Cerebral Invasive Aspergillosis in a Case of Chronic Lymphocytic Leukemia with Bruton Tyrosine Kinase Inhibitor
Abstract
1. Introduction
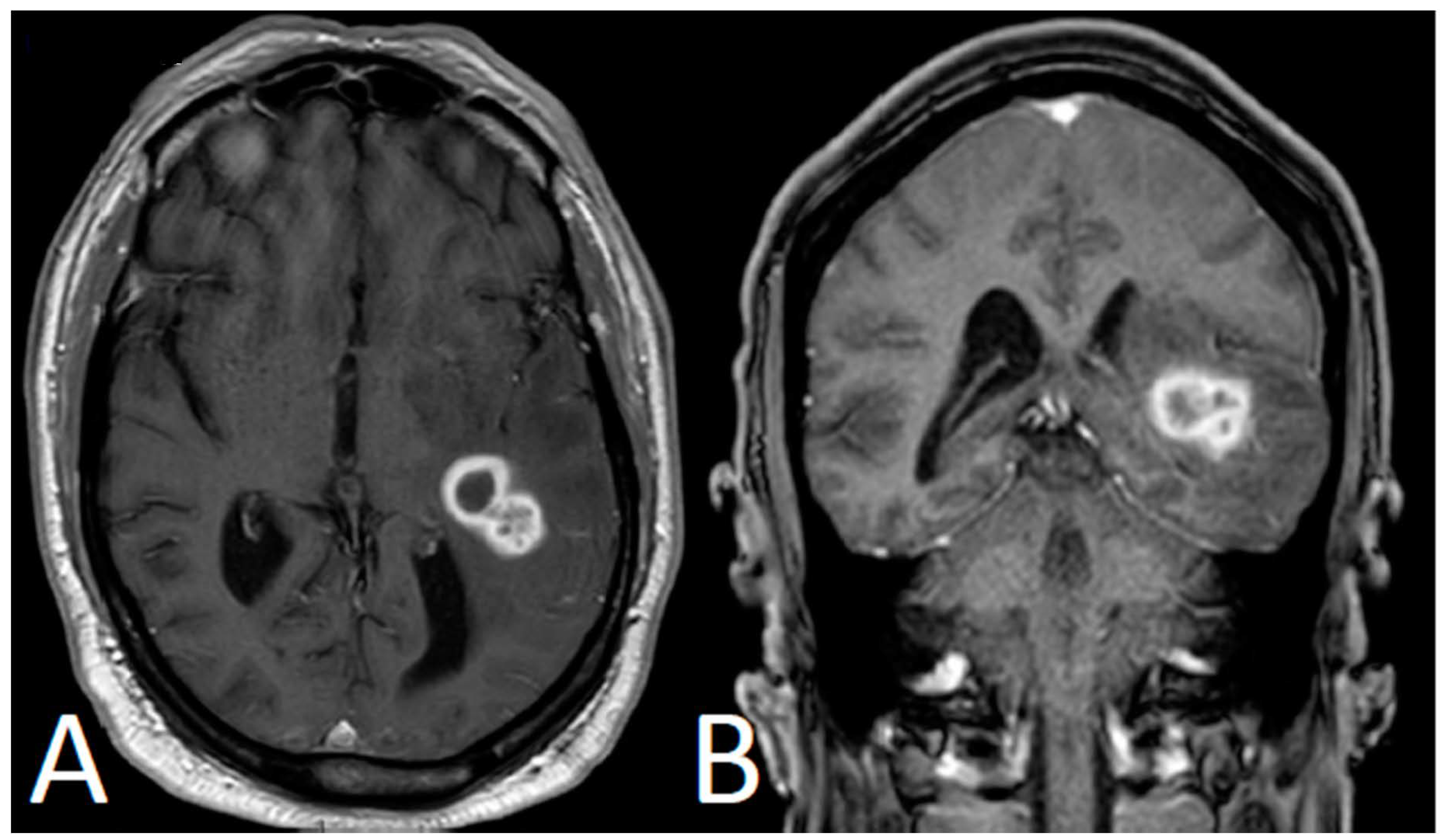
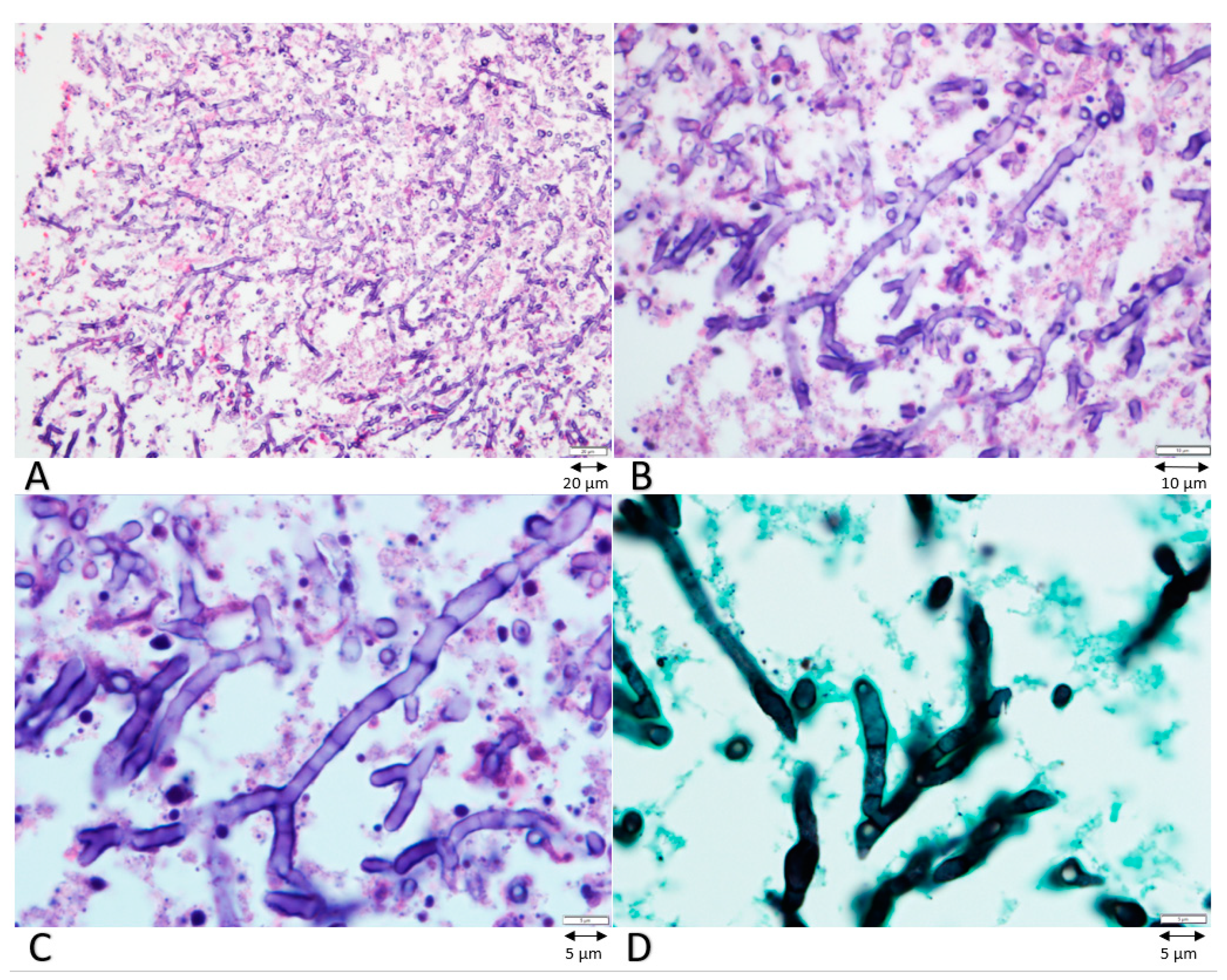
2. Case Presentation
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hallek, M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2019, 94, 1266–1287. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, R.; Roldan Galiacho, V.; Llorente, L. Immunological aspects in chronic lymphocytic leukemia (CLL) development. Ann. Hematol. 2012, 91, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, E.M.; Saullo, J.; Brander, D.; Wang, S.H.; Perfect, J.R.; Messina, J.A. A case of CNS aspergillosis in a patient with chronic lymphocytic leukemia on first-line ibrutinib therapy. Med. Mycol. Case Rep. 2020, 27, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.S.; Shaikh, H.; Khattab, A.; Albrethsen, M.; Fazal, S. Cerebral aspergillosis in a patient on ibrutinib therapy-A predisposition not to overlook. J. Oncol. Pharm. Pract. 2019, 25, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Gaye, E.; Le Bot, A.; Talarmin, J.P.; Le Calloch, R.; Belaz, S.; Dupont, M.; Tattevin, P. Cerebral aspergillosis: An emerging opportunistic infection in patients receiving ibrutinib for chronic lymphocytic leukemia? Med. Mal. Infect. 2018, 48, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; Ledoux, M.P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Varughese, T.; Taur, Y.; Cohen, N.; Palomba, M.L.; Seo, S.K.; Hohl, T.M.; Redelman-Sidi, G. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clin. Infect. Dis. 2018, 67, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Ruchlemer, R.; Ben-Ami, R.; Bar-Meir, M.; Brown, J.R.; Malphettes, M.; Mous, R.; Tonino, S.H.; Soussain, C.; Barzic, N.; Messina, J.A.; et al. Ibrutinib-associated invasive fungal diseases in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: An observational study. Mycoses 2019, 62, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.T.; Schuh, A.; Brown, J.R.; Furman, R.R.; Pagel, J.M.; Hillmen, P.; Stephens, D.M.; Woyach, J.; Bibikova, E.; Charuworn, P.; et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019, 3, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Lewis, R.E.; Kontoyiannis, D.P. Enemy of the (immunosuppressed) state: An update on the pathogenesis of Aspergillus fumigatus infection. Br. J. Haematol. 2010, 150, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lionakis, M.S.; Kontoyiannis, D.P. Call for Action: Invasive Fungal Infections Associated With Ibrutinib and Other Small Molecule Kinase Inhibitors Targeting Immune Signaling Pathways. Clin. Infect. Dis. 2018, 66, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.A.B.; Melville, K.B. Disseminated Cryptococcal Infection in a Patient Receiving Acalabrutinib for Chronic Lymphocytic Leukemia. Infect. Dis. Clin. Pract. 2019, 27, 160–162. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Morrison, V.A. Infectious complications of chronic lymphocytic leukemia. Semin. Oncol. 2006, 33, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, K.; Sindrilaru, A.; Terszowski, G.; Kokai, E.; Feyerabend, T.B.; Bullinger, L.; Rodewald, H.R.; Brunner, C. Neutrophil development and function critically depend on Bruton tyrosine kinase in a mouse model of X-linked agammaglobulinemia. Blood 2011, 117, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Fiorcari, S.; Maffei, R.; Vallerini, D.; Scarfò, L.; Barozzi, P.; Maccaferri, M.; Potenza, L.; Ghia, P.; Luppi, M.; Marasca, R. BTK Inhibition Impairs the Innate Response Against Fungal Infection in Patients With Chronic Lymphocytic Leukemia. Front. Immunol. 2020, 11, 2158. [Google Scholar] [CrossRef] [PubMed]
- Stiff, A.; Trikha, P.; Wesolowski, R.; Kendra, K.; Hsu, V.; Uppati, S.; McMichael, E.; Duggan, M.; Campbell, A.; Keller, K.; et al. Myeloid-Derived Suppressor Cells Express Bruton’s Tyrosine Kinase and Can Be Depleted in Tumor-Bearing Hosts by Ibrutinib Treatment. Cancer Res. 2016, 76, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Perkhofer, S.; Kehrel, B.E.; Dierich, M.P.; Donnelly, J.P.; Nussbaumer, W.; Hofmann, J.; von Eiff, C.; Lass-Florl, C. Human platelets attenuate Aspergillus species via granule-dependent mechanisms. J. Infect. Dis. 2008, 198, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tian, X.; Lee, Y.S.; Gunti, S.; Lipsky, A.; Herman, S.E.M.; Salem, D.; Stetler-Stevenson, M.; Yuan, C.; Kardava, L.; et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood 2015, 126, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Teh, B.W.; Micklethwaite, K.; Slavin, M. Azole antifungals and new targeted therapies for hematological malignancy. Curr. Opin. Infect. Dis. 2019, 32, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Facchinelli, D.; Marchesini, G.; Nadali, G.; Pagano, L. Invasive Fungal Infections in Patients with Chronic Lymphoproliferative Disorders in the Era of Target Drugs. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018063. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkharabsheh, O.; Alsayed, A.; Morlote, D.M.; Mehta, A. Cerebral Invasive Aspergillosis in a Case of Chronic Lymphocytic Leukemia with Bruton Tyrosine Kinase Inhibitor. Curr. Oncol. 2021, 28, 837-841. https://doi.org/10.3390/curroncol28010081
Alkharabsheh O, Alsayed A, Morlote DM, Mehta A. Cerebral Invasive Aspergillosis in a Case of Chronic Lymphocytic Leukemia with Bruton Tyrosine Kinase Inhibitor. Current Oncology. 2021; 28(1):837-841. https://doi.org/10.3390/curroncol28010081
Chicago/Turabian StyleAlkharabsheh, Omar, Alhareth Alsayed, Diana M. Morlote, and Amitkumar Mehta. 2021. "Cerebral Invasive Aspergillosis in a Case of Chronic Lymphocytic Leukemia with Bruton Tyrosine Kinase Inhibitor" Current Oncology 28, no. 1: 837-841. https://doi.org/10.3390/curroncol28010081
APA StyleAlkharabsheh, O., Alsayed, A., Morlote, D. M., & Mehta, A. (2021). Cerebral Invasive Aspergillosis in a Case of Chronic Lymphocytic Leukemia with Bruton Tyrosine Kinase Inhibitor. Current Oncology, 28(1), 837-841. https://doi.org/10.3390/curroncol28010081





