Lung Cancer Pre-Diagnostic Pathways from First Presentation to Specialist Referral
Abstract
1. Introduction
2. Methods
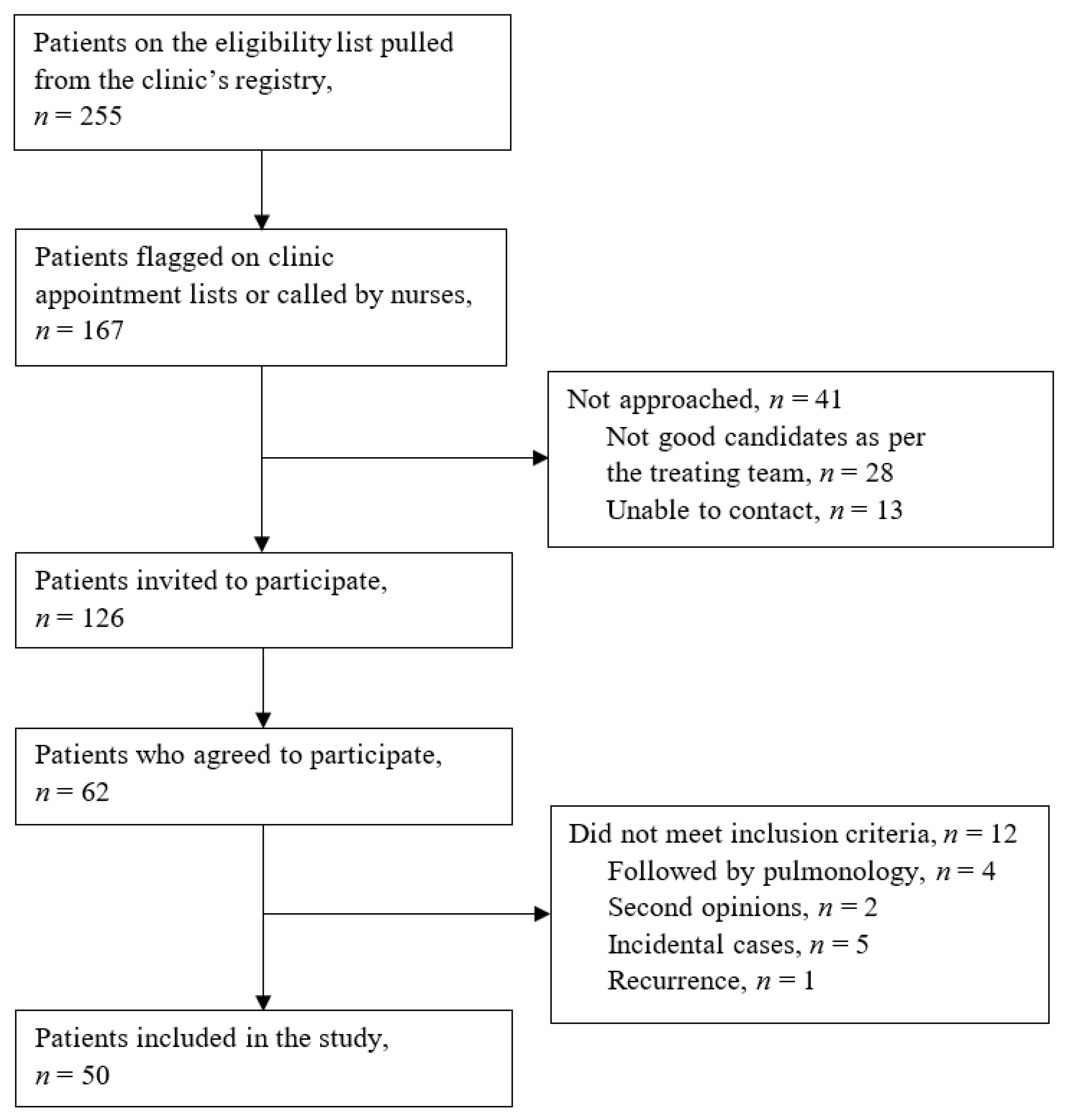
2.1. Study Design and Population
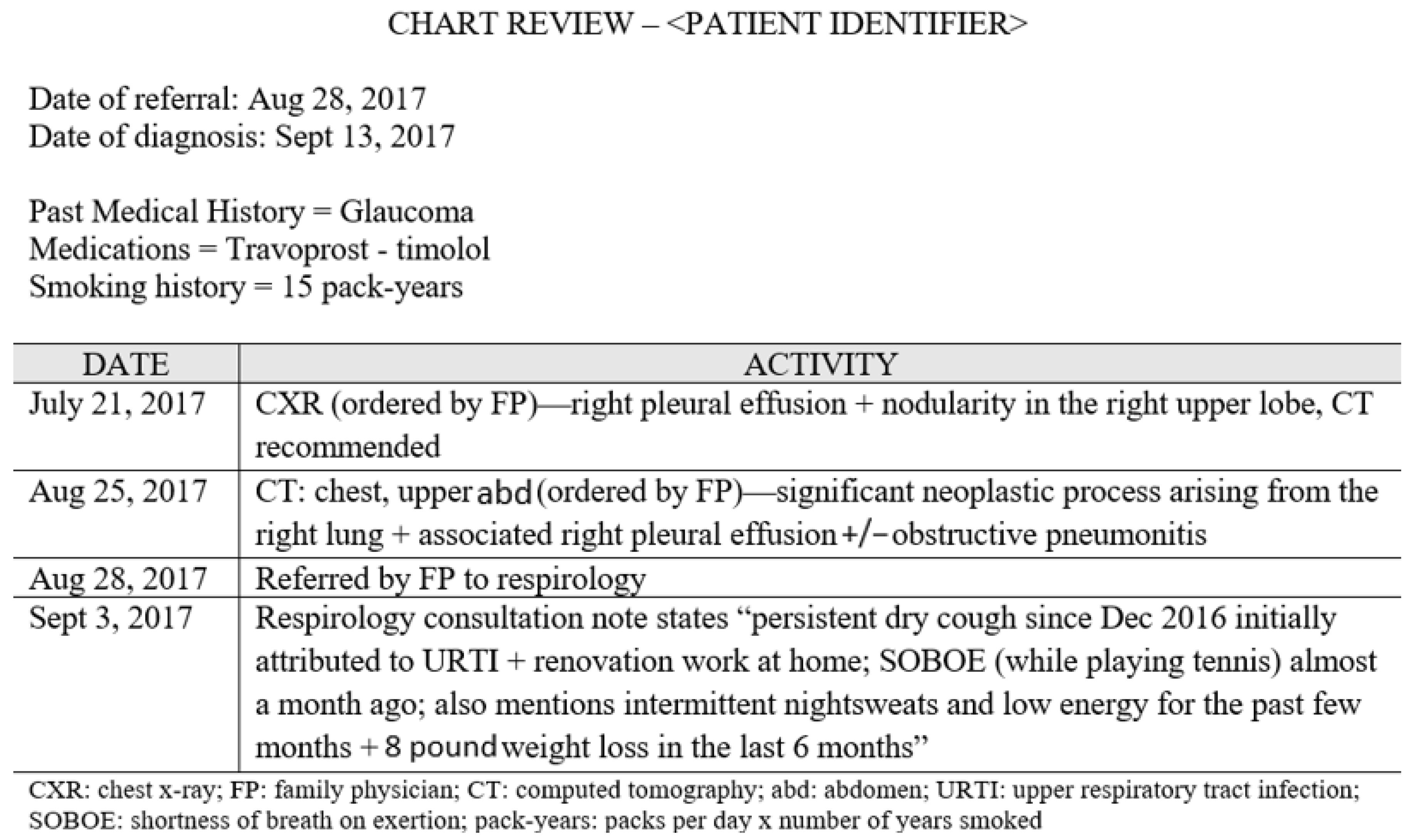
