Abstract
Natural killer (NK) cells can be widely applied for cancer immunotherapy due to their ability to lyse tumor targets without prior sensitization or human leukocyte antigens-matching. Several NK-based therapeutic approaches have been attempted in clinical practice, but their efficacy is not sufficient to suppress tumor development mainly because of lacking specificity. To this end, the engineering of NK cells with T cell receptor along with CD3 subunits (TCR-NK) has been developed to increase the reactivity and recognition specificity of NK cells toward tumor cells. Here, we review recent advances in redirecting NK cells for cancer immunotherapy and discuss the major challenges and future explorations for their clinical applications.
1. Introduction
Natural killer (NK) cells are known as the non-specific immune system that screen cell surfaces of autologous cells for abnormal expression of MHC class I molecules and cell stress marker [1]. NK cells were first identified in mice in 1975 as a subgroup of lymphocytes endowed with the capacity to eliminate cancerous cells without presenting the MHC class I molecule [2]. Since then, NK cells became a main ideology in terms of their unspecific killer machines and vital catalyzers of adaptive T-cell responses.
NK cells have been investigated clinically in several immunotherapeutic strategies for various cancers. Evidence has shown high efficacy of NK cells mediating direct killing of freshly isolated human tumor cells from hematopoietic and solid tumors [3,4]. Moreover, adoptive cell therapy (ACT) treatment using alloreactivity NK cells was safe and effective for patients with metastatic melanoma, colon carcinoma, refractory Hodgkin’s disease, and recurrent acute myeloid leukemia (AML) [5,6,7,8]. However, not all tumors appeared to respond to this type of ACT therapy. In some cases, tumor cells can evade NK cell clearance due to lacking antigen specificity. Gene-modified NK cells with chimeric antigen receptor (CAR) have been shown to enhance the effector cell function and antigen-specificity against several tumor targets, including anti-CD19 CAR-NK for targeting and chronic lymphocytic leukemia (CLL) [9] and anti-CD138 CAR-NK for targeting multiple myeloma patients [10]. Although the therapeutic effectiveness and safety of CAR-NK cell therapy have been reported, the usage of CAR-NK cell-based therapy is still faced with several obstacles, including low efficiency of CAR-transduction, limited cell expansion, and lack of available targets [11]. TCR-transduced T cells (TCR-T) have been used in clinical trials against a wide variety of tumor antigens, particularly the cancer-testis antigens (CTA) [12,13,14]. Recently, two reports tested the efficacy of TCR in combining with NK cell lines for targeting malignant cancer [15,16]. However, a concern of TCR gene transfer redirecting T cells is the mispairing of introduced TCR chains with endogenous chains [17]. Herein, we discuss the major challenges and future directions for the clinical application of NK cells.
2. Interplay between NK Cells and Cancer Cells
NK cell population is about 10–15% in a whole of human peripheral blood lymphocytes and is regarded as a natural killer as they have cytotoxic properties against tumor cells without any prior priming (e.g., as required by CD8 T cells) [18]. They have been considered of great importance in terms of immunosurveillance, as they recognize and kill different types of target cells, such as virus-infected cells and malignant cells. The majority (~90%) CD56dim of the total NK cell population in peripheral blood expresses high levels of FcγRIII (CD16), whereas a small population (~10%) CD56bright of NK cells are mostly involved in the production of cytokines [18,19]. NK cells do not undergo antigen-specific receptor rearrangement as T and B lymphocytes, instead of the functional activities of NK cells to lyse the target process through their germline-encoded immunoreceptors [20]. NK cells protect the host from infectious or cancers by expressing activating and inhibitory receptors. Activated NK cells can recognize and eliminate the target cells by the balance of the signaling derived from inhibitory receptors (e.g., KIRS or NKG2A) and activating receptors (e.g., NCRs or NKG2D) [21,22]. Moreover, NK cells are involved in the regulation of the immune response by the expression of different chemokines and chemokine receptors such as CCL4, CXCL8, and CXCR3 [23,24]. Klas Karre, the first person, as part of his doctoral thesis, proposed that NK cytolysis of a target cell could be triggered by a decrease or absence of host major histocompatibility class I molecules (MHC-I) on the surface of a target cell [25]. This hypothesis was then confirmed by several other groups [26,27,28]. NK cells are inactivated when their inhibitory receptors identify the self-MHC class I molecule, and thus they can protect the cells from host cell attacking [29]. In cancer patients who are low/deficient of MHC-class I or bear “altered-self” stress-inducible proteins can be targeted by NK cell killing and cytotoxicity "the missing self-hypothesis" of Klas Karre [25]. NK cells kill tumor cells through several mechanisms, including the release of cytoplasmic granules containing perforin and granzyme, secretion of immunoregulatory cytokines such as nitric oxide (NO), and expression of other TNF-family members such as Fas-L or TRAIL (Figure 1A). However, tumor cells can evade host immune response via multiple strategies, including weak immunogenicity of target antigens and the creation of an immune-suppressive tumor environment (Figure 1B).
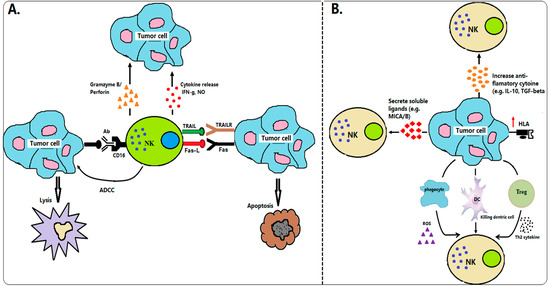
Figure 1.
Interactions between NK cells and cancer cells in immunosurveillance. (A) Mechanism of NK cells in tumor immunosurveillance. NK cells can identify the tumor cells by stress or danger signals. Upon stimulation, NK cells directly killed tumor cells through many tactics, including the release of cytokine productions (e.g., IFN-g, NO), cytoplasmic granule release (e.g., granzyme B, perforin), death receptor-induced apoptosis (e.g., Fas-L, TRAIL), and ADCC. (B) Mechanism of tumor cells invading NK cells. Tumor cells defend themselves from NK cell attack through several techniques, including secretion soluble ligand of NK cell receptors (e.g., MICA/B), upregulation of HLA molecules, secretion immunosuppressive factor products (e.g., TGF-β or IL-10), and activation of Treg or phagocyte-derived inhibitory cytokines (ROS).
3. NK Cells in Cancer Immunotherapy
The ability to recognize and lyse tumor cells via a variety of recognition receptors make NK cells a candidate for cancer immunotherapy. Several strategies of using NK cells have been attempted for clinical practice in cancer immunotherapy (Table 1) [30,31,32]. Some of these approaches include isolation of immune cells and expansion cell with cytokines (e.g., autologous and allogeneic NK cells, and NK cell lines), and other strategies involved genetically modified immune cells with specific-target genes (e.g., CAR-NK and TCR-NK). Despite some successes, a large proportion of patients failed to respond to NK cell-based immunotherapy.

Table 1.
