Blood Cholesterol and Outcome of Patients with Cancer under Regular Cardiological Surveillance
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Parameters
2.2. Endpoints
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
4. Discussion
- Cholesterol levels were significantly lower in patients who died.
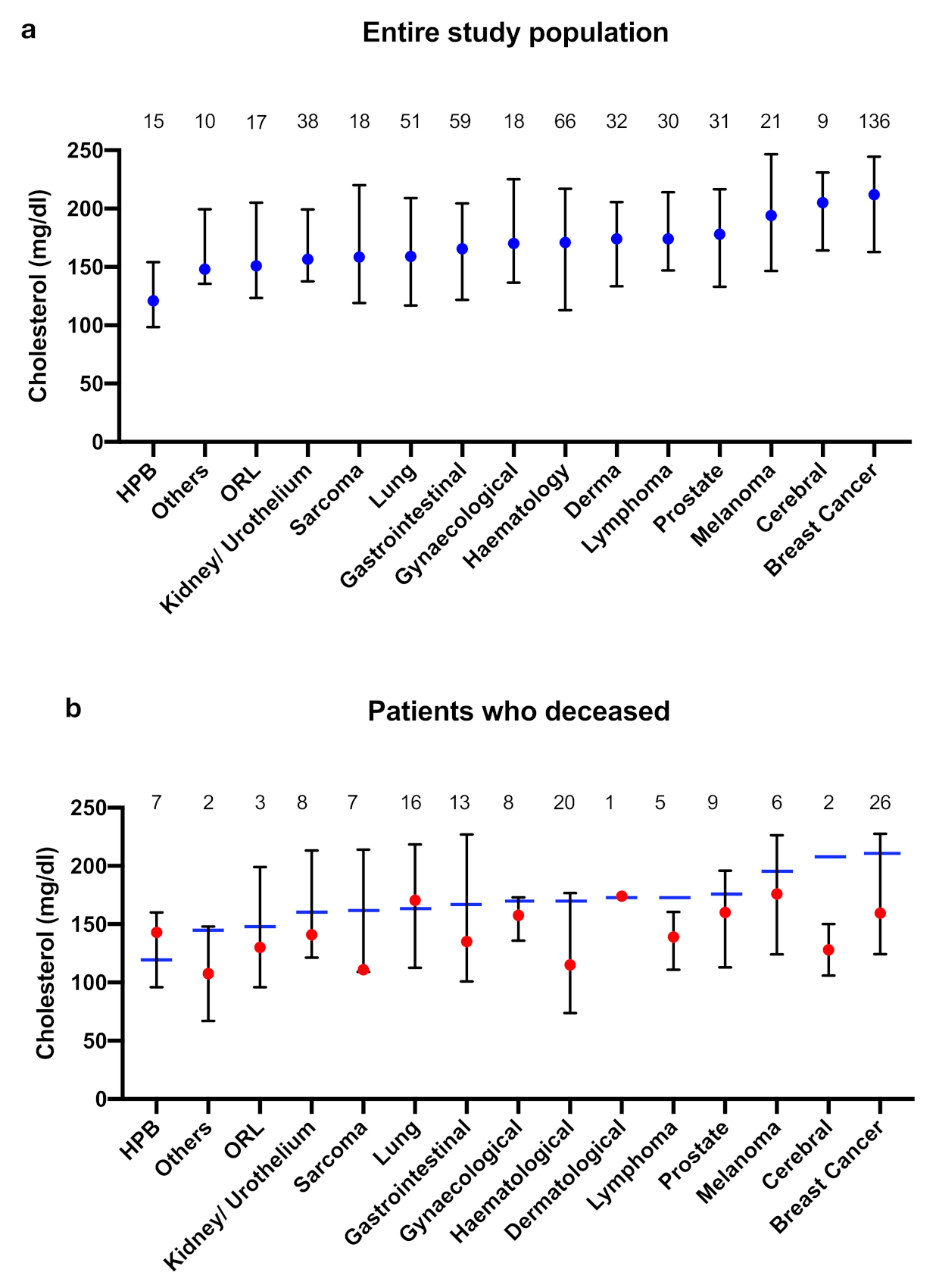
- Cholesterol levels varied between different tumour entities; lowest levels were found in patients with HPB tumours (median 121 mg/dL), while patients with melanoma, cerebral tumours, and breast cancer had rather high cholesterol levels (median > 190 mg/dL).