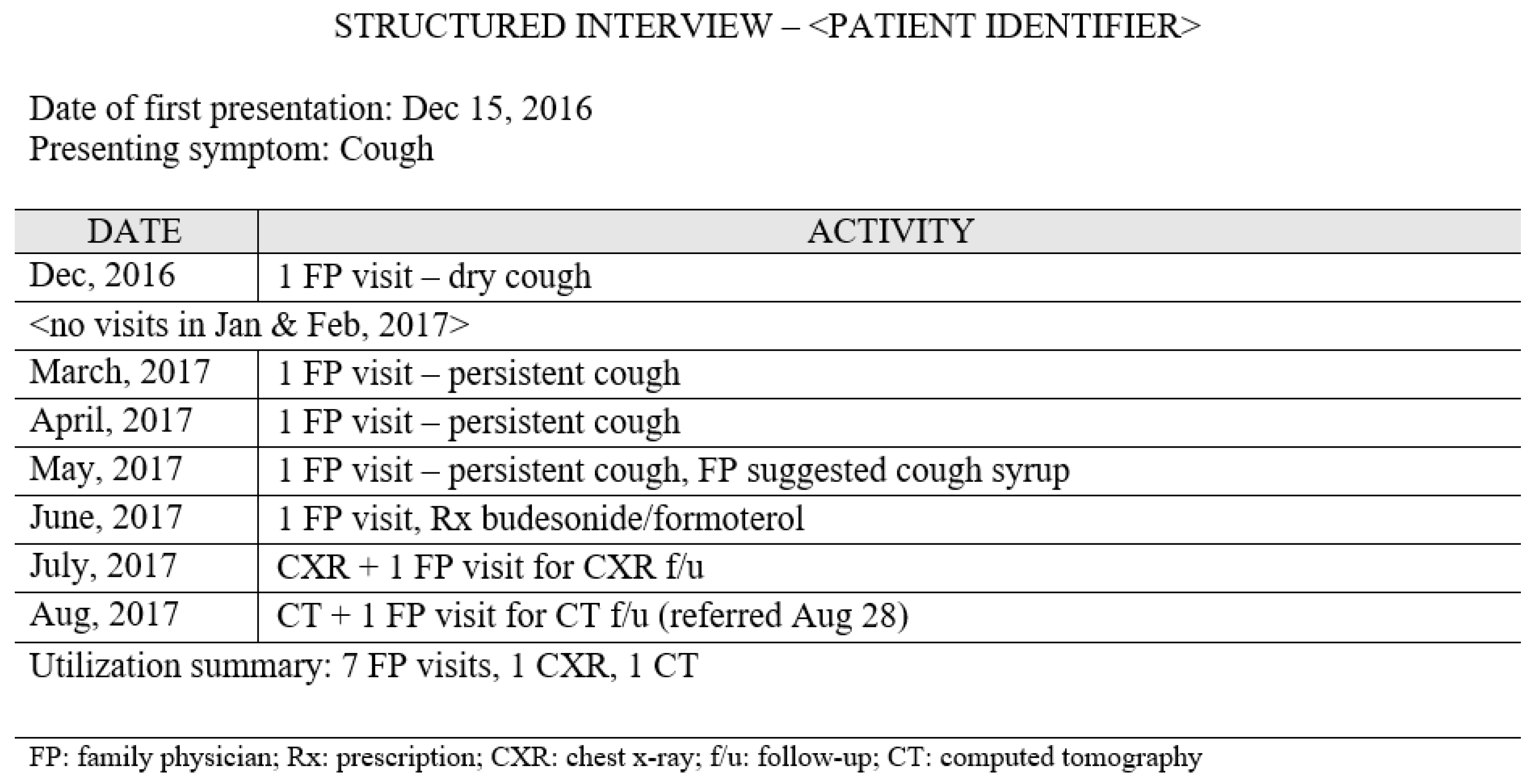
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethics Approval
3. Results
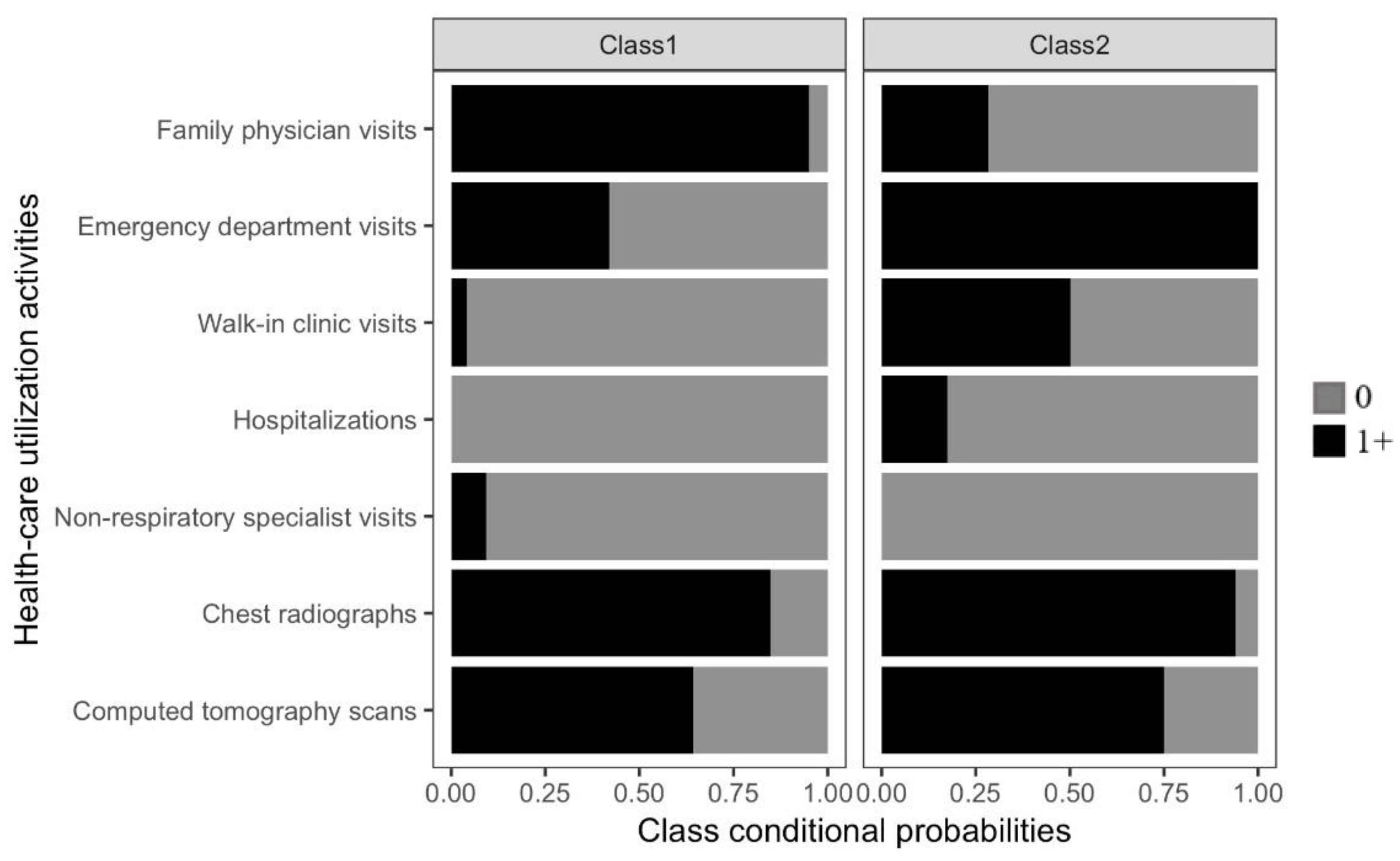
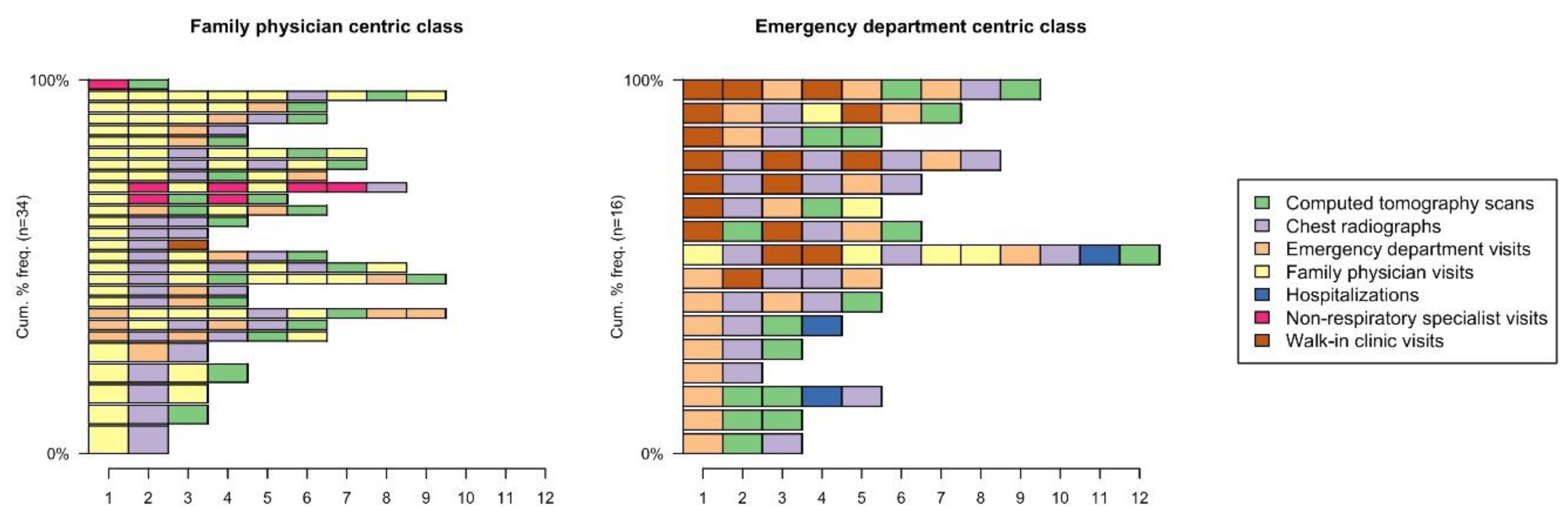
3.1. Identifying Pre-Diagnostic Pathways
3.2. Characteristics of Patients by Pre-Diagnostic Pathway Group
3.3. Sequence of Events within Pre-Diagnostic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Society. Lung Cancer Statistics; Canadian Cancer Society: Toronto, ON, Canada, 2019; Available online: http://www.cancer.ca/en/cancer-information/cancer-type/lung/statistics/?region=qc (accessed on 14 May 2020).
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2017; Canadian Cancer Society: Toronto, ON, Canada, 2017; Available online: cancer.ca/Canadian-CancerStatistics-2017-EN.pdf (accessed on 11 March 2020).
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2018; Canadian Cancer Society: Toronto, ON, Canada, 2018; Available online: cancer.ca/Canadian-Cancer-Statistics-2018-EN (accessed on 14 May 2020).