Strategies to eliminate tumors and some limitations.
3.1. Autologous NK Cell Therapy
Early trials of autologous NK cell therapy from a leukapheresis product have demonstrated high potency against several advanced metastatic cancers [59,60]. In one clinical trial (UMIN000007527), autologous NK cell therapy was very effective in patients with advanced digestive, colon, and lung cancer, and adverse event was not observed [61]. However, some other studies showed that these autologous NK cells failed to demonstrate clinical responses or efficacy [34,62]. This failure was mainly due to the inhibitory receptors on autologous NK cells matched self MHC class I presented on cancer cells, which subsequently suppressed the activation of NK cells [63,64]. In addition, autologous NK cells derived from cancer patients were usually in an immunosuppression state with impaired functions, making these cells difficult to demonstrate antitumor functionality.
Previous studies proposed that NK cell antitumor activity could be enhanced by the systemic administration of cytokines. Systemic cytokine administration in combination with NK cells was a major strategy for adoptive cancer immunotherapy [65,66,67,68]. Optimization by using adoptive transfer of ex vivo IL-2 activated NK cells showing better outcomes than the systemic administration of IL-2 [61]. Although cytokine IL-2-activated autologous NK cells can boost NK cell activation and exhibit high efficacy against malignant tumors, existing studies showed that this strategy had limited success [35]. Lymphokine-activated killer (LAK) cells in combination with IL-2 displayed upregulated adhesion molecules and activating receptors and secreted inflammatory cytokines to adhere and lyse metastatic renal cell carcinoma (RCC) [69]. However, after adoptively transferring LAK cells, patients received toxic side effects of vascular leak syndrome due to a high level of IL-2 [70]. Another study demonstrated that transferring of ex vivo expanded autologous NK cells with IL-15 was potent regression in non-Hodgkin lymphoma (NHL) patients [71]. However, extremely high doses of IL-15 were associated with cytokine release syndrome in the patients with advanced acute myeloid leukemia (AML) [72] and dose-limiting toxicities in the patient malignant melanoma or renal cell cancer [73]. In contrast to autologous NK cell therapy, stimulation human haploidentical NK cells with IL-15 cytokine showed complete hematologic remission in poor-prognosis AML patients [5].
3.2. Allogeneic NK Cell Therapy
Unlike T cells, adaptive transferring of NK cells does not promote GVHD, and therefore life-threatening toxicity of patients of allogeneic donor NK cell administration is negligible. KIR-ligand mismatches have been trialed in patients with AML and showed increasing overall survival, better engraftment, and a reduced incidence of GVHD after receiving haploidentical T cell-depleted allogeneic stem cell transplantation [74]. Allogeneic NK cells with KIR mismatch offer greater cytotoxicity than autologous NK cells and can be effective at controlling AML relapse [5,75]. Moreover, clinical evidence also agrees with the therapeutic effect of allogeneic NK cells in controlling human malignancies, including high-risk leukemia, renal cell carcinoma, and others [5,46,76,77]. Allogeneic NK cells expanded with IL-15 and hydrocortisone are being tested for the treatment of non-small cell lung carcinoma in a Phase I safety clinical trial [40]. The result of other Phase I clinical trials also showed high efficacy and safety of allogeneic NK cells in combing with interleukin 21 (mbIL-21) expansions for targeting the advanced myeloid malignancies [39]. The advantages of allogeneic NK cell transfusion include that these cells are cultivated or well-educated in healthy hosts and have high-efficiency killing cancer cells. However, using KIR mismatched allogeneic NK cells sometimes created immune-mediated rejection due to MHC mismatch. The study of KIR/HLA genotype has been demonstrated that a large population of patients was associated with acute rejection after kidney transplantation because of KIR/HLA polymorphism [42]. In Phase II clinical trials (NCT00703820), adaptive allogeneic NK cells from KIR–HLA-mismatched donors failed to respond to the intermediate- or high-risk AML. The failure could be the outcome of insufficient numbers and limited persistence of alloreactive donor NK cells [44]. The major limitation of using allogeneic NK cells in therapy is the problem of yielding an adequate cell number. Therefore, the optimization ex vivo expansion and activation strategies remain a major focus [44]. In addition, allogeneic NK cells from donors may be risky for cancer immunotherapy due to unpredictable T or B lymphocyte presenting.
3.3. NK Cell Lines
The NK cell lines, such as NK-92 or NK-92MI, NKL, NKG, KHYG-1, and YT, have been generated from malignant NK cell clones [78]. NK cell lines are purity NK cells, which are unlimited cell expansion and proliferation, and can obtain a sufficient amount of the cells in a short period, and hence it can transplant to patients on a regular schedule. Only NK-92 has shown a high antitumor activity in several types of tumor and has worked well in pre-clinical development [78,79,80]. NK-92 was generated from a non-Hodgkin Lymphoma patient and could kill hematopoietic cancers in vitro [81]. NK-92 cells expressed most of the activating receptor but rarely expressed inhibitory killer cell immunoglobulin-like receptors (KIRs) except for KIR2DL4, which can hamper NK cell activation and killing the target cells [80]. Alternatively, NK-92MI cells have similar characteristics of activated NK cells as their origin NK-92 cells and obtain the gene expression by transduction with human IL-2 cDNA [82].
NK-92 has received US FDA approval for testing in patients with solid tumors, and the non-modified NK-92 has completed Phase I trials [45,46,47,83]. For minor risk factors, cell lines need to irradiate priority before transfusion to patients. Thus, NK-92 cells are unable to expand severely in vivo, which can decrease their efficacy to target the tumor. Several Phase I clinical trials data have shown the safety and tolerability of NK-92 cells, however, the results are still unsatisfied with their clinical benefit [47,48]. For example, no antitumor response of the adoptive transfer of NK-92 cells was observed in all patients with refractory/relapsed AML of Phase I clinical trial (NCT00900809) [45].