- In patients who died lower cholesterol levels were observed in patients with tumours with higher mitotic rate (mesenchymal tumours, cerebral tumours, breast cancer). This was particularly remarkable in patients with breast cancer, although cholesterol levels of the total population of patients with breast cancer were significantly elevated.
- Patients with stem cell transplantation (HR 4.31) and metastasised tumour stages (HR 3.31) showed the highest mortality risk, while cardiac risk factors were also associated with a worse outcome, whereby the best discriminative performance was found for total cholesterol (p = 0.002).
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lerman, A.; Sandhu, N.P.; Villarraga, H.R.; Mulvagh, S.L.; Kohli, M. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clin. Proc. 2014, 89, 1287–1306. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Tobe, S.W.; Stone, J.A.; Walker, K.M.; Anderson, T.; Bhattacharyya, O.; Cheng, A.Y.; Gregoire, J.; Gubitz, G.; L’Abbé, M.; Lau, D.C.; et al. Canadian Cardiovascular Harmonized National Guidelines Endeavour (C-CHANGE): 2014 update. CMAJ 2014, 186, 1299–1305. [Google Scholar] [CrossRef]
- Nydegger, U.E.; Butler, R.E. Serum lipoprotein levels in patients with cancer. Cancer Res. 1972, 32, 1756–1760. [Google Scholar]
- Rose, G.; Blackburn, H.; Keys, A.; Taylor, H.L.; Kannel, W.B.; Paul, O.; Reid, D.; Stamler, J. Colon cancer and blood-cholesterol. Lancet 1974, 1, 181–183. [Google Scholar] [CrossRef]
- Cambien, F.; Ducimetiere, P.; Richard, J. Total serum cholesterol and cancer mortality in a middle-aged male population. Am. J. Epidemiol. 1980, 112, 388–394. [Google Scholar] [CrossRef]
- Kannel, W.B.; Castelli, W.P.; Gordon, T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann. Intern. Med. 1979, 90, 85–91. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. How cells handle cholesterol. Science 2000, 290, 1721–1726. [Google Scholar] [CrossRef]
- Silvente-Poirot, S.; Poirot, M. Cholesterol metabolism and cancer: The good, the bad and the ugly. Curr. Opin. Pharmacol. 2012, 12, 673–676. [Google Scholar] [CrossRef]
