Safe Administration of Cemiplimab to a Kidney Transplant Patient with Locally Advanced Squamous Cell Carcinoma of the Scalp
Abstract
1. Introduction
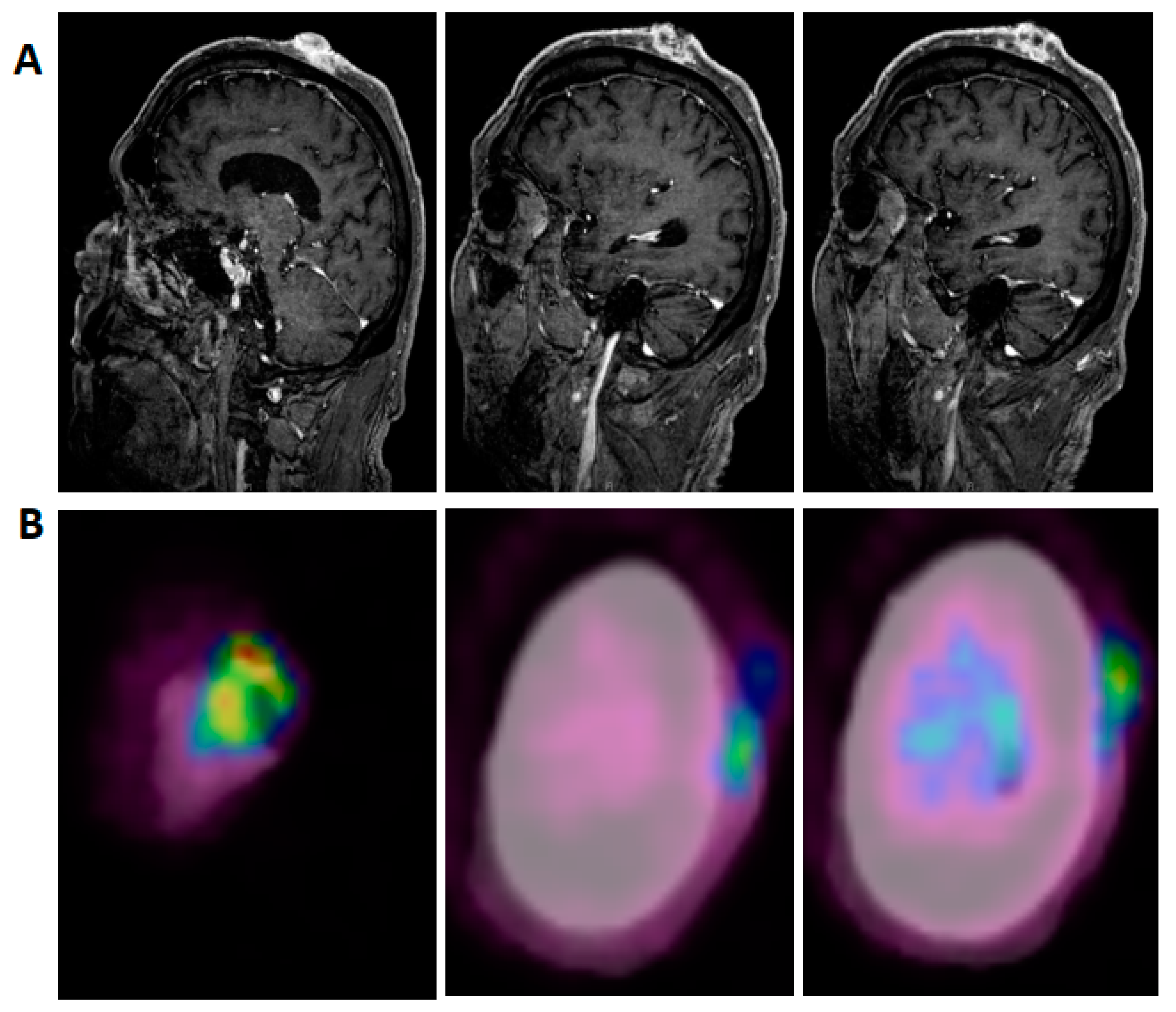
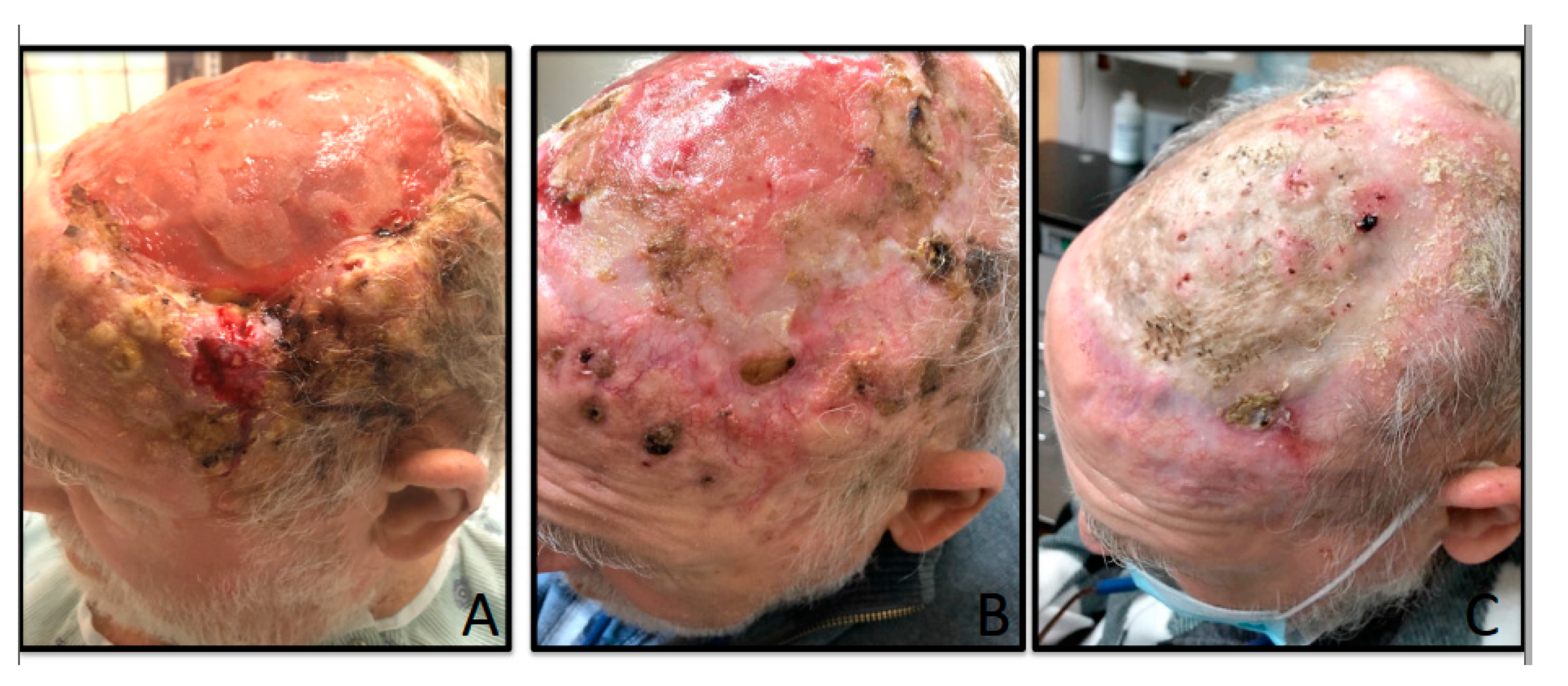
2. Case Presentation
3. Discussion
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of Cancer Risk among U.S. Solid Organ Transplant Recipients: The Transplant Cancer Match Study. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.A.; Mesher, D.; McGregor, J.M.; Mitchell, L.; Leedham-Green, M.; Raftery, M.; Cerio, R.; Leigh, I.M.; Sasieni, P.; Proby, C.M. A surveillance model for skin cancer in organ transplant recipients: A 22-year prospective study in an ethnically diverse population. Am. J. Transplant. 2013, 13, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Vajdic, C.M.; McDonald, S.P.; McCredie, M.R.; van Leeuwen, M.T.; Stewart, J.H.; Law, M.; Chapman, J.R.; Webster, A.C.; Kaldor, J.M.; Grulich, A.E. Cancer incidence before and after kidney transplantation. JAMA 2006, 296, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.C.; Otley, C.C.; Stasko, T.; Euvrard, S.; Brown, C.; Schanbacher, C.F.; Weaver, A.L. Defining the clinical course of metastatic skin cancer in organ transplant recipients: A multicenter collaborative study. Arch. Dermatol. 2003, 139, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Euvrard, S.; Kanitakis, J.; Claudy, A.S.O. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Otley, C.C.; Berg, D.; Ulrich, C.; Stasko, T.; Murphy, G.M.; Salasche, S.J.; Christenson, L.J.; Sengelmann, R.; Loss, G.E., Jr.; Garces, J.; et al. Reduction of immunosuppression for transplant-associated skin cancer: Expert consensus survey. Br. J. Dermatol. 2006, 54, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.M.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 Axis Converts Human Th1 Cells into Regulatory T Cells. Sci. Transl. Med. 2011, 3, 111ra120. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Hubbard-Lucey, V.M.; Tang, J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 2019, 18, 899–900. [Google Scholar]