3.4. Antibody-Based NK Cell Therapy
Antibody-based drugs are widely used in cancer immunotherapy. The activating type IIIa Fc receptor (FcγRIIIA) or CD16 expression by NK cells enables them to bind to antibody-coated targets initiating ADCC pathway that eventually results in the elimination of target cells [84,85,86]. The roles of ADCC in the efficacy of therapeutic antitumor monoclonal antibodies have shown in the patients with non-Hodgkin’s lymphoma (NHL) for rituximab (anti-CD20) and metastatic breast and gastric carcinoma for herceptin (anti-HER2) [87,88]. Currently, the use of margetuximab (anti-HER2) plus pembrolizumab (anti-PD1 checkpoint blockade) has been demonstrated highly effective for treating patients of HER2-positive gastro-oesophageal adenocarcinoma [89]. Moreover, in the clinical trial (NCT03248492), trastuzumab deruxtecan (antibody-drug conjugate) has been demonstrated high efficacy, prolong progression-free survival and safety in the patients with Her-2 positive metastatic breast cancer [90]. Alemtuzumab (anti-CD52), which activates NK cell effectors, has been shown to have high-efficiency in patients with B-CLL, and GVHD post-HSCT [91,92]. Dinutuximab is a product of human-mouse chimeric mAb (ch14.18 mAb), which can mediate ADCC through NK cell receptors. This product has demonstrated high efficacy against GD2-positive neuroblastoma cells in vitro and melanoma cells in vivo [93,94,95]. Use of cetuximab in combination with irradiated high affinity (ha) NK cells has shown highly lysed chordoma cancer [53]. Daratumumab (a mAb against CD38)-induced NK cells via ADCC mechanism have demonstrated effective elimination of multiple myeloma (MM) in pre-clinical and clinical studies [49,96,97]. In clinical trials (NCT03158688 and NCT01998971), Daratumumab plus carfilzomib and dexamethasone have shown effective clinical response and prolong progression-free survival (PFS) in patients with relapsed or refractory MM [97,98]. Moreover, monoclonal antibodies (zalutumumab and necitumumab), anti-EGFR mAb, have been shown efficacy for treating patients with squamous cell carcinoma of the head and neck and have been approved by the FDA [99,100]. In MHC-I expressing tumor cells, the effector functions of autologous NK cells are often inhibited by KIR. Therefore, strategies to block KIR expression using antibodies have been developed to potentiate NK cell cytotoxicity. The anti-KIR (IPH2101) mAb is being tested in Phase I clinical trial (EUDRACT: 2005-005298-31). Interestingly, IPH2101 mAb can block KIR-mediated inhibition of NK cells to enhance cytotoxicity against AML blasts [51] and multiple myeloma [52]. Moreover, several clinical trials of using therapeutic mAb in combination with NK cells for cancer treatments have been reported in elsewhere reviews [101,102,103,104,105,106,107]. Although the therapeutic monoclonal antibodies have shown a promising therapeutic efficacy for many cancer types, dose optimization and optimal management of toxicity are still needed to be determined before infusing them into patients [54].
3.5. Genetic Modification of CAR-NK Cells
The cytokine gene transfer approaches, including interleukins and stem cell factor (SCF), have been shown to induce NK cell proliferation and increases survival capacity in vivo functional activity [108,109]. However, these strategies are still limited due to NK cell specificity. Genetic manipulation of chimeric antigen receptors (CAR) redirecting NK cells (CAR-NK) could be an effective approach to mediate specific NK cell antitumor effects against different targets. This technique is based on the transduction of NK cells to target tumor cells by gene transfer of CAR-specific receptors through recombinant a single-chain variable fragment receptor (Fv) specific of a tumor-associated specific antigen to downstream intracellular signaling machinery [110].
The use of primary CAR-NK and CAR-NK lines in pre-clinical and clinical trials for targeting specific tumor have been reported in elsewhere reviews [56,111,112]. Pre-clinical studies in hematological tumors showed high specificity and cytotoxicity toward the target cells [55,113,114,115,116,117,118,119,120,121,122,123]. For example, the transduction of cord blood (CB)-derived NK cells with a retroviral vector of CAR-CD19 containing IL-15 and suicide caspase-9-based suicide (iC9/CAR.19/IL-15 CB-NK cells) have demonstrated to be efficient in killing CD19-expressing cell lines and primary leukemia cells in vitro, xenograft model, and in the clinical trial (NCT03056339) [9,124]. Moreover, the CAR-NK-based showed a potent antitumor effect against several solid tumors in pre-clinical studies [125,126,127].
So far, only a few clinical trial studies of CAR-NK for targeting hematological or solid tumors have been registered on ClinicalTrials.gov (Table 2). Two clinical trials were the origin of NK cell lines, including BCMA (NCT03940833) for targeting malignant multiple myeloma (MM) and CD33 (NCT02944162) for targeting AML. The other trials were the origin from human primary NK cells, including ROBO1 for targeting solid tumor (NCT03940820), Mesothelin for targeting epithelial ovarian cancer (NCT03692637), PSMA for targeting prostate cancer (NCT03692663), CD22 for targeting refractory B cell lymphoma (NCT03692767), CD19 for targeting refractory B cell lymphoma (NCT03690310), CD19/CD22 for targeting metastatic solid tumors (NCT03824964), NKG2D for targeting relapsed and refractory B cell lymphoma (NCT03415100), and CD19/iCasp9/IL15 for targeting B cell non-Hodgkin lymphoma (NHL) (NCT03579927).

Table 2.
Clinical study of CAR-NK in hematological and solid tumors.
Although CAR-NK therapy has successfully entered into clinical trials, its clinical application is often associated with some challenges. First, the obstacle of the clinical application of primary CAR-NK is the limitation of cell expansion for flexible schedule transfusion [56]. Stimulation of NK cells with feeder cells has been shown to expand the number of NK cells. However, the final NK cell products are possible containing the feeder cells, which could be a potential risk in clinical usage [128]. Second, NK cells are hard to achieve high transduction efficiency than other cells of the hematopoietic system [57]. The transfection efficiency of peripheral blood (PB) and cord blood (CB) with mRNA showed low efficacy: less than 10% (PB and CB), while lentiviral transduction showed ~8–16% in PB and ~12–73% in CB [115]. Although electroporation mRNA transfection has seen better efficiency in CB, the expression of CAR molecule is unstable and losses expression within a few days, which are the barrier for ACT [118]. Third, the freeze-thaw process of NK cells was associated with loss of functional activity [58]. There have been reported the severe loss of cytolytic function of cryopreservation IL-15-activated NK cells (92–98% reduction), followed by overnight culturing the cell without cytokine IL-15 [58]. Hence, similar to CAR-NK immunotherapy, genetically modified NK cells using TCR molecules could be a better option for cancer treatment.
3.6. TCR Transduced NK Cells in Cancer Immunotherapy
Recent technology of genetically modified NK cells could be used to study different pathways involved in NK cell tumor targeting and to improve their tumor cytotoxicity [129]. NK cells lack the expression of the TCR complex subunits (except CD3ζ); however, they do express all necessary molecules for downstream signaling [130]. The possibility to apply such gene-transfer strategies to NK cells appears fascinating. It is possible to give extra tumor-antigen specificity to NK cell, which is already MHC-independent antitumor activity. TCR, as a natural antigen receptor, is effectively recognizing several proteins of the tumor. Therefore, it can redirect to cells such as NK cells against the tumors. The wild-type TCR can generate from the low avidity of T cells, and its affinity can enhance by using the phage display library [13,131]. Techniques used to transfer the gene redirecting NK cells, including viral transduction (retroviral/lentiviral) and transfection (DNA/RNA electroporation) [129]. Lentiviral transduction was a potential approach and safe in the clinic and showed efficient transduction and stable integration of transgenes [132,133]. Similar to CAR-NK products, TCR-NK products also could be prepared for clinical use. It has been known that loss or downregulation of HLA Ia in human tumors usually facilitates immune evasion from CD8+ CTL-mediated killing [134]. However, the absence or downregulation of HLA class Ia can promote the expression of new tumor peptides presented by HLA-E, therefore activating unconventional CD8+ T cells through HLA-E–peptide–specific recognition by TCRs. This feature endows an advantage of TCR-NK in recognizing tumor cells with HLA I downregulation.
