Lung Metastasis Probability in Ewing Sarcoma: A Nomogram Based on the SEER Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ES | Ewing sarcoma |
| OS | overall survival |
| SEER | the Surveillance, Epidemiology, and End Results |
| LASSO | least absolute shrinkage and selection operator |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
| DCA | decision curve analysis |
| CXCR6 | CXC-chemokine receptor 6 |
| CXCL16 | CXC-chemokine ligand 16 |
| CHM1 | chondromodulin 1 |
References
- Ranft, A.; Seidel, C.; Hoffmann, C.; Paulussen, M.; Warby, A.C.; van den Berg, H.; Ladenstein, R.; Rossig, C.; Dirksen, U.; Rosenbaum, D.; et al. Quality of survivorship in a rare disease: Clinicofunctional outcome and physical activity in an observational cohort study of 618 long-term survivors of ewing sarcoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1704–1712. [Google Scholar] [CrossRef]
- Stiller, C.A.; Bielack, S.S.; Jundt, G.; Steliarova-Foucher, E. Bone tumours in European children and adolescents, 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur. J. Cancer 2006, 42, 2124–2135. [Google Scholar] [CrossRef]
- Balamuth, N.J.; Womer, R.B. Ewing’s sarcoma. Lancet Oncol. 2010, 11, 184–192. [Google Scholar] [CrossRef]
- Gaspar, N.; Hawkins, D.S.; Dirksen, U.; Lewis, I.J.; Ferrari, S.; Le Deley, M.C.; Kovar, H.; Grimer, R.; Whelan, J.; Claude, L.; et al. Ewing sarcoma: Current management and future approaches through collaboration. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3036–3046. [Google Scholar] [CrossRef]
- Whelan, J.S.; Burcombe, R.J.; Janinis, J.; Baldelli, A.M.; Cassoni, A.M. A systematic review of the role of pulmonary irradiation in the management of primary bone tumours. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2002, 13, 23–30. [Google Scholar] [CrossRef]
- Esiashvili, N.; Goodman, M.; Marcus, R.B., Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance epidemiology and end results data. J. Pediatric Hematol. Oncol. 2008, 30, 425–430. [Google Scholar] [CrossRef]
