1 | n = 557 | n = 150 | n = 407 | 0.021 |
| Yes | 229 (41%) | 70 (47%) | 159 (39%) | |
| No | 328 (59%) | 80 (53%) | 248 (61%) | |
| HR for + superiority RCTs 2 | ||||
| Median (IQR) | 0.65 (0.52–0.75) | 0.67 (0.51–0.77) | 0.63 (0.52–0.75) | 0.571 |
| ESMO-MCBS grade 3 | n = 145 | n = 49 | n = 96 | 0.137 |
| Substantial benefit (A,B,4,5) | 45 (31%) | 14 (29%) | 31 (32%) | |
| Not substantial benefit (C,1,2,3) | 100 (69%) | 35 (71%) | 65 (68%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Wells, J.C.; Hopman, W.M.; Del Paggio, J.C.; Gyawali, B.; Hammad, N.; Hay, A.E.; Booth, C.M. Cancer, Clinical Trials, and Canada: Our Contribution to Worldwide Randomized Controlled Trials. Curr. Oncol. 2021, 28, 1518-1527. https://doi.org/10.3390/curroncol28020143
Sharma S, Wells JC, Hopman WM, Del Paggio JC, Gyawali B, Hammad N, Hay AE, Booth CM. Cancer, Clinical Trials, and Canada: Our Contribution to Worldwide Randomized Controlled Trials. Current Oncology. 2021; 28(2):1518-1527. https://doi.org/10.3390/curroncol28020143
Chicago/Turabian StyleSharma, Shubham, J. Connor Wells, Wilma M. Hopman, Joseph C. Del Paggio, Bishal Gyawali, Nazik Hammad, Annette E. Hay, and Christopher M. Booth. 2021. "Cancer, Clinical Trials, and Canada: Our Contribution to Worldwide Randomized Controlled Trials" Current Oncology 28, no. 2: 1518-1527. https://doi.org/10.3390/curroncol28020143
APA StyleSharma, S., Wells, J. C., Hopman, W. M., Del Paggio, J. C., Gyawali, B., Hammad, N., Hay, A. E., & Booth, C. M. (2021). Cancer, Clinical Trials, and Canada: Our Contribution to Worldwide Randomized Controlled Trials. Current Oncology, 28(2), 1518-1527. https://doi.org/10.3390/curroncol28020143