- Lipson, E.J.; Bagnasco, S.M.; Moore, J., Jr.; Jang, S.; Patel, M.J.; Zachary, A.A.; Pardoll, D.M.; Taube, J.M.; Taube, C.G. Tumor Regression and Allograft Rejection after Administration of Anti-PD-1. N. Engl. J. Med. 2016, 374, 896–898. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, P.; Van Gestel, D.; Ost, P.; Kruse, V.; Brochez, L.; Van Vlierberghe, H.; Arnaud, D.; del Véronique, M.; Alain, L.M.; Sandrine, A. Immune checkpoint blockade for organ transplant patients with advanced cancer: How far can we go. Curr. Opin. Oncol. 2019, 31, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, N.; Safa, H.; Abudayyeh, A.; Johnson, D.H.; Zobniw, C.M.; Lin, H.; Wong, M.K.; Abdelrahim, M.; Gaber, A.O.; Suarez-Almazor, M.E.; et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: An institutional experience and a systematic review of the literature. J. Immunother. Cancer 2019, 7, 106. [Google Scholar] [CrossRef]
- Manohar, S.; Thongprayoon, C.; Cheungpasitporn, W.; Markovic, S.N.; Herrmann, S.M. Systematic Review of the Safety of Immune Checkpoint Inhibitors among Kidney Transplant Patients. Kidney Int. Rep. 2019, 5, 149–158. [Google Scholar] [CrossRef] [PubMed]
- d’Izarni-Gargas, T.; Durrbach, A.; Zaidan, M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: A systematic review. Am. J. Transplant. 2020, 20, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Trager, M.H.; Coley, S.M.; Dube, G.; Khan, S.; Ingham, M.; Samie, F.H.; Geskin, L.J.; McDonnell, D.; Brouder, D.; Saenger, Y.; et al. Combination of checkpoint blockade for metastatic cutaneous malignancies in kidney transplant recipients. J. Immunother. Cancer 2020, 8, e000908. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Arman, H.E.; Patel, A.A.; Birhiray, R.E. Successful Administration of Cemiplimab to a Patient With Advanced Cutaneous Squamous Cell Carcinoma After Renal Transplantation. JCO Oncol. Pract. 