- Cotterill, S.J.; Ahrens, S.; Paulussen, M.; Jürgens, H.F.; Voûte, P.A.; Gadner, H.; Craft, A.W. Prognostic factors in Ewing’s tumor of bone: Analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 3108–3114. [Google Scholar] [CrossRef]
- Cangir, A.; Vietti, T.J.; Gehan, E.A.; Burgert, E.O., Jr.; Thomas, P.; Tefft, M.; Nesbit, M.E.; Kissane, J.; Pritchard, D. Ewing’s sarcoma metastatic at diagnosis. Results and comparisons of two intergroup Ewing’s sarcoma studies. Cancer 1990, 66, 887–893. [Google Scholar] [CrossRef]
- Sandoval, C.; Meyer, W.H.; Parham, D.M.; Kun, L.E.; Hustu, H.O.; Luo, X.; Pratt, C.B. Outcome in 43 children presenting with metastatic Ewing sarcoma: The St. Jude Children’s Research Hospital experience, 1962 to 1992. Med. Pediatric Oncol. 1996, 26, 180–185. [Google Scholar] [CrossRef]
- Paulussen, M.; Ahrens, S.; Burdach, S.; Craft, A.; Dockhorn-Dworniczak, B.; Dunst, J.; Fröhlich, B.; Winkelmann, W.; Zoubek, A.; Jürgens, H. Primary metastatic (stage IV) Ewing tumor: Survival analysis of 171 patients from the EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1998, 9, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Dai, M.; Zhang, B. Risk factors for metastasis at initial diagnosis with Ewing sarcoma. Front. Oncol. 2019, 9, 1043. [Google Scholar] [CrossRef]
- Raciborska, A.; Bilska, K.; Rychłowska-Pruszyńska, M.; Duczkowski, M.; Duczkowska, A.; Drabko, K.; Chaber, R.; Sobol, G.; Wyrobek, E.; Michalak, E. Management and follow-up of Ewing sarcoma patients with isolated lung metastases. J. Pediatric Surg. 2016, 51, 1067–1071. [Google Scholar] [CrossRef]
- Meybaum, C.; Graff, M.; Fallenberg, E.M.; Leschber, G.; Wormanns, D. Contribution of CAD to the sensitivity for detecting lung metastases on thin-section CT—A prospective study with surgical and histopathological correlation. RoFo Fortschr. Geb. Rontgenstrahlen Nukl. 2020, 192, 65–73. [Google Scholar] [CrossRef]