- Allgar, V.L.; Neal, R.D. General practictioners’ management of cancer in England: Secondary analysis of data from the National Survey of NHS Patients-Cancer. Eur. J. Cancer Care 2005, 14, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.; Hamilton, W. Pathways to the diagnosis of lung cancer in the UK: A cohort study. BMC Fam. Pract. 2008, 9, 31. [Google Scholar] [CrossRef]
- Weller, D.; Vedsted, P.; Rubin, G.; Walter, F.M.; Emery, J.; Scott, S.; Campbell, C.; Andersen, R.S.; Hamilton, W.; Olesen, F.; et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer 2012, 106, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Rankin, N.M.; McGregor, D.; Stone, E.; Butow, P.N.; Young, J.M.; White, K.; Shaw, T. Evidence-practice gaps in lung cancer: A scoping review. Eur. J. Cancer Care 2018, 27, e12588. [Google Scholar] [CrossRef] [PubMed]
- Koyi, H.; Hillerdal, G.; Branden, E. Patient’s and doctors’ delays in the diagnosis of chest tumors. Lung Cancer 2002, 35, 53–57. [Google Scholar] [CrossRef]
- Rubin, G.; Berendsen, A.; Crawford, S.M.; Dommett, R.; Earle, C.; Emery, J.; Fahey, T.; Grassi, L.; Grunfeld, E.; Gupta, S.; et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015, 16, 1231–1272. [Google Scholar] [CrossRef]
- Shim, J.; Brindle, L.; Simon, M.; George, S. A systematic review of symptomatic diagnosis of lung cancer. Fam. Pract. 2014, 31, 137–148. [Google Scholar] [CrossRef]
- Olsson, J.K.; Schultz, E.M.; Gould, M.K. Timeliness of care in patients with lung cancer: A systematic review. Thorax 2009, 64, 749–756. [Google Scholar] [CrossRef]
- Rose, P.W.; Rubin, G.; Perera-Salazar, R.; Almberg, S.S.; Barisic, A.; Dawes, M.; Grunfeld, E.; Hart, N.; Neal, R.D.; Pirotta, M.; et al. Explaining variation in cancer survival between 11 jurisdictions in the International Cancer Benchmarking Partnership: A primary care vignette survey. BMJ Open 2015, 5, e007212. [Google Scholar] [CrossRef]
- Canadian Partnership against Cancer. The 2015 Cancer System Performance Report; System Performance Reports; Canadian Partnership against Cancer: Toronto, ON, Canada, 2015; pp. 1–161. [Google Scholar]
- Kasymjanova, G.; Small, D.; Cohen, V.; Jagoe, R.T.; Batist, G.; Sateren, W.; Ernst, P.; Pepe, C.; Sakr, L.; Agulnik, J. Lung cancer care trajectory at a Canadian centre: An evaluation of how wait times affect clinical outcomes. Curr. Oncol. 2017, 24, 302–309. [Google Scholar] [CrossRef]
- Pampalon, R.; Hamel, D.; Gamache, P. Health inequalities in urban and rural Canada: Comparing inequalities in survival according to an individual and area-based deprivation index. Health Place 2010, 16, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Brusco, N.K.; Watts, J.J. Empirical evidence of recall bias for primary health care visits. BMC Health Serv. Res. 2015, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.R.; Vedel, I. Recall bias and reduction measures: An example in primary health care service utilization. Fam. Pract. 2019, 36, 672–676. [Google Scholar] [CrossRef]
- Flaherty, B.P.; Kiff, C.J. Latent class and latent profile models. In APA Handbook of Research Methods in Psychology: Vol 3 Data Analysis and Research Publication: American Psychological Association; Cooper, H., Ed.; American Psychological Association: Washington, DC, USA, 2012; pp. 391–404. [Google Scholar]
- UCLA: Statistical Consulting Group. Latent Class Analysis in Mplus California; Statistical Computing Seminars: Los Angeles, CA, USA, 2016; Available online: http://www.ats.ucla.edu/stat/mplus/seminars/lca/ (accessed on 9 April 2017).