A schematic presents the techniques for the preparation of TCR-NK product is shown in Figure 2. Tumor-specific TCRs (e.g., NY-ESO-1 gene) and the four subsets of the CD3 gene were constructed into a lentivirus vector. Lentiviral particles can produce by transfection of the viral vector containing the TCR/CD3 gene into the package cells (e.g., 293 T cell). For producing TCR and CD3 stable expression in NK cell line (e.g., NK-92), the NK-92 cells were transduced with the lentiviral particles of CD3 (NK-92-CD3). NK-92-CD3 clone can achieve by shorting and seeding in 96 well-plaque. NK-92-CD3 cells were transduced with lentiviral particles of the tumor-specific TCR (TCR-NK-92-CD3, we called TCR-NK). The positive TCR-NK were shorted and seeded in 96 well-plaque to obtain the pure products. The TCR-NK products have tested the effect against the target cells and manufactured under GMP-compliant conditions. Therefore, the final TCR-products could be frozen and preserved in a therapeutic biobank, and they would be readily available for transfusion to patients.
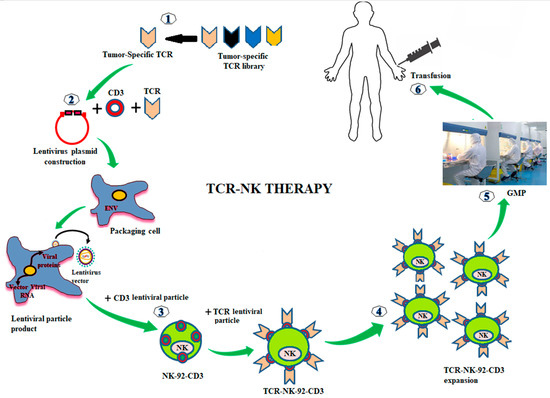
Figure 2.
Genetic modifications of NK-92 cells with TCR for cancer immunotherapy. Tumor-specific TCRs are generated from the tumor-specific TCR library. TCRs are constructed into a lentivirus vector. The lentiviral particles of TCR are produced by transfection with the TCRs lentivirus vector into packing cells (293T cell). To make a stable expression of TCR and CD3 in NK-92 cells, the NK-92 cells are transduced with the lentiviral particles of CD3 (NK-92-CD3). Then, the stable expression of NK-92-CD3 is transduced with lentiviral particles of TCR (TCR-NK-92-CD3, in the short term called TCR-NK). The positive TCRs expressing in NK-92 cells are expanded ex vivo. TCR-NK products are manufactured under GMP-compliant conditions (e.g., irradiated cells prior infusion to patients). The final products of TCR-NK are infused into patients.
Currently, two independent studies have shown that the transduction of TCRs along with all CD3 subunits redirected NK cell lines were specifically recognized tumor cells expressing the relevant antigen [15,16]. Parlar et al. [16] showed that TCR-NK (origin of NK-92 and YTS cell lines) against the HLA-A2-restricted tyrosinase-derived melanoma epitope (Tyr368-377) is MHC-restricted with the antigen-specific killing of tumor cells both in vitro and in vivo. Mensali et al. [15] also have reported the functional activities of TCR-NK (origin of NK-92 cell) against a TGF-βRII frameshift mutation peptide (TGFβRII131–139) and the melanoma-associated antigen melan-A peptide (Melan-A26-35) with a similar approach using all four CD3 chains. TCR-NK demonstrated enhanced antigen-specific recognition of target cells. The results from these pre-clinical studies showed the potential therapeutic efficacy of TCR-NK (Table 3).

Table 3.
TCR-NK cell-based therapy in pre-clinical study.
As compared to other sources of universal T cells, the TCR-NK line can be genetically modified and expanded in vitro with less effort; furthermore, a pure proportion of TCR-NK products is much safer for clinical usage. TCR-NK can replace the high-cost TCR-T, which becomes effectively personalized for cancer immunotherapy. Moreover, without the expression of endogenous α/β TCR, infusion TCR-NK can be safe from severe toxicities, poorly immunogenic, or rejected by the host. Therefore, TCR-NK-based therapy might be more efficient for patients who failed several rounds of therapies, and might not qualify for autologous treatment conditions due to the poor quality of their immune cells
4. Conclusions
Despite the promising results of non-genetic- and genetic modified NK cells, NK cell-based cancer immunotherapy still has several challenges to overcome. Adoptive transfer of autologous-and allogeneic NK cells is hard to succeed in clinics due to the limitation of the number of infused cells. The optimization protocols may need to boost the number of the cell population for clinic usage. Although NK cell lines are unlimited cell expansions, which can provide benefit for cancer immunotherapy. The use of NK cell lines in the clinic has been demonstrated in low efficacy, and the lines needed to be irradiated in prior infusion to the patients. Even though NK cell-based monoclonal antibodies have been shown promising antitumor activity in patients with diverse tumor types, dose-limiting toxicities may need to manage and improve for the patient safety profile. Moreover, it remains some barriers for CAR-NK to accomplish in clinics, including the efficiency of CAR-transduction, limitation of cell expansion, lack of available targets, and weak elimination of the solid tumor. Therefore, some efforts should create to boost the efficiency of CAR-NK products. For TCR-NK-based therapy, many further studies must be continuing to investigate for the clinical application of TCR-NK in gene therapy, including engineering TCRs with primary NK cells or engineering NK cells with a variety range of TCR affinities for clinical benefit.
