Oncology Clinicians’ Challenges to Providing Palliative Cancer Care—A Theoretical Domains Framework, Pan-Cancer System Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Instrument
2.2. Survey Process
2.3. Data Analysis
3. Results
3.1. Demographics
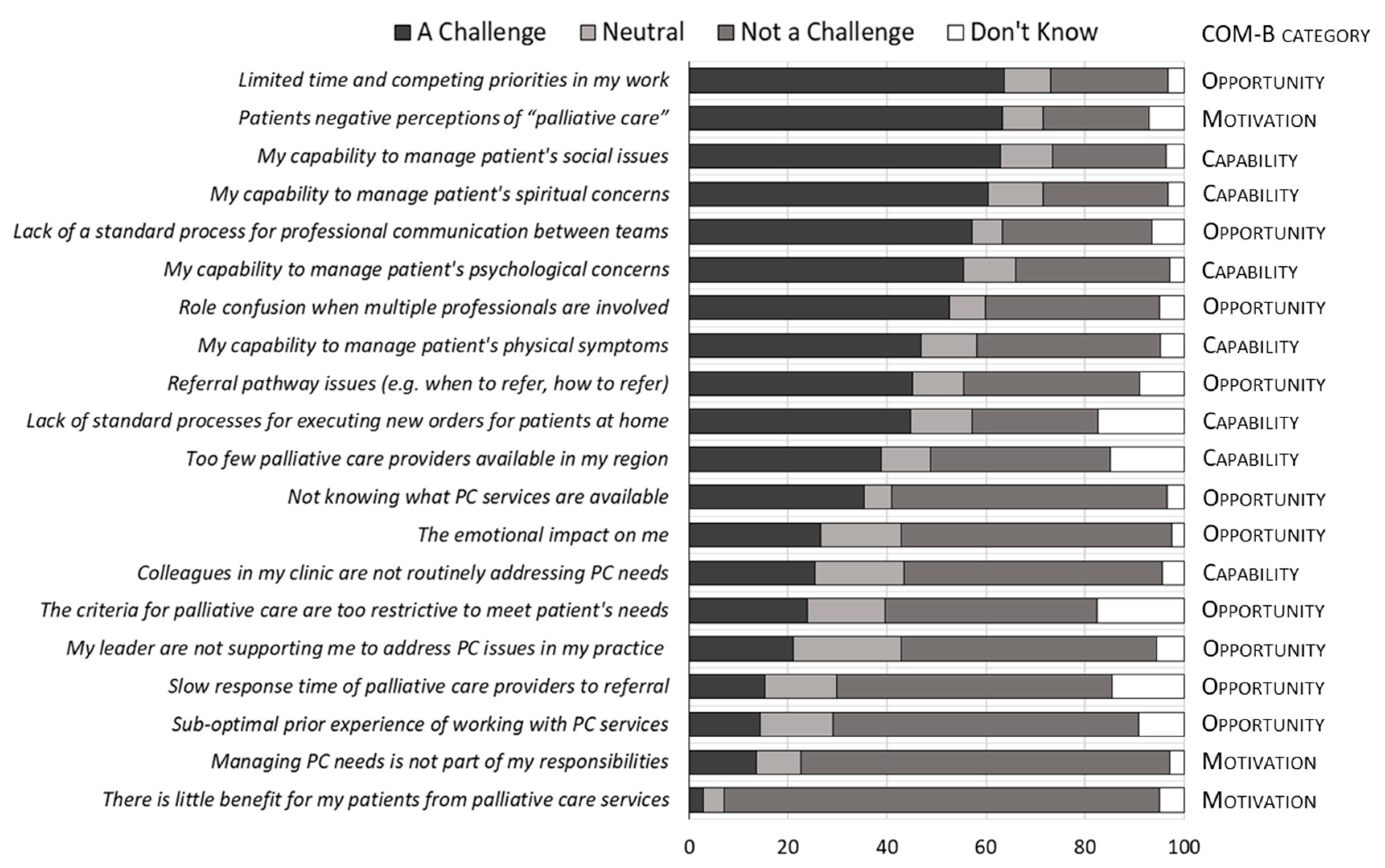
3.2. Challenges in Providing PC
3.3. Clinician Characteristics and Challenge Perceptions
3.3.1. Professional Role
3.3.2. Practice Location
3.3.3. Tumour Specialty
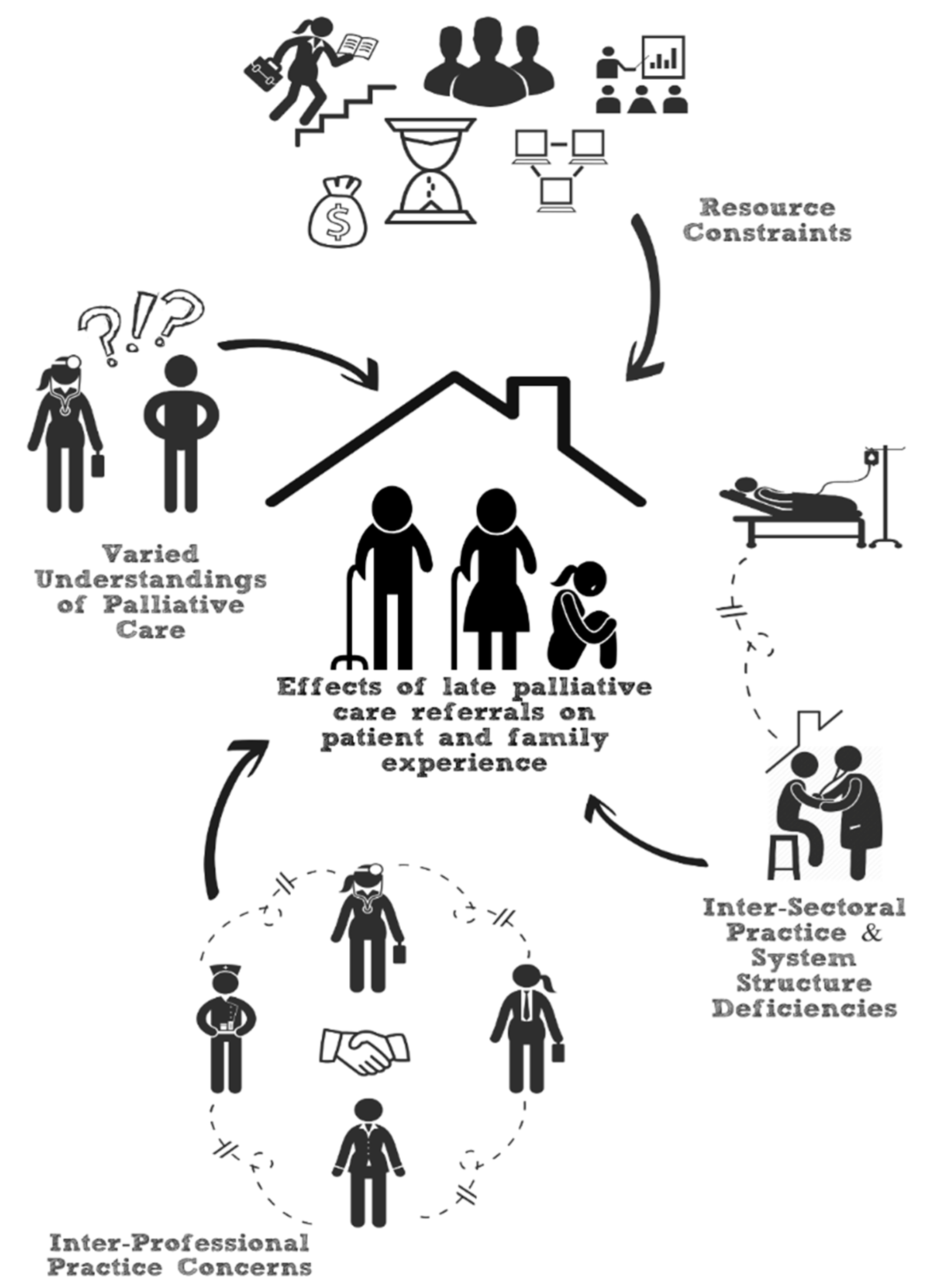
3.4. Content Analysis
3.4.1. Patients’ Varied Perceptions of PC
3.4.2. Inter-Professional Practice Challenges
3.4.3. Inter-Sectoral Practice Challenges
“Our patients are reliant on transfusion support until the very last days of their life [.] PC practitioners will often refuse to see patients until these transfusions are discontinued, making the time for support incredibly short... there is a lack of understanding from PC practitioners that although these patients might have failed treatment, they may still have good quality of life for a period of time with supportive care.”