- Ciccarese, F.; Bazzocchi, A.; Ciminari, R.; Righi, A.; Rocca, M.; Rimondi, E.; Picci, P.; Reggiani, M.L.B.; Albisinni, U.; Zompator, M. The many faces of pulmonary metastases of osteosarcoma: Retrospective study on 283 lesions submitted to surgery. Eur. J. Radiol. 2015, 84, 2679–2685. [Google Scholar] [CrossRef]
- Shi, J.; Yang, J.; Ma, X.; Wang, X. Risk factors for metastasis and poor prognosis of Ewing sarcoma: A population based study. J. Orthop. Surg. Res. 2020, 15, 88. [Google Scholar] [CrossRef]
- Shariat, S.F.; Karakiewicz, P.I.; Suardi, N.; Kattan, M.W. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: A critical analysis of the literature. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 4400–4407. [Google Scholar] [CrossRef]
- Cronin, K.A.; Ries, L.A.; Edwards, B.K. The surveillance, epidemiology, and end results (SEER) program of the National Cancer Institute. Cancer 2014, 120, 3755–3757. [Google Scholar] [CrossRef]
- Pavlou, M.; Ambler, G.; Seaman, S.; De Iorio, M.; Omar, R.Z. Review and evaluation of penalised regression methods for risk prediction in low-dimensional data with few events. Stat. Med. 2016, 35, 1159–1177. [Google Scholar] [CrossRef]
- Rousson, V.; Zumbrunn, T. Decision curve analysis revisited: Overall net benefit, relationships to ROC curve analysis, and application to case-control studies. BMC Med. Inform. Decis. Mak. 2011, 11, 45. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Mikulić, D.; Ilić, I.; Cepulić, M.; Giljević, J.S.; Orlić, D.; Zupancić, B.; Fattorini, I.; Seiwerth, S. Angiogenesis and Ewing sarcoma—Relationship to pulmonary metastasis and survival. J. Pediatric Surg. 2006, 41, 524–529. [Google Scholar] [CrossRef]
- Von Heyking, K.; Calzada-Wack, J.; Göllner, S.; Neff, F.; Schmidt, O.; Hensel, T.; Schirmer, D.; Fasan, A.; Esposito, I.; Muller-Tidow, C.; et al. The endochondral bone protein CHM1 sustains an undifferentiated, invasive phenotype, promoting lung metastasis in Ewing sarcoma. Mol. Oncol. 2017, 11, 1288–1301. [Google Scholar] [CrossRef]