- Vitols, S.; Gahrton, G.; Ost, A.; Peterson, C. Elevated low density lipoprotein receptor activity in leukemic cells with monocytic differentiation. Blood 1984, 63, 1186–1193. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; Backer, G.G.D.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Sleijfer, S.; van der Gaast, A.; Planting, A.S.; Stoter, G.; Verweij, J. The potential of statins as part of anti-cancer treatment. Eur. J. Cancer 2005, 41, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Clendening, J.W.; Penn, L.Z. Targeting tumor cell metabolism with statins. Oncogene 2012, 31, 4967–4978. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.M.; Mo, H.; McConathy, W.J.; Sabnis, N.; Lacko, A.G. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: A review of scientific findings, relevant to future cancer therapeutics. Front. Pharmacol. 2013, 4, 119. [Google Scholar] [CrossRef]
- Jain, M.K.; Ridker, P.M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005, 4, 977–987. [Google Scholar] [CrossRef]
- Solomon, K.R.; Pelton, K.; Boucher, K.; Joo, J.; Tully, C.; Zurakowski, D.; Schaffner, C.P.; Kim, J.; Freeman, M.R. Ezetimibe is an inhibitor of tumor angiogenesis. Am. J. Pathol. 2009, 174, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Warner, M.; Gustafsson, J.-A. On estrogen, cholesterol metabolism, and breast cancer. N. Engl. J. Med. 2014, 370, 572–573. [Google Scholar] [CrossRef]
- Llaverias, G.; Danilo, C.; Mercier, I.; Daumer, K.; Capozza, F.; Williams, T.M.; Sotgia, F.; Lisanti, M.P.; Frank, P.G. Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 2011, 178, 402–412. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, R.; Hotta, M.; Oikawa, S.; Takano, K. Etiology of hypercholesterolemia in patients with anorexia nervosa. Int. J. Eat. Disord. 2006, 39, 598–601. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 551) | Deceased during Follow-Up (n = 140, 25.4%) | Alive during Follow-Up (n = 411, 74.6%) | p-Value (Univariable) | R2 | |
|---|---|---|---|---|---|
| Sex, m (%) | 273 (49.5) | 63 (45.0) | 210 (51.1) | 0.23 | - |
| Age at first diagnosis | 61.6 ± 15.1 | 64.3 ± 14.1 | 60.6 ± 15.3 | 0.01 | 0.01 |
| Median follow-up (months, 95% CI) | 41 (40, 43) | 23 (18, 29) | 43 (42, 45) | <0.0001 | 0.06 |
| Advanced tumour disease (metastasised) | 175 (31.8) | 86 (61.4) | 89 (21.7) | <0.0001 | 0.14 |
| Relapse | 44 (8.0) | 19 (13.6) | 25 (6.1) | 0.9 | - |
| Secondary tumour | 91 (16.5) | 27 (19.3) | 64 (15.6) | 0.31 | - |
| Palliative treatment | 263 (47.7) | 112 (80.0) | 151 (36.7) | <0.0001 | 0.14 |
| Karnofsky-Index | 77.3 ± 18.9 | 74.1 ± 19.8 | 78.4 ± 18.5 | 0.02 | 0.01 |
| Shared risk factors, n (%) | |||||
| Hypertension | 324 (58.8) | 83 (59.3) | 241 (58.6) | 0.89 | - |