“[A] large component of the benefit from PC Services… is the relationship that develops between the care provider and patient. Under the current system, patients and families can meet different PC providers in hospital, in clinic (and this may vary week to week in clinic), and in the community. I feel the lack of continuity is a huge drawback of the current system.”
3.4.4. Resource Constraints
4. Discussion
4.1. Next Steps
4.2. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haun, M.W.; Estel, S.; Rucker, G.; Friederich, H.C.; Villalobos, M.; Thomas, M.; Hartmann, M. Early palliative care for adults with advanced cancer. Cochrane Database Syst. Rev. 2017, 6, CD011129. [Google Scholar] [CrossRef]
- Kaasa, S.; Loge, J.H.; Aapro, M.; Albreht, T.; Anderson, R.; Bruera, E.; Brunelli, C.; Caraceni, A.; Cervantes, A.; Currow, D.C.; et al. Integration of oncology and palliative care: A Lancet Oncology Commission. Lancet Oncol. 2018, 19, e588–e653. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Mitera, G.; Earle, C.; Latosinsky, S.; Booth, C.; Bezjak, A.; Desbiens, C.; Delouya, G.; Laing, K.; Camuso, N.; Porter, G. Choosing Wisely Canada cancer list: Ten low-value or harmful practices that should be avoided in cancer care. J. Oncol. Pract. 2015, 11, e296–e303. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Aapro, M.; Kaasa, S.; Ripamonti, C.; Scotté, F.; Strasser, F.; Young, A.; Bruera, E.; Herrstedt, J.; Keefe, D.; et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann. Oncol. 2018, 29, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, J.; Siemens, W.; Meerpohl, J.J.; Antes, G.; Meffert, C.; Xander, C.; Stock, S.; Mueller, D.; Schwarzer, G.; Becker, G. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: Systematic review and meta-analysis. BMJ 2017, 357, j2925. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.; Sered, H. Advance Directives in Family Practice. Einstein Q. J. Biol. Med. 2001, 18, 67. [Google Scholar]
- Canadian Hospice Palliative Care Association. Synthesis of Recommendations from National Report on Hospice Palliative Care: A Discussion Paper; Canadian Hospice Palliative Care Association: Ottawa, ON, Canada, 2013. [Google Scholar]
- Sinnarajah, A.; Earp, M.; Cai, P.; Fong, A.; Blacklaws, K.; Karim, S.; Cheung, W.Y.; Kerba, M. Impact of specialist palliative care delivered over three months prior to death on a colorectal cancer patient’s risk of experiencing aggressive end-of-life care. J. Clin. Oncol. 2019, 37, 6614. [Google Scholar] [CrossRef]
- Cripe, J.C.; Mills, K.A.; Kuroki, L.K.; Wan, L.; Hagemann, A.R.; Fuh, K.C.; Mutch, D.G.; Powell, M.A.; Thaker, P.H. Gynecologic Oncologists’ Perceptions of Palliative Care and Associated Barriers: A Survey of the Society of Gynecologic Oncology. Gynecol. Obstet. Investig. 2018, 84, 50–55. [Google Scholar] [CrossRef]
- Wentlandt, K.; Krzyzanowska, M.K.; Swami, N.; Rodin, G.M.; Le, L.W.; Zimmermann, C. Referral practices of oncologists to specialized palliative care. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 4380–4386. [Google Scholar] [CrossRef]
- Hui, D.; Cerana, M.A.; Park, M.; Hess, K.; Bruera, E. Impact of Oncologists’ Attitudes Toward End-of-Life Care on Patients’ Access to Palliative Care. Oncologist 2016, 21, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, M.D.; Hasselaar, J.; Garralda, E.; van der Eerden, M.; Stevenson, D.; McKendrick, K.; Centeno, C.; Meier, D.E. Education, implementation, and policy barriers to greater integration of palliative care: A literature review. Palliat. Med. 2016, 30, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, K.; Watanabe, S.M. Early palliative care and its translation into oncology practice in Canada: Barriers and challenges. Ann. Palliat. Med. 2015, 4, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, R.; Nevedal, A.; Patel, M.; Blayney, D.W.; Timko, C.; Ramchandran, K.; Kelly, P.A.; Asch, S.M. The Appropriate Provision of Primary versus Specialist Palliative Care to Cancer Patients: Oncologists’ Perspectives. J. Palliat. Med. 2017, 20, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Earp, M.A.; Sinnarajah, A.; Kerba, M.; Tang, P.A.; Rodriguez-Arguello, J.; King, S.; Watanabe, S.M.; Simon, J.E. Opportunity is the greatest barrier to providing palliative care to advanced colorectal cancer patients: A survey of oncology clinicians. Curr. Oncol. 2018, 25, e480–e485. [Google Scholar] [CrossRef]