- Na, K.Y.; Kim, H.S.; Jung, W.W.; Sung, J.Y.; Kalil, R.K.; Kim, Y.W.; Park, Y.K. CXCL16 and CXCR6 in Ewing sarcoma family tumor. Hum. Pathol. 2014, 45, 753–760. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, Z.; Yang, J.; Yan, X.; Li, Y.; Lyu, J. A nomogram for determining the disease-specific survival in Ewing sarcoma: A population study. BMC Cancer 2019, 19, 667. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, K.H.; Kim, H.Y.; Cho, Y.J.; Noh, J.K.; Suh, J.S.; Yang, W.I. Postoperative nomogram to predict the probability of metastasis in Enneking stage IIB extremity osteosarcoma. BMC Cancer 2014, 14, 666. [Google Scholar] [CrossRef]
- Karski, E.E.; Matthay, K.K.; Neuhaus, J.M.; Goldsby, R.E.; Dubois, S.G. Characteristics and outcomes of patients with Ewing sarcoma over 40 years of age at diagnosis. Cancer Epidemiol. 2013, 37, 29–33. [Google Scholar] [CrossRef]
- Ramkumar, D.B.; Ramkumar, N.; Miller, B.J.; Henderson, E.R. Risk factors for detectable metastatic disease at presentation in Ewing sarcoma—An analysis of the SEER registry. Cancer Epidemiol. 2018, 57, 134–139. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, Z.Y.; Lin, Z.Q. A nomogram to predict prognosis in Ewing sarcoma of bone. J. Bone Oncol. 2019, 15, 100223. [Google Scholar] [CrossRef]
- Chen, L.; Long, C.; Liu, J.; Xing, F.; Duan, X. Characteristics and prognosis of pelvic Ewing sarcoma: A SEER population-based study. PeerJ 2019, 7, e7710. [Google Scholar] [CrossRef]
- Hence, H.W.; Ahrens, S.; Paulussen, M.; Lehnert, M.; Jurgens, H. Factors associated with tumor volume and primary metastases in Ewing tumors: Results from the (EI)CESS studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1999, 10, 1073–1077. [Google Scholar] [CrossRef]
- Miller, B.J.; Cram, P.; Lynch, C.F.; Buckwalter, J.A. Risk factors for metastatic disease at presentation with osteosarcoma: An analysis of the SEER database. J. Bone Jt. Surg. Am. Vol. 2013, 95, e89. [Google Scholar] [CrossRef]