Author Contributions
Original draft preparation, S.K.; review and editing, L.Z., E.Y., and Y.L.; supervision, X.G. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by gr ants “Major New Drug Development Project” from Ministry of Science and Technology of China (2019ZX09201-002-003),State Key Program of National Natural Science of China (82030076), National Natural Science Foundation of China (82070161, 81970151, and 81870134), Beijing Natural Science Foundation (7202186), Natural Science Foundation of Shenzhen University General Hospital (SUGH2020QD008),and The Science and Technology Foundation of Shenzhen (JCYJ20200109113810154).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef]
- Cho, D.; Kim, S.-K.; Carson, W.E. NK cell-based immunotherapy for treating cancer: Will it be promising? Korean J. Hematol. 2011, 46, 3–5. [Google Scholar] [CrossRef]
- Re, F.; Staudacher, C.; Zamai, L.; Vecchio, V.; Bregni, M. Killer cell Ig-like receptors ligand-mismatched, alloreactive natural killer cells lyse primary solid tumors. Cancer 2006, 107, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Malmberg, K.J.; Ljunggren, H.G. Natural killer cell-mediated lysis of freshly isolated human tumor cells. Int. J. Cancer 2009, 124, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.; Wang, C.; Yan, X.; Wang, Y.; Niu, C.; Zhang, X.; Li, M.; Tian, H.; Yao, C.; et al. Adoptive transfer of natural killer cells in combination with chemotherapy improves outcomes of patients with locally advanced colon carcinoma. Cytotherapy 2017. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Koh, Y.; Park, H.; Hwang, Y.k.; Kim, W.S. A Phase 1 Study of the Combination of MG4101, Ex Vivo-Expanded Allogeneic NK Cells and Rituximab for Relapsed or Refractory Non-Hodgkin Lymphoma. Blood 2020, 136, 14–15. [Google Scholar] [CrossRef]
- Tarazona, R.; Duran, E.; Solana, R. Natural Killer Cell Recognition of Melanoma: New Clues for a More Effective Immunotherapy. Front. Immunol. 2016, 6, 649. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Shang, P.; Zhang, H.; Fu, W.; Ye, F.; Zeng, T.; Huang, H.; Zhang, X.; Sun, W.; et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol. Oncol. 2014, 8, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Cui, H.; Caligiuri, M.A.; Yu, J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, C.S.; Rosenberg, S.A. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol. Rev. 2014, 257, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Moysey, R.; Molloy, P.E.; Vuidepot, A.L.; Mahon, T.; Baston, E.; Dunn, S.; Liddy, N.; Jacob, J.; Jakobsen, B.K.; et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat. Biotechnol. 2005, 23, 349–354. [Google Scholar] [CrossRef]
- Morgan, R.A.; Chinnasamy, N.; Abate-Daga, D.; Gros, A.; Robbins, P.F.; Zheng, Z.; Dudley, M.E.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013, 36, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Mensali, N.; Dillard, P.; Hebeisen, M.; Lorenz, S.; Theodossiou, T.; Myhre, M.R.; Fåne, A.; Gaudernack, G.; Kvalheim, G.; Myklebust, J.H.; et al. NK cells specifically TCR-dressed to kill cancer cells. EBioMedicine 2019, 40, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Parlar, A.; Sayitoglu, E.C.; Ozkazanc, D.; Georgoudaki, A.M.; Pamukcu, C.; Aras, M.; Josey, B.J.; Chrobok, M.; Branecki, S.; Zahedimaram, P.; et al. Engineering antigen-specific NK cell lines against the melanoma-associated antigen tyrosinase via TCR gene transfer. Eur. J. Immunol. 2019, 49, 1278–1290. [Google Scholar] [CrossRef]
- Cameron, B.J.; Gerry, A.B.; Dukes, J.; Harper, J.V.; Kannan, V.; Bianchi, F.C.; Grand, F.; Brewer, J.E.; Gupta, M.; Plesa, G.; et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 2013, 5, 197ra103. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol. Oncol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Raulet, D.H.; Guerra, N. Oncogenic stress sensed by the immune system: Role of natural killer cell receptors. Nat. Rev. Immunol. 2009, 9, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Fritsch, K.; Kohrt, H.E. Natural Killer Cell Immunomodulation: Targeting Activating, Inhibitory, and Co-stimulatory Receptor Signaling for Cancer Immunotherapy. Front. Immunol. 2015, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011, 89, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Montaldo, E.; Vitale, C.; Cottalasso, F.; Conte, R.; Glatzer, T.; Ambrosini, P.; Moretta, L.; Mingari, M.C. Human NK cells at early stages of differentiation produce CXCL8 and express CD161 molecule that functions as an activating receptor. Blood 2012, 119, 3987–3996. [Google Scholar] [CrossRef] [PubMed]
- Inngjerdingen, M.; Damaj, B.; Maghazachi, A.A. Expression and regulation of chemokine receptors in human natural killer cells. Blood 2001, 97, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.S.; Hasegawa, J. Natural killer cell biology: An update and future directions. J. Allergy Clin. Immunol. 2013, 132, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Parikh, B.A.; Bern, M.D.; Piersma, S.J.; Yang, L.; Beckman, D.L.; Poursine-Laurent, J.; Plougastel-Douglas, B.; Yokoyama, W.M. Control of Viral Infection by Natural Killer Cell Inhibitory Receptors. Cell Rep. 2020, 32, 107969. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tian, Z. NK cell education via nonclassical MHC and non-MHC ligands. Cell. Mol. Immunol. 2017, 14, 321–330. [Google Scholar] [CrossRef]
- Malmberg, K.J.; Sohlberg, E.; Goodridge, J.P.; Ljunggren, H.G. Immune selection during tumor checkpoint inhibition therapy paves way for NK-cell “missing self” recognition. Immunogenetics 2017, 69, 547–556. [Google Scholar] [CrossRef]
- Karre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. 1986. J. Immunol. 2005, 174, 6566–6569. [Google Scholar]
- Cheng, M.; Chen, Y.; Xiao, W.; Sun, R.; Tian, Z. NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 2013, 10, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Domogala, A.; Madrigal, J.A.; Saudemont, A. Natural Killer Cell Immunotherapy: From Bench to Bedside. Front. Immunol. 2015, 6, 264. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Pulliam, S.R.; Uzhachenko, R.V.; Adunyah, S.E.; Shanker, A. Common gamma chain cytokines in combinatorial immune strategies against cancer. Immunol. Lett. 2016, 169, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Ishikawa, T.; Kokura, S.; Okayama, T.; Oka, K.; Ideno, M.; Sakai, F.; Kato, A.; Tanabe, M.; Enoki, T.; et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J. Transl. Med. 2015, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, M.R.; Riley, J.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 6287–6297. [Google Scholar] [CrossRef]
- Rueff, J.; Medinger, M.; Heim, D.; Passweg, J.; Stern, M. Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol Blood Marrow Transpl. 2014, 20, 896–899. [Google Scholar] [CrossRef]