- Howard, M.; Day, A.G.; Bernard, C.; Tan, A.; You, J.; Klein, D.; Heyland, D.K. Development and Psychometric Properties of a Survey to Assess Barriers to Implementing Advance Care Planning in Primary Care. J. Pain Symptom Manag. 2018, 55, 12–21. [Google Scholar] [CrossRef]
- You, J.J.; Downar, J.; Fowler, R.A.; Lamontagne, F.; Ma, I.W.; Jayaraman, D.; Kryworuchko, J.; Strachan, P.H.; Ilan, R.; Nijjar, A.P.; et al. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: A multicenter survey of clinicians. JAMA Intern. Med. 2015, 175, 549–556. [Google Scholar] [CrossRef]
- Cane, J.; O’Connor, D.; Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 2012, 7, 37. [Google Scholar] [CrossRef]
- Michie, S.; Johnston, M.; Abraham, C.; Lawton, R.; Parker, D.; Walker, A. Making psychological theory useful for implementing evidence based practice: A consensus approach. Qual. Saf. Health Care 2005, 14, 26–33. [Google Scholar] [CrossRef]
- Michie, S.; van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef]
- Collaborative, Palliative Care Early and Systematic Project Resources. Available online: https://cumming.ucalgary.ca/research/paces-project (accessed on 30 March 2021).
- Charters, E. The Use of Think-aloud Methods in Qualitative Research an Introduction to Think-aloud Methods. Brock Educ. 2003, 12, 68–82. [Google Scholar] [CrossRef]
- Dillman, D.A.; Smyth, J.D.; Christian, L.M. Internet, Phone, Mail, and Mixed-Mode Surveys: The Tailored Design Method, 4th ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Spinger: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Hsieh, H.F.; Shannon, S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- Booker, R.; Dunn, S.; Earp, M.A.; Sinnarajah, A.; Biondo, P.D.; Simon, J.E. Hematology oncology clinicians’ perspectives on integrating palliative care in oncology. Curr. Oncol. 2020, 27, 313. [Google Scholar] [CrossRef]
- Sommerbakk, R.; Haugen, D.F.; Tjora, A.; Kaasa, S.; Hjermstad, M.J. Barriers to and facilitators for implementing quality improvements in palliative care—Results from a qualitative interview study in Norway. BMC Palliat. Care 2016, 15, 61. [Google Scholar] [CrossRef]
- Fels, J.; Pigorsch, S.; Vorwerk, H.; Engenhart-Cabillic, R.; van Oorschot, B. Palliative care in everyday practice of radiation oncologists: Results from a web-based survey among medical members of the German Society for Radiation Oncology (DEGRO). Strahlenther. Onkol. 2019, 195, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.L.; Mattes, M.D.; Yu, J.; Thrasher, A.; Shu, H.K.; Paganetti, H.; De Los Santos, J.; Koontz, B.; Abraham, C.; Balboni, T. Attitudes of radiation oncologists toward palliative and supportive care in the United States: Report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract. Radiat. Oncol. 2017, 7, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Naqvi, S.F.; Sinnarajah, A.; McGhan, G.; Simon, J.; Santana, M. Patient and caregiver experiences with advanced cancer care: A qualitative study informing the development of an early palliative care pathway. BMJ Support. Palliat. Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Park, M.; Liu, D.; Reddy, A.; Dalal, S.; Bruera, E. Attitudes and Beliefs toward Supportive and Palliative Care Referral Among Hematologic and Solid Tumor Oncology Specialists. Oncologist 2015, 20, 1326–1332. [Google Scholar] [CrossRef]
- Zimmermann, C.; Swami, N.; Krzyzanowska, M.; Leighl, N.; Rydall, A.; Rodin, G.; Tannock, I.; Hannon, B. Perceptions of palliative care among patients with advanced cancer and their caregivers. CMAJ 2016, 188, E217–E227. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, T.W.; O’Donnell, J.D.; Crowley-Matoka, M.; Rabow, M.W.; Smith, C.B.; White, D.B.; Tiver, G.A.; Arnold, R.M.; Schenker, Y. Perceptions of palliative care among hematologic malignancy specialists: A mixed-methods study. J. Oncol. Pract. 2015, 11, e230–e238. [Google Scholar] [CrossRef]
- AHS. Cancer Guidelines, Guidelines Resource Unit, Information for Health Professionals. 2021. Available online: https://www.albertahealthservices.ca/info/cancerguidelines.aspx (accessed on 31 May 2020).