- Duchman, K.R.; Gao, Y.; Miller, B.J. Prognostic factors for survival in patients with Ewing’s sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015, 39, 189–195. [Google Scholar] [CrossRef]
- Thewes, B.; Husson, O.; Poort, H.; Custers, J.A.E.; Butow, P.N.; McLachlan, S.A.; Prins, J.B. Fear of Cancer Recurrence in an Era of Personalized Medicine. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3275–3278. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef]
- Guimarães, J.B.; Rigo, L.; Lewin, F.; Emerick, A. The importance of PET/CT in the evaluation of patients with Ewing tumors. Radiol. Bras. 2015, 48, 175–180. [Google Scholar] [CrossRef]
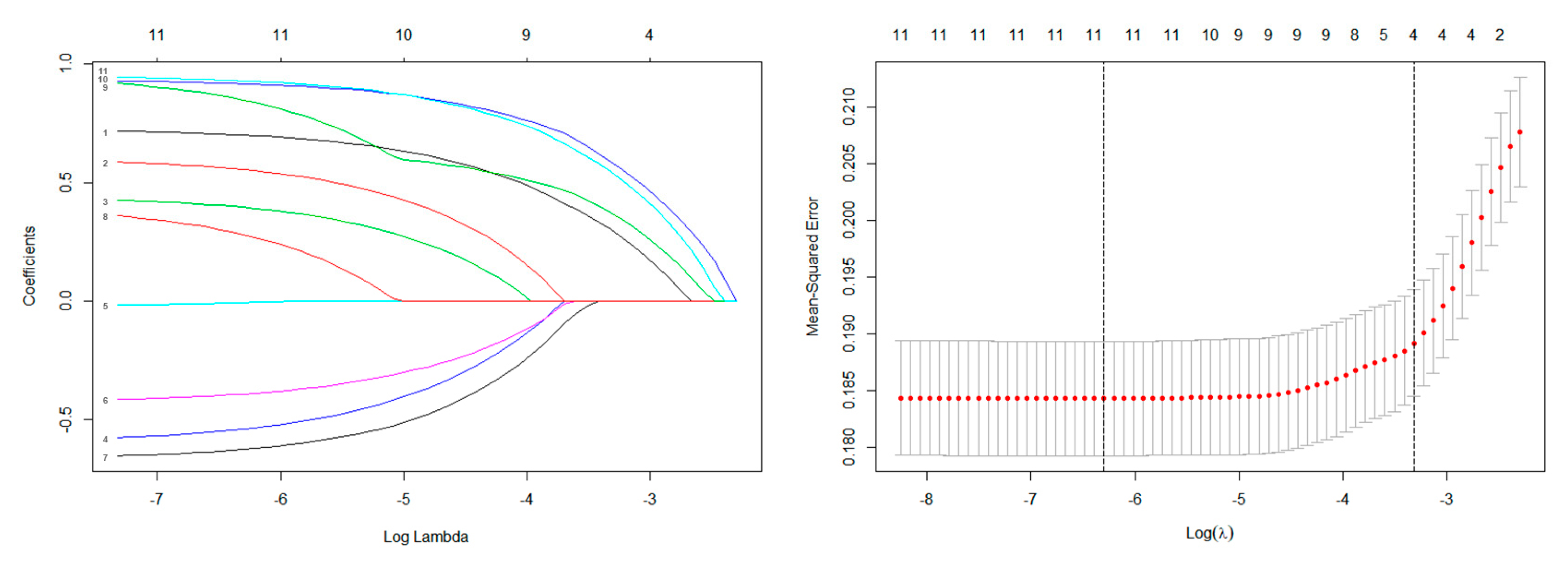
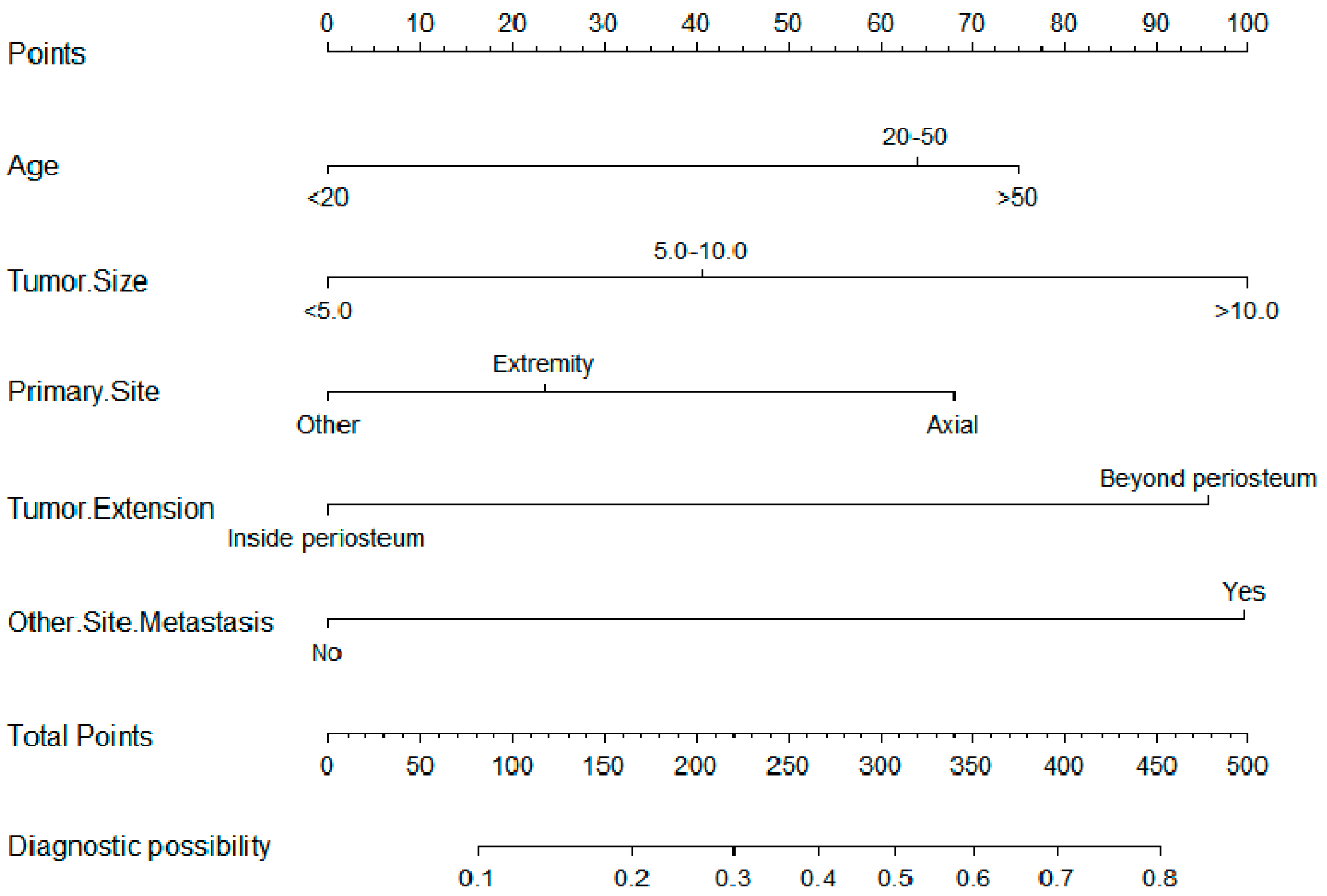
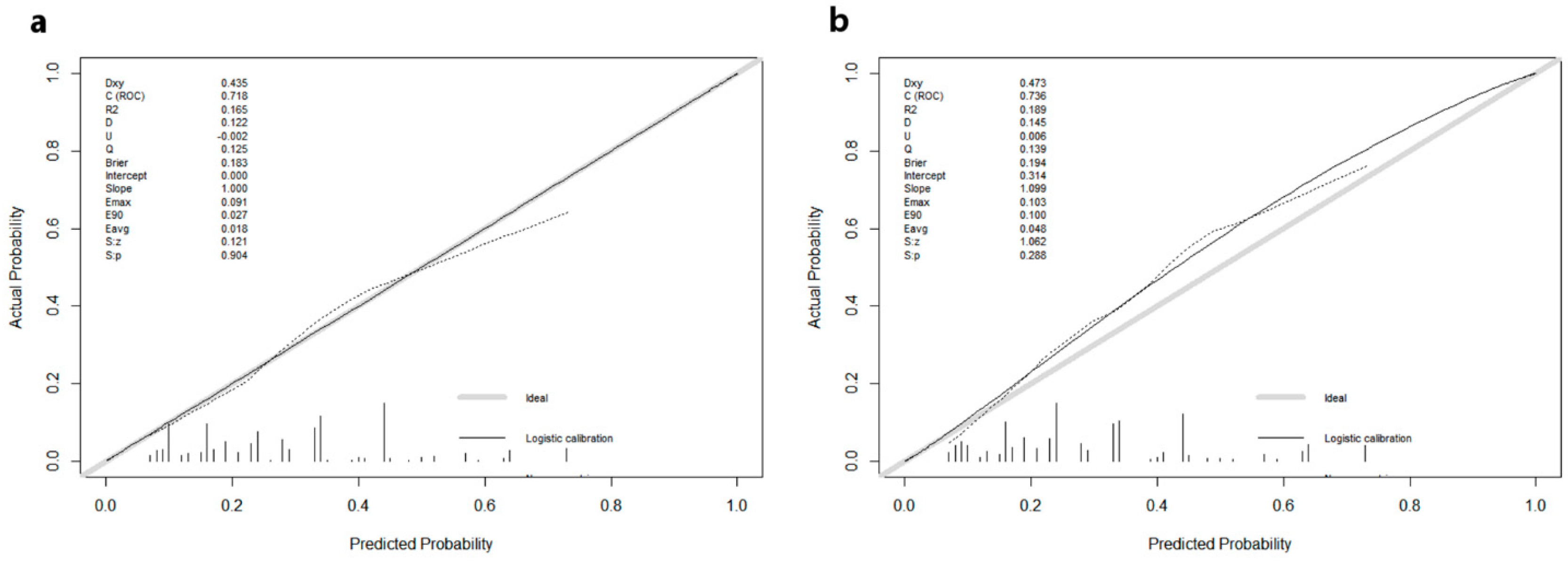

| Variables | Total Population (N = 1157; 100.0%) | Training Cohort (N = 812; 70.1%) | Validation Cohort (N = 345; 29.9%) | p-Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Lung Metastasis | 0.616 | ||||||
| No | 1039 | 89.8 | 716 | 88.2 | 308 | 89.3 | |
| Yes | 118 | 10.2 | 96 | 11.8 | 37 | 10.7 | |
| Age (years) | 0.376 | ||||||
| 20 | 751 | 64.9 | 537 | 66.1 | 214 | 62.0 | |
| 20–50 | 336 | 29.0 | 229 | 28.2 | 107 | 31.0 | |
| 50 | 70 | 6.1 | 46 | 5.7 | 24 | 7.0 | |
| Race | 0.619 | ||||||
| White | 1029 | 88.9 | 726 | 89.4 | 303 | 87.8 | |
| Black | 44 | 3.8 | 31 | 3.8 | 13 | 3.8 | |
| Other | 84 | 7.3 | 55 | 6.8 | 29 | 8.4 | |
| Sex | 0.719 | ||||||
| Male | 722 | 62.4 | 504 | 62.1 | 218 | 63.2 | |
| Female | 435 | 37.6 | 308 | 37.9 | 127 | 36.8 | |
| Primary Site | 0.893 | ||||||
| Axial | 406 | 35.1 | 287 | 35.3 | 119 | 34.5 | |
| Extremity | 506 | 43.7 | 356 | 43.8 | 150 | 43.5 | |
| Other | 245 | 21.2 | 169 | 20.8 | 76 | 22.0 | |
| Tumor Size(cm) | 0.088 | ||||||
| <5 | 176 | 15.2 | 113 | 13.9 | 63 | 18.3 | |
| 5–10 | 410 | 35.4 | 284 | 35.0 | 126 | 36.5 | |