- Baluna, R.; Vitetta, E.S. Vascular leak syndrome: A side effect of immunotherapy. Immunopharmacology 1997, 37, 117–132. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Schafer, J.R.; Bassett, R.; Denman, C.J.; Cao, K.; Willis, D.; Rondon, G.; Chen, J.; Soebbing, D.; Kaur, I.; et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood 2017, 130, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulou, E.G.; Kountourakis, P.; Karamouzis, M.V.; Doufexis, D.; Ardavanis, A.; Baxevanis, C.N.; Rigatos, G.; Papamichail, M.; Perez, S.A. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol. Immunother. CII 2010, 59, 1781–1789. [Google Scholar] [CrossRef]
- Lundqvist, A.; McCoy, J.P.; Samsel, L.; Childs, R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49-mismatched NK cells from MHC-matched donors. Blood 2007, 109, 3603–3606. [Google Scholar] [CrossRef] [PubMed]
- Jafari, D.; Nafar, M.; Yekaninejad, M.S.; Abdolvahabi, R.; Lesan Pezeshki, M.; Razaghi, E.; Amirzargar, A.A. Investigation of Killer Immunoglobulin-like Receptor (KIR) and HLA Genotypes to Predict the Occurrence of Acute Allograft Rejection after Kidney Transplantation. Iran. J. Allergy Asthma Immunol. 2017, 16, 245–255. [Google Scholar] [PubMed]
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.; Panoskaltsis-Mortari, A.; Verneris, M.R.; et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Wu, H.; Pounds, S.; Inaba, H.; Ribeiro, R.C.; Cullins, D.; Rooney, B.; Bell, T.; Lacayo, N.J.; Heym, K.; et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J. Immunother. Cancer 2019, 7, 81. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Agha, M.; Redner, R.L.; Sehgal, A.; Im, A.; Hou, J.Z.; Farah, R.; Dorritie, K.A.; Raptis, A.; Lim, S.H.; et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 2017, 19, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Meagher, R.; Swearingen, M.; Myint, H.; Rich, E.; Martinson, J.; Klingemann, H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: A phase I trial. Cytotherapy 2008, 10, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Suck, G.; Odendahl, M.; Nowakowska, P.; Seidl, C.; Wels, W.S.; Klingemann, H.G.; Tonn, T. NK-92: An ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. CII 2016, 65, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Hughes, T.; Zhang, J.; Caligiuri, M.A.; Benson, D.M.; Yu, J. Fratricide of NK Cells in Daratumumab Therapy for Multiple Myeloma Overcome by Ex Vivo-Expanded Autologous NK Cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4006–4017. [Google Scholar] [CrossRef]
- Alderson, K.L.; Sondel, P.M. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J. Biomed. Biotechnol. 2011, 2011, 379123. [Google Scholar] [CrossRef]
- Vey, N.; Bourhis, J.H.; Boissel, N.; Bordessoule, D.; Prebet, T.; Charbonnier, A.; Etienne, A.; Andre, P.; Romagne, F.; Benson, D.; et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 2012, 120, 4317–4323. [Google Scholar] [CrossRef]
- Benson, D.M., Jr.; Hofmeister, C.C.; Padmanabhan, S.; Suvannasankha, A.; Jagannath, S.; Abonour, R.; Bakan, C.; Andre, P.; Efebera, Y.; Tiollier, J.; et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012, 120, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R.; Schlom, J.; Hodge, J.W. A potential therapy for chordoma via antibody-dependent cell-mediated cytotoxicity employing NK or high-affinity NK cells in combination with cetuximab. J. Neurosurg. 2018, 128, 1419–1427. [Google Scholar] [CrossRef]
- Sahin, U.; Schuler, M.; Richly, H.; Bauer, S.; Krilova, A.; Dechow, T.; Jerling, M.; Utsch, M.; Rohde, C.; Dhaene, K.; et al. A phase I dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-oesophageal junction cancer. Eur. J. Cancer 2018, 100, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gang, M.; Marin, N.D.; Wong, P.; Neal, C.C.; Marsala, L.; Foster, M.; Schappe, T.; Meng, W.; Tran, J.; Schaettler, M.; et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 2020, 136, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tian, Z.G.; Zhang, C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol. Sin. 2018, 39, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Sutlu, T.; Nyström, S.; Gilljam, M.; Stellan, B.; Applequist, S.E.; Alici, E. Inhibition of intracellular antiviral defense mechanisms augments lentiviral transduction of human natural killer cells: Implications for gene therapy. Hum. Gene Ther. 2012, 23, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Van Ostaijen-ten Dam, M.M.; Prins, H.J.; Boerman, G.H.; Vervat, C.; Pende, D.; Putter, H.; Lankester, A.; van Tol, M.J.; Zwaginga, J.J.; Schilham, M.W. Preparation of Cytokine-activated NK Cells for Use in Adoptive Cell Therapy in Cancer Patients: Protocol Optimization and Therapeutic Potential. J. Immunother. 2016, 39, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Leitman, S.; Chang, A.E.; Ettinghausen, S.E.; Matory, Y.L.; Skibber, J.M.; Shiloni, E.; Vetto, J.T.; et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Chang, A.E.; Avis, F.P.; Leitman, S.; Linehan, W.M.; Robertson, C.N.; Lee, R.E.; Rubin, J.T.; et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med. 1987, 316, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Barkholt, L.; Alici, E.; Conrad, R.; Sutlu, T.; Gilljam, M.; Stellan, B.; Christensson, B.; Guven, H.; Björkström, N.K.; Söderdahl, G.; et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: A phase I clinical study. Immunotherapy 2009, 1, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.W.; Gastpar, R.; Andreesen, R.; Gross, C.; Ullrich, H.; Thonigs, G.; Pfister, K.; Multhoff, G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: A clinical phase i trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Geller, M.A.; Cooley, S.; Judson, P.L.; Ghebre, R.; Carson, L.F.; Argenta, P.A.; Jonson, A.L.; Panoskaltsis-Mortari, A.; Curtsinger, J.; McKenna, D.; et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 2011, 13, 98–107. [Google Scholar] [CrossRef]
- Mantovani, S.; Oliviero, B.; Varchetta, S.; Mele, D.; Mondelli, M.U. Natural Killer Cell Responses in Hepatocellular Carcinoma: Implications for Novel Immunotherapeutic Approaches. Cancers 2020, 12, 926. [Google Scholar] [CrossRef]
- Farag, S.S.; Caligiuri, M.A. Cytokine modulation of the innate immune system in the treatment of leukemia and lymphoma. Adv. Pharmacol. 2004, 51, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.J.; Weisdorf, D.J.; DeFor, T.E.; Vesole, D.H.; Repka, T.L.; Blazar, B.R.; Burger, S.R.; Panoskaltsis-Mortari, A.; Keever-Taylor, C.A.; Zhang, M.J.; et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: A phase I/II trial. Bone Marrow Transplant. 2003, 32, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Robertson, M.J.; Gordon, M.; Lotze, M.T.; DeCoste, M.; DuBois, J.S.; Ritz, J.; Sandler, A.B.; Edington, H.D.; Garzone, P.D.; et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997, 3, 409–417. [Google Scholar]
- Nakamura, S.; Otani, T.; Ijiri, Y.; Motoda, R.; Kurimoto, M.; Orita, K. IFN- -Dependent and -Independent Mechanisms in Adverse Effects Caused by Concomitant Administration of IL-18 and IL-12. J. Immunol. 2000, 164, 3330–3336. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Hatano, T.; Ogawa, Y.; Gakiya, M.; Ogura, H.; Osawa, A. Treatment of advanced renal cell carcinoma using regional arterial administration of lymphokine-activated killer cells in combination with low doses of rIL-2. Urol. Int. 1994, 53, 117–124. [Google Scholar] [CrossRef]