| Variable | n (%) | |
|---|---|---|
| All Respondents | 263 (100%) | |
| Primary role | Nurse | 109 (41) |
| Physician | 65 (25) | |
| Allied healthcare professional | 48 (18) | |
| Radiation Therapist | 28 (11) | |
| Administration 1 | 8 (3) | |
| Educator/Facilitator | 5 (2) | |
| Primary location | Tertiary centre—Edmonton | 78 (30) |
| Tertiary centre—Calgary | 99 (38) | |
| Community centre/Other 1 | 86 (33) | |
| Primary Oncological Discipline | Medical Oncology | 128 (49) |
| Radiation Oncology | 55 (21) | |
| Surgical Oncology | 7 (3) | |
| Other Oncology Disciplines 2 | 10 (4) | |
| Not applicable 3 | 63 (24) | |
| Tumour lens | Breast | 51 (19) |
| Palliative care | 42 (16) | |
| Gastrointestinal | 37 (14) | |
| Lung | 36 (14) | |
| Hematological | 30 (11) | |
| Head and Neck | 20 (8) | |
| Gynecological | 16 (6) | |
| Genito-urinary | 14 (5) | |
| Neurological | 7 (3) | |
| All cancers | 6 (2) | |
| Other cancers 4 | 4 (2) | |
| Work with advanced cancer patients | Most of the time | 145 (55) |
| Sometimes | 108 (41) | |
| Rarely | 10 (4) | |
| Gender | Female | 210 (80) |
| Male | 52 (20) | |
| Not Reported | 1 (0) | |
| Years in role | ≥10 years | 155 (59) |
| <10 year | 108 (41) | |
| Count (%) Who “Agree” Is a Challenge; OR (95% CI) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Professional Role | Tumour lens | Location | ||||||||||||||
| Question | Physician | Nurse | Allied HCP 1 | RT | Other 2 | GI | Lung | Breast | Blood | H&N | Palliative | Other 3 | Tertiary—Calgary | Comm./ Other | Tertiary—Edmonton | |
| 1 | Limited time and competing priorities in my work. | 41/64 = 64%; ref. | 73/104 = 70%; 1.14 (0.56–2.3) | 27/43 = 63%; 0.79 (0.33–1.93) | 14/21 = 67%; 1.34 (0.46–4.11) | 5/10 = 50%; 0.91 (0.26–3.44) | 26/37 = 70%; ref. | 28/34 = 82%; 2.07 (0.67–6.97) | 28/47 = 60%; 0.75 (0.29–1.93) | 18/28 = 64%; 0.85 (0.28–2.57) | 12/18 = 67%; 0.89 (0.24–3.5) | 24/36 = 67%; 0.87 (0.29–2.57) | 24/42 = 57%; 0.62 (0.22–1.71) | 54/87 = 62%; ref. | 55/84 = 65%; 1.19 (0.59–2.4) | 51/71 = 72%; 1.55 (0.75–3.23) |
| 2 | Patients have negative perceptions of “palliative care”. | 32/64 = 50%; ref. | 79/105 = 75%; 3.4 (1.68–7.03) | 33/45 = 73%; 3.46 (1.44–8.7) | 15/18 = 83%; 6.93 (1.82–34.97) | 8/12 = 67%; 2.75 (0.76–11.7) | 26/36 = 72%; ref. | 23/33 = 70%; 0.96 (0.32–2.9) | 34/45 = 76%; 1.35 (0.46–3.95) | 20/26 = 77%; 1.38 (0.4–5.06) | 15/20 = 75%; 1.64 (0.41–7.18) | 28/41 = 68%; 0.81 (0.27–2.43) | 21/43 = 49%; 0.47 (0.16–1.32) | 64/90 = 71%; ref. | 57/81 = 70%; 0.63 (0.29–1.35) | 47/73 = 64%; 0.43 (0.2–0.91) |