| >10 | 571 | 49.4 | 415 | 51.1 | 156 | 45.2 | |
| Tumor Extension | 0.160 | ||||||
| Inside periosteum | 397 | 34.3 | 289 | 35.6 | 108 | 31.3 | |
| Beyond periosteum | 760 | 65.7 | 523 | 64.4 | 237 | 68.7 | |
| Other Sites Metastases | 0.233 | ||||||
| No | 991 | 85.7 | 702 | 86.5 | 289 | 83.8 | |
| Yes | 166 | 14.3 | 110 | 13.5 | 56 | 16.2 | |
| Variables | Training Cohort (N = 812) | |
|---|---|---|
| OR (95% CI) | p-Value | |
| Age | ||
| 20 | 1 (reference) | |
| 20–50 | 1.852 (0.944–5.320) | 0.068 |
| 50 | 2.059 (1.459–4.886) | 0.003 * |
| Race | ||
| White | 1 (reference) | |
| Black | 0.352 (0.120–1.013) | 0.075 |
| Other | 0.640 (0.288–1.463) | 0.053 |
| Tumor Size(cm) | ||
| 5 | 1 (reference) | |
| 5–10 | 2.620 (1.494–4.823) | 0.001 * |
| 10 | 1.478 (0.814–2.800) | 0.000 * |
| Primary Site | ||
| Other | 1 (reference) | |
| Extremity | 0.798 (0.496–1.267) | 0.344 |
| Axial | 1.535 (1.064–2.218) | 0.022 * |
| Tumor Extension | ||
| Inside periosteum | 1 (reference) | |
| Beyond periosteum | 0.398 (0.269–0.581) | 0.000 * |
| Other Sites Metastases | ||
| No | 1 (reference) | |
| Yes | 2.610 (1.677–4.072) | 0.000 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Fan, Y.; Xia, L. Lung Metastasis Probability in Ewing Sarcoma: A Nomogram Based on the SEER Database. Curr. Oncol. 2021, 28, 69-77. https://doi.org/10.3390/curroncol28010009
Wang J, Fan Y, Xia L. Lung Metastasis Probability in Ewing Sarcoma: A Nomogram Based on the SEER Database. Current Oncology. 2021; 28(1):69-77. https://doi.org/10.3390/curroncol28010009
Chicago/Turabian StyleWang, Jie, Yonggang Fan, and Lei Xia. 2021. "Lung Metastasis Probability in Ewing Sarcoma: A Nomogram Based on the SEER Database" Current Oncology 28, no. 1: 69-77. https://doi.org/10.3390/curroncol28010009
APA StyleWang, J., Fan, Y., & Xia, L. (2021). Lung Metastasis Probability in Ewing Sarcoma: A Nomogram Based on the SEER Database. Current Oncology, 28(1), 69-77. https://doi.org/10.3390/curroncol28010009