| Diabetes mellitus | 96 (17.4) | 26 (18.6) | 70 (17.0) | 0.68 | - |
| Hyperlipidaemia | 169 (30.7) | 31 (22.1) | 138 (33.6) | 0.01 | 0.01 |
| BMI (kg/m2) | 26.4 ± 5.6 | 25.6 ± 5.0 | 26.7 ± 5.8 | 0.07 | - |
| Smoking history | 155 (28.1) | 44 (31.4) | 111 (27.0) | 0.32 | - |
| Known cardiac disease | 120 (21.8) | 53 (37.9) | 67 (16.3) | 0.008 | 0.03 |
| Prior stroke/ TIA | 42 (7.6) | 21 (15.0) | 21 (5.1) | 0.0001 | 0.03 |
| Blood fat profile | |||||
| Cholesterol (mg/dL) | 179.9 ± 32.7 | 156.5 ± 59.4 | 188.1 ± 53.4 | <0.0001 | 0.06 |
| LDL cholesterol (mg/dL) | 110.0 ± 20.0 | 106.4 ± 43.1 | 111.4 ± 37.8 | 0.44 | - |
| HDL cholesterol (mg/dL) | 50.2 ± 9.1 | 48.9 ± 22.8 | 50.8 ± 16.5 | 0.54 | - |
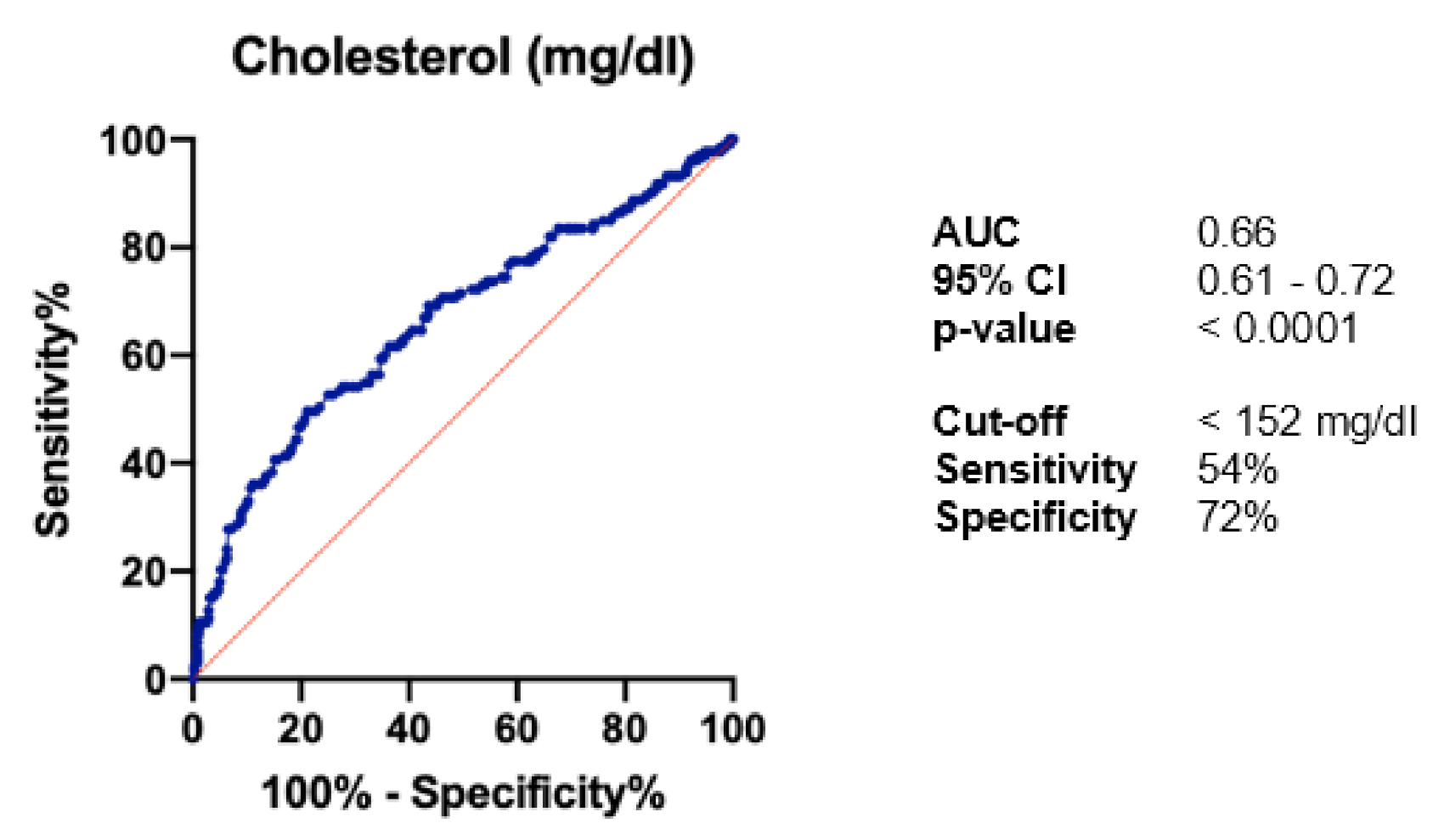
| Total cholesterol <152 mg/dL | 169 (30.7) | 69 (49.3) | 100 (24.3) | <0.0001 | 0.06 |
| Low cholesterol due to statin therapy | 84 (15.2) | 12 (8.6) | 72 (17.5) | 0.01 | 0.01 |
| Concomitant medication | |||||
| Betablocker | 206 (37.4) | 48 (34.3) | 158 (38.4) | 0.34 | - |
| ACE inhibitor/ARB | 226 (41.0) | 52 (37.1) | 174 (42.3) | 0.25 | - |
| Anticoagulant | 172 (31.2) | 55 (39.3) | 117 (28.5) | 0.02 | 0.01 |
| Platelet inhibition | 118 (21.4) | 32 (22.9) | 86 (20.9) | 0.66 | - |
| Vit. D | 109 (19.8) | 30 (21.4) | 79 (19.2) | 0.59 | - |
| Diabetes medication | 77 (14.0) | 21 (15.0) | 56 (13.6) | 0.66 | - |
| Statin | 136 (24.7) | 29 (20.7) | 107 (26.0) | 0.19 | - |
| Cancer treatment | |||||
| Platin | 105 (19.1) | 29 (20.7) | 76 (18.5) | 0.56 | - |
| Taxane | 157 (28.5) | 39 (27.9) | 118 (28.7) | 0.85 | - |
| Anthrazycline | 147 (26.7) | 44 (31.4) | 103 (25.1) | 0.14 | - |
| Gemcitabine | 24 (4.4) | 11 (7.9) | 13 (3.2) | 0.02 | 0.01 |
| 5-FU | 46 (8.3) | 21 (15.0) | 25 (6.1) | 0.001 | 0.02 |
| Aromatase inhibitors | 45 (8.2) | 11 (7.9) | 34 (8.3) | 0.88 | - |
| Bortezomib/Lenalidomid | 15 (2.7) | 1 (0.7) | 14 (3.4) | 0.09 | - |
| Surgical treatment | 304 (55.2) | 66 (47.1) | 238 (57.9) | 0.03 | 0.009 |
| Radiation | 213 (38.7) | 69 (49.3) | 144 (35.0) | 0.003 | 0.02 |
| Stem cell transplantation | 26 (4.7) | 12 (8.6) | 14 (3.4) | 0.01 | 0.01 |
| Entire Study Population | Patients Who Died | Alive during Follow-Up | p-Value | |
|---|---|---|---|---|
| HPB | 121 [99; 154] | 143 [96; 160] | 109 [98; 158] | 0.84 |
| Others | 148 [136; 200] | 108 [67; 148] | 148 [147; 227] | 0.13 |
| ORL | 151 [124; 205] | 130 [96; 199] | 152 [139; 211] | 0.41 |
| Kidney/Urothelium | 157 [138; 199] | 141 [121; 213] | 160 [147; 199] | 0.36 |