- Rosenberg, S.A. The development of new immunotherapies for the treatment of cancer using interleukin-2. A review. Ann Surg 1988, 208, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Porrata, L.F.; Inwards, D.J.; Micallef, I.N.; Johnston, P.B.; Ansell, S.M.; Hogan, W.J.; Markovic, S.N. Interleukin-15 affects patient survival through natural killer cell recovery after autologous hematopoietic stem cell transplantation for non-Hodgkin lymphomas. Clin. Dev. Immunol. 2010, 2010, 914945. [Google Scholar] [CrossRef]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.M.; DeFor, T.E.; Curtsinger, J.M.; Robertson, P.; Grzywacz, B.; Conlon, K.C.; Waldmann, T.A.; et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019, 3, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Cooley, S.; Parham, P.; Farag, S.S.; Verneris, M.R.; McQueen, K.L.; Guethlein, L.A.; Trachtenberg, E.A.; Haagenson, M.; Horowitz, M.M.; et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood 2007, 109, 5058–5061. [Google Scholar] [CrossRef] [PubMed]
- Lim, O.; Jung, M.Y.; Hwang, Y.K.; Shin, E.-C. Present and Future of Allogeneic Natural Killer Cell Therapy. Front. Immunol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Denman, C.J.; Rondon, G.; Woodworth, G.; Chen, J.; Fisher, T.; Kaur, I.; Fernandez-Vina, M.; Cao, K.; Ciurea, S.; et al. Haploidentical Natural Killer Cells Infused before Allogeneic Stem Cell Transplantation for Myeloid Malignancies: A Phase I Trial. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1290–1298. [Google Scholar] [CrossRef]
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural Killer Cells for Immunotherapy - Advantages of the NK-92 Cell Line over Blood NK Cells. Front. Immunol. 2016, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; You, F.; Jiang, L.; Li, J.; Zhu, X.; Bao, Y.; Sun, X.; Tang, X.; Meng, H.; An, G.; et al. Gene-modified NK-92MI cells expressing a chimeric CD16-BB-ζ or CD64-BB-ζ receptor exhibit enhanced cancer-killing ability in combination with therapeutic antibody. Oncotarget 2017, 8, 37128–37139. [Google Scholar] [CrossRef] [PubMed]
- Tonn, T.; Becker, S.; Esser, R.; Schwabe, D.; Seifried, E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J. Hematotherapy Stem Cell Res. 2001, 10, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Klingemann, H.G.; Wong, E.; Maki, G. A cytotoxic NK-cell line (NK-92) for ex vivo purging of leukemia from blood. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 1996, 2, 68–75. [Google Scholar]
- Favors, S.E.; Curd, L.M.; Gregg, R.K. Use of the anti-inflammatory cytokine interleukin-11 to reverse HIV-1gp120 repression of a natural killer cell line. Cell. Immunol. 2012, 276, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Law, A.D.; Routy, B.; denHollander, N.; Gupta, V.; Wang, X.-H.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 8, 89256–89268. [Google Scholar] [CrossRef]
- Sconocchia, G.; Titus, J.A.; Segal, D.M. Signaling pathways regulating CD44-dependent cytolysis in natural killer cells. Blood 1997, 90, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.H.W.; Koch, J.; Götz, J.J.; Arnold, A.; Reusch, U.; Gantke, T.; Rajkovic, E.; Treder, M.; Cerwenka, A. CD16A Activation of NK Cells Promotes NK Cell Proliferation and Memory-Like Cytotoxicity against Cancer Cells. Cancer Immunol. Res. 2018, 6, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Pfreundschuh, M.; Ho, A.D.; Cavallin-Stahl, E.; Wolf, M.; Pettengell, R.; Vasova, I.; Belch, A.; Walewski, J.; Zinzani, P.L.; Mingrone, W.; et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: An exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet. Oncol. 2008, 9, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Iannello, A.; Ahmad, A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005, 24, 487–499. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Kang, Y.K.; Park, H.; Uronis, H.E.; Lee, K.W.; Ng, M.C.H.; Enzinger, P.C.; Park, S.H.; Gold, P.J.; Lacy, J.; et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): A single-arm, phase 1b-2 trial. Lancet Oncol. 2020, 21, 1066–1076. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Ratzinger, G.; Reagan, J.L.; Heller, G.; Busam, K.J.; Young, J.W. Differential CD52 expression by distinct myeloid dendritic cell subsets: Implications for alemtuzumab activity at the level of antigen presentation in allogeneic graft-host interactions in transplantation. Blood 2003, 101, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Lundin, J.; Kimby, E.; Björkholm, M.; Broliden, P.A.; Celsing, F.; Hjalmar, V.; Möllgård, L.; Rebello, P.; Hale, G.; Waldmann, H.; et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 2002, 100, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Kendra, K.; Malkovska, V.; Allen, M.; Guzman, J.; Albertini, M. In vivo binding and antitumor activity of Ch14.18. J. Immunother. 1999, 22, 423–430. [Google Scholar] [CrossRef]
- Barker, E.; Mueller, B.M.; Handgretinger, R.; Herter, M.; Yu, A.L.; Reisfeld, R.A. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991, 51, 144–149. [Google Scholar]
- Terzic, T.; Cordeau, M.; Herblot, S.; Teira, P.; Cournoyer, S.; Beaunoyer, M.; Peuchmaur, M.; Duval, M.; Sartelet, H. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated With Anti-GD2 Immunotherapy. Pediatric Dev. Pathol. Off. J. Soc. Pediatric Pathol. Paediatr. Pathol. Soc. 2018, 21, 355–362. [Google Scholar] [CrossRef]
- Plesner, T.; Arkenau, H.T.; Gimsing, P.; Krejcik, J.; Lemech, C.; Minnema, M.C.; Lassen, U.; Laubach, J.P.; Palumbo, A.; Lisby, S.; et al. Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood 2016, 128, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Martinez-Lopez, J.; Mateos, M.V.; Bladé, J.; Benboubker, L.; Oriol, A.; Arnulf, B.; Rodriguez-Otero, P.; Pineiro, L.; Jakubowiak, A.; et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 2019, 134, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Quach, H.; Mateos, M.V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Yang, H.; Klippel, Z.; Zahlten-Kumeli, A.; et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020, 396, 186–197. [Google Scholar] [CrossRef]
- Saloura, V.; Cohen, E.E.; Licitra, L.; Billan, S.; Dinis, J.; Lisby, S.; Gauler, T.C. An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother. Pharmacol. 2014, 73, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Chigutsa, E.; Long, A.J.; Wallin, J.E. Exposure-Response Analysis of Necitumumab Efficacy in Squamous Non-Small Cell Lung Cancer Patients. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 560–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front. Immunol. 2015, 6, 368. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.C.; Minute, L.; Rodriguez, I.; Garasa, S.; Perez-Ruiz, E.; Inogés, S.; Melero, I.; Berraondo, P. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell Biol. 2017, 95, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.J.; Wu, J.; Walcheck, B. Engineering Anti-Tumor Monoclonal Antibodies and Fc Receptors to Enhance ADCC by Human NK Cells. Cancers 2021, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shmuel, A.; Biber, G.; Barda-Saad, M. Unleashing Natural Killer Cells in the Tumor Microenvironment-The Next Generation of Immunotherapy? Front. Immunol. 2020, 11, 275. [Google Scholar] [CrossRef]