| 3 | My capability to manage patients’ social issues (e.g., lives alone). | 49/64 = 77%; ref. | 68/105 = 65%; 0.39 (0.18–0.82) | 21/42 = 50%; 0.18 (0.07–0.46) | 16/21 = 76%; 0.82 (0.24–3.03) | 4/9 = 44%; 0.28 (0.07–1.12) | 27/37 = 73%; ref. | 24/34 = 71%; 1.11 (0.37–3.38) | 29/45 = 64%; 0.89 (0.32–2.42) | 17/28 = 61%; 0.8 (0.26–2.48) | 12/18 = 67%; 1.1 (0.28–4.46) | 24/36 = 67%; 0.76 (0.24–2.38) | 25/43 = 58%; 0.61 (0.21–1.74) | 53/87 = 61%; ref. | 57/82 = 70%; 2.09 (1–4.43) | 48/72 = 67%; 2.05 (1.01–4.25) |
| 4 | My capability to manage patients’ spiritual concerns (e.g., meaning of life). | 41/64 = 64%; ref. | 68/105 = 65%; 0.79 (0.39–1.55) | 21/43 = 49%; 0.31 (0.13–0.73) | 18/21 = 86%; 3.16 (0.87–15.26) | 3/9 = 33%; 0.51 (0.14–1.86) | 23/37 = 62%; ref. | 25/34 = 74%; 2.49 (0.87–7.49) | 27/46 = 59%; 1.21 (0.48–3.07) | 17/28 = 61%; 1.51 (0.52–4.44) | 13/18 = 72%; 3.49 (0.94–14.5) | 22/36 = 61%; 1.03 (0.36–2.97) | 24/43 = 56%; 1.06 (0.4–2.81) | 50/87 = 57%; ref. | 55/83 = 66%; 2.17 (1.07–4.47) | 46/72 = 64%; 1.53 (0.76–3.13) |
| 5 | Lack of standard processes for professional communication between teams. | 27/64 = 42%; ref. | 70/104 = 67%; 3.59 (1.82–7.29) | 30/43 = 70%; 4.53 (1.86–11.58) | 13/19 = 68%; 3.26 (1.02–11.35) | 8/12 = 67%; 3.5 (0.93–15.32) | 23/37 = 62%; ref. | 19/33 = 58%; 0.7 (0.25–1.96) | 25/42 = 60%; 0.96 (0.36–2.55) | 17/28 = 61%; 0.68 (0.22–2.09) | 14/19 = 74%; 1.41 (0.36–5.91) | 28/39 = 72%; 1.22 (0.41–3.68) | 22/44 = 50%; 0.47 (0.17–1.3) | 55/87 = 63%; ref. | 41/80 = 51%; 0.36 (0.17–0.76) | 52/75 = 69%; 0.73 (0.34–1.52) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunn, S.; Earp, M.A.; Biondo, P.; Cheung, W.Y.; Kerba, M.; Tang, P.A.; Sinnarajah, A.; Watanabe, S.M.; Simon, J.E. Oncology Clinicians’ Challenges to Providing Palliative Cancer Care—A Theoretical Domains Framework, Pan-Cancer System Survey. Curr. Oncol. 2021, 28, 1483-1494. https://doi.org/10.3390/curroncol28020140
Dunn S, Earp MA, Biondo P, Cheung WY, Kerba M, Tang PA, Sinnarajah A, Watanabe SM, Simon JE. Oncology Clinicians’ Challenges to Providing Palliative Cancer Care—A Theoretical Domains Framework, Pan-Cancer System Survey. Current Oncology. 2021; 28(2):1483-1494. https://doi.org/10.3390/curroncol28020140
Chicago/Turabian StyleDunn, Sharlette, Madelene A. Earp, Patricia Biondo, Winson Y. Cheung, Marc Kerba, Patricia A. Tang, Aynharan Sinnarajah, Sharon M. Watanabe, and Jessica E. Simon. 2021. "Oncology Clinicians’ Challenges to Providing Palliative Cancer Care—A Theoretical Domains Framework, Pan-Cancer System Survey" Current Oncology 28, no. 2: 1483-1494. https://doi.org/10.3390/curroncol28020140
APA StyleDunn, S., Earp, M. A., Biondo, P., Cheung, W. Y., Kerba, M., Tang, P. A., Sinnarajah, A., Watanabe, S. M., & Simon, J. E. (2021). Oncology Clinicians’ Challenges to Providing Palliative Cancer Care—A Theoretical Domains Framework, Pan-Cancer System Survey. Current Oncology, 28(2), 1483-1494. https://doi.org/10.3390/curroncol28020140