2020, 16, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.L.; Hojo, M.; Maluccio, M.; Yamaji, K.; Suthanthiran, M. Rapamycin blocks tumor progression: Unlinking immunosuppression from antitumor efficacy. Transplantation 2002, 73, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Karia, P.S.; Azzi, J.R.; Heher, E.C.; Hills, V.M.; Schmults, C.D. Association of Sirolimus Use With Risk for Skin Cancer in a Mixed-Organ Cohort of Solid-Organ Transplant Recipients With a History of Cancer. JAMA Dermatol. 2016, 152, 533–540. [Google Scholar] [CrossRef] [PubMed]
| Cemiplimab | Creatinine (Ref Range < l.5 mg/dL) | GFR (Ref Range > 60 mL/min/BSA) |
|---|---|---|
| C#1 | 1.2 | 60 |
| C#2 | 1.1 | 66 |
| C#3 | 1.0 | 74 |
| C#4 | 1.2 | 60 |
| C#5 | 1.2 | 60 |
| C#6 | 1.0 | 74 |
| C#7 | 0.97 | 78 |
| C#8 | 0.88 | 86 |
| C#9 | 0.91 | 84 |
| C#10 | 0.85 | 87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paoluzzi, L.; Ow, T.J. Safe Administration of Cemiplimab to a Kidney Transplant Patient with Locally Advanced Squamous Cell Carcinoma of the Scalp. Curr. Oncol. 2021, 28, 574-580. https://doi.org/10.3390/curroncol28010057
Paoluzzi L, Ow TJ. Safe Administration of Cemiplimab to a Kidney Transplant Patient with Locally Advanced Squamous Cell Carcinoma of the Scalp. Current Oncology. 2021; 28(1):574-580. https://doi.org/10.3390/curroncol28010057
Chicago/Turabian StylePaoluzzi, Luca, and Thomas J Ow. 2021. "Safe Administration of Cemiplimab to a Kidney Transplant Patient with Locally Advanced Squamous Cell Carcinoma of the Scalp" Current Oncology 28, no. 1: 574-580. https://doi.org/10.3390/curroncol28010057
APA StylePaoluzzi, L., & Ow, T. J. (2021). Safe Administration of Cemiplimab to a Kidney Transplant Patient with Locally Advanced Squamous Cell Carcinoma of the Scalp. Current Oncology, 28(1), 574-580. https://doi.org/10.3390/curroncol28010057