- Van der Horst, H.J.; Nijhof, I.S.; Mutis, T.; Chamuleau, M.E.D. Fc-Engineered Antibodies with Enhanced Fc-Effector Function for the Treatment of B-Cell Malignancies. Cancers 2020, 12, 3041. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Khalili, S.; Baradaran, B.; Bidar, N.; Shahbazi, M.A.; Mosafer, J.; Hashemzaei, M.; Mokhtarzadeh, A.; Hamblin, M.R. Bispecific monoclonal antibodies for targeted immunotherapy of solid tumors: Recent advances and clinical trials. Int. J. Biol. Macromol. 2021, 167, 1030–1047. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Mailliard, R.; Kashii, Y.; Reichert, T.E.; Herberman, R.B.; Robbins, P.; Whiteside, T.L. Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. Blood 1998, 91, 3850–3861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, R.; Wei, H.; Zhang, J.; Tian, Z. Characterization of stem cell factor gene-modified human natural killer cell line, NK-92 cells: Implication in NK cell-based adoptive cellular immunotherapy. Oncol. Rep. 2004, 11, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, D.L.; Kaufman, D.S. Utilizing chimeric antigen receptors to direct natural killer cell activity. Front. Immunol. 2015, 6, 195. [Google Scholar] [CrossRef]
- Mehta, R.S.; Rezvani, K. Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Front. Immunol. 2018, 9, 283. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, J.; Wu, C. CAR-NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer Lett. 2020, 472, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tassev, D.V.; Cheng, M.; Cheung, N.K. Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer Gene Ther. 2012, 19, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Romanski, A.; Uherek, C.; Bug, G.; Seifried, E.; Klingemann, H.; Wels, W.S.; Ottmann, O.G.; Tonn, T. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J. Cell. Mol. Med. 2016, 20, 1287–1294. [Google Scholar] [CrossRef]
- Boissel, L.; Betancur, M.; Lu, W.; Wels, W.S.; Marino, T.; Van Etten, R.A.; Klingemann, H. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk. Lymphoma 2012, 53, 958–965. [Google Scholar] [CrossRef]
- Altvater, B.; Landmeier, S.; Pscherer, S.; Temme, J.; Schweer, K.; Kailayangiri, S.; Campana, D.; Juergens, H.; Pule, M.; Rossig, C. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin. Cancer Res. 2009, 15, 4857–4866. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, N.; Fujisaki, H.; Cho, D.; Masselli, M.; Lockey, T.; Eldridge, P.; Leung, W.; Campana, D. A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. Cytotherapy 2012, 14, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, L.N.; Feller, S.; Allen, C.; Shivakumar, R.; Fratantoni, J.; Wolfraim, L.A.; Fujisaki, H.; Campana, D.; Chopas, N.; et al. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther. 2009, 17, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Hochberg, J.; Yahr, A.; Ayello, J.; van de Ven, C.; Barth, M.; Czuczman, M.; Cairo, M.S. Targeting CD20+ Aggressive B-cell Non-Hodgkin Lymphoma by Anti-CD20 CAR mRNA-Modified Expanded Natural Killer Cells In Vitro and in NSG Mice. Cancer Immunol. Res. 2015, 3, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Grote, S.; Mittelstaet, J.; Baden, C.; Chan, K.C.; Seitz, C.; Schlegel, P.; Kaiser, A.; Handgretinger, R.; Schleicher, S. Adapter chimeric antigen receptor (AdCAR)-engineered NK-92 cells: An off-the-shelf cellular therapeutic for universal tumor targeting. Oncoimmunology 2020, 9, 1825177. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.; Stikvoort, A.; Nolan, E.; Kirkham-McCarthy, L.; Khoruzhenko, S.; Shivakumar, R.; Zweegman, S.; Van de Donk, N.; Mutis, T.; Szegezdi, E.; et al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica 2020. [Google Scholar] [CrossRef]
- Quintarelli, C.; Sivori, S.; Caruso, S.; Carlomagno, S.; Falco, M.; Boffa, I.; Orlando, D.; Guercio, M.; Abbaszadeh, Z.; Sinibaldi, M.; et al. Efficacy of third-party chimeric antigen receptor modified peripheral blood natural killer cells for adoptive cell therapy of B-cell precursor acute lymphoblastic leukemia. Leukemia 2020, 34, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Ravi, D.; Sarkar, S.; Purvey, S.; Passero, F.; Beheshti, A.; Chen, Y.; Mokhtar, M.; David, K.; Konry, T.; Evens, A.M. Interaction kinetics with transcriptomic and secretory responses of CD19-CAR natural killer-cell therapy in CD20 resistant non-hodgkin lymphoma. Leukemia 2020, 34, 1291–1304. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018, 32, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Kruschinski, A.; Moosmann, A.; Poschke, I.; Norell, H.; Chmielewski, M.; Seliger, B.; Kiessling, R.; Blankenstein, T.; Abken, H.; Charo, J. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc. Natl. Acad. Sci. USA 2008, 105, 17481–17486. [Google Scholar] [CrossRef] [PubMed]
- Esser, R.; Müller, T.; Stefes, D.; Kloess, S.; Seidel, D.; Gillies, S.D.; Aperlo-Iffland, C.; Huston, J.S.; Uherek, C.; Schönfeld, K.; et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J. Cell. Mol. Med. 2012, 16, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Montagner, I.M.; Penna, A.; Fracasso, G.; Carpanese, D.; Dalla Pietà, A.; Barbieri, V.; Zuccolotto, G.; Rosato, A. Anti-PSMA CAR-engineered NK-92 Cells: An Off-the-shelf Cell Therapy for Prostate Cancer. Cells 2020, 9, 1382. [Google Scholar] [CrossRef]
- Shah, N.N.; Baird, K.; Delbrook, C.P.; Fleisher, T.A.; Kohler, M.E.; Rampertaap, S.; Lemberg, K.; Hurley, C.K.; Kleiner, D.E.; Merchant, M.S.; et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood 2015, 125, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Childs, R.W. Genetic Manipulation of NK Cells for Cancer Immunotherapy: Techniques and Clinical Implications. Front. Immunol. 2015, 6, 266. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Nunès, J.A.; Vély, F. Natural killer cell signaling pathways. Science 2004, 306, 1517–1519. [Google Scholar] [CrossRef]
- Zhao, Y.; Bennett, A.D.; Zheng, Z.; Wang, Q.J.; Robbins, P.F.; Yu, L.Y.; Li, Y.; Molloy, P.E.; Dunn, S.M.; Jakobsen, B.K.; et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J. Immunol. 2007, 179, 5845–5854. [Google Scholar] [CrossRef] [PubMed]
- Lana, M.G.; Strauss, B.E. Production of Lentivirus for the Establishment of CAR-T Cells. Methods Mol. Biol. 2020, 2086, 61–67. [Google Scholar] [CrossRef]
- Dasgupta, A.; Tinch, S.; Szczur, K.; Ernst, R.; Shryock, N.; Kaylor, C.; Lewis, K.; Day, E.; Truong, T.; Swaney, W. Phase I/II Manufacture of Lentiviral Vectors Under GMP in an Academic Setting. Methods Mol. Biol. 2020, 2086, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).