| Sarcoma | 159 [119; 220] | 111 [109; 214] | 193 [153; 228] | 0.14 |
| Lung | 159 [117; 209] | 171 [113; 219] | 154 [118; 209] | 0.91 |
| Gastrointestinal | 166 [122; 205] | 135 [101; 227] | 166 [127; 203] | 0.9 |
| Gynaecological | 170 [137; 225] | 158 [136; 173] | 199 [134; 242] | 0.17 |
| Haematological | 171 [113; 217] | 115 [74; 177] | 188 [137; 223] | 0.005 |
| Dermatological | 174 [134; 206] | 174 | 172 [133; 206] | 0.97 |
| Lymphoma | 174 [147; 214] | 139 [111; 161] | 186 [165; 228] | 0.02 |
| Prostate | 178 [133; 217] | 160 [113; 196] | 185 [148; 231] | 0.18 |
| Melanoma | 194 [147; 247] | 176 [124; 226] | 194 [164; 256] | 0.39 |
| Cerebral | 205 [164; 231] | 128 [106; 150] | 221 [204; 233] | 0.002 |
| Mamma | 212 [163; 245] | 160 [124; 228] | 216 [180; 248] | 0.0004 |
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Age at first diagnosis | 1.02 | 1.01 to 1.03 | 0.007 |
| Metastasised tumour stage | 3.31 | 2.24 to 4.88 | <0.0001 |
| Known cardiac disease | 1.58 | 1.07 to 2.3 | 0.02 |
| Prior stroke/TIA | 1.73 | 1.06 to 2.82 | 0.03 |
| Total cholesterol <152 mg/dL | 1.70 | 1.21 to 2.39 | 0.002 |
| Treatment with 5-FU | 1.32 | 0.81 to 2.15 | 0.26 |
| Radiation | 0.95 | 0.66 to 1.37 | 0.78 |
| Stem cell transplantation | 4.31 | 2.27 to 8.20 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohneck, A.L.; Rosenkaimer, S.; Hofheinz, R.-D.; Akin, I.; Borggrefe, M.; Gerhards, S. Blood Cholesterol and Outcome of Patients with Cancer under Regular Cardiological Surveillance. Curr. Oncol. 2021, 28, 863-872. https://doi.org/10.3390/curroncol28010085
Hohneck AL, Rosenkaimer S, Hofheinz R-D, Akin I, Borggrefe M, Gerhards S. Blood Cholesterol and Outcome of Patients with Cancer under Regular Cardiological Surveillance. Current Oncology. 2021; 28(1):863-872. https://doi.org/10.3390/curroncol28010085
Chicago/Turabian StyleHohneck, Anna Lena, Stephanie Rosenkaimer, Ralf-Dieter Hofheinz, Ibrahim Akin, Martin Borggrefe, and Stefan Gerhards. 2021. "Blood Cholesterol and Outcome of Patients with Cancer under Regular Cardiological Surveillance" Current Oncology 28, no. 1: 863-872. https://doi.org/10.3390/curroncol28010085
APA StyleHohneck, A. L., Rosenkaimer, S., Hofheinz, R.-D., Akin, I., Borggrefe, M., & Gerhards, S. (2021). Blood Cholesterol and Outcome of Patients with Cancer under Regular Cardiological Surveillance. Current Oncology, 28(1), 863-872. https://doi.org/10.3390/curroncol28010085