- Hagenaars, J.A.; McCutcheon, A.L. (Eds.) Applied Latent Class Analysis; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Brzinsky-Fay, C.; Kohler, U. New Developments in Sequence Analysis. SMR Sociol. Methods Res. 2010, 38, 359–364. [Google Scholar] [CrossRef]
- Allgar, V.L.; Neal, R.D. Delays in the diagnosis of six cancers: Analysis of data from the National Survey of NHS Patients: Cancer. Br. J. Cancer 2005, 92, 1959–1970. [Google Scholar] [CrossRef]
- Lyratzopoulos, G.; Wardle, J.; Rubin, G. Rethinking diagnostic delay in cancer: How difficult is the diagnosis? BMJ 2014, 349, g7400. [Google Scholar] [CrossRef]
- Hamilton, W.; Peters, T.J.; Round, A.; Sharp, D. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax 2005, 60, 1059–1065. [Google Scholar] [CrossRef]
- Stapley, S.; Sharp, D.W.H. Negative chest X-rays in primary care patients with lung cancer. Br. J. Gen. Pract. 2006, 56, 570–573. [Google Scholar]
- Yap, S.; Goldsbury, D.; Yap, M.L.; Susan, Y.; Rankin, N.M.; Weber, M.; Karen, C.; Dianne, L.O. Patterns of care and emergency presentations for people with non-small cell lung cancer in New South Wales, Australia: A population-based study. Lung Cancer 2018, 122, 171–179. [Google Scholar] [CrossRef]
- Mitchell, E.D.; Rubin, G.; Macleod, U. Understanding diagnosis of lung cancer in primary care: Qualitative synthesis of significant event audit reports. Br. J. Gen. Pract. 2013, 63, 37–46. [Google Scholar] [CrossRef]
- Grunfeld, E. It takes a team: CanIMPACT: Canadian Team to Improve Community-Based Cancer Care along the Continuum. Can. Fam. Physician Med. Fam. Can. 2016, 62, 781–782. [Google Scholar]
- Ezer, N.; Navasakulpong, A.; Schwartzman, K.; Ofiara, L.; Gonzalez, A.V. Impact of rapid investigation clinic on timeliness of lung cancer diagnosis and treatment. BMC Pulm. Med. 2017, 17, 178. [Google Scholar] [CrossRef] [PubMed]
- Keely, E.; Liddy, C. Transforming the specialist referral and consultation process in Canada. Can. Med. Assoc. J. 2019, 191, E408–E409. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Health Fact Sheet: Primary Health Care Providers, 2016: Government of Canada 2017. Available online: https://www150.statcan.gc.ca/n1/pub/82-625-x/2017001/article/54863-eng.htm (accessed on 19 December 2019).
- Canadian Institute for Health Information. Canada’s Health Care Providers: Provincial Profiles, 2008 to 2017—Data Tables: CIHI. 2019. Available online: https://www.cihi.ca/en/access-data-reports/results?f%5B0%5D=field_primary_theme%3A2047&f%5B1%5D=field_professions%3A2010 (accessed on 29 December 2019).
- Del Giudice, M.E.; Young, S.; Vella, E.T.; Ash, M.; Bansal, P.; Robinson, A.; Skrastins, R.; Ung, Y.; Zeldin, R.; Levitt, C. Guideline for referral of patients with suspected lung cancer by family physicians and other primary care providers. Can. Fam. Physician 2014, 60, 711–716. [Google Scholar] [PubMed]
- Casal-Mouriño, A.; Valdés, L.; Barros-Dios, J.M.; Ruano-Ravina, A. Lung cancer survival among never smokers. Cancer Lett. 2019, 451, 142–149. [Google Scholar] [CrossRef]
- Kjær, T.K.; Mellemgaard, A.; Oksen, M.S.; Rix, B.A.; Karlsen, R.; Johansen, C.; Dalton, S.O. Recruiting newly referred lung cancer patients to a patient navigator intervention (PACO): Lessons learnt from a pilot study. Acta Oncol. 2017, 56, 335–341. [Google Scholar] [CrossRef]
- Spiro, S.G.; Gower, N.H.; Facchini, F.M.; Rudd, R.M.; Evans, M.T. Recruitment of patients with lung cancer into a randomised clinical trial: Experience at two centres. Thorax 2000, 55, 463–465. [Google Scholar] [CrossRef][Green Version]

| Characteristic | Total Median (IQR) of Patients, * n = 50 |
|---|---|
| Age at diagnosis, yr | 66 (57.2–76.7) |
| Sex, female, n (%) | 28 (56) |
| Smoking history, pack-yr | 20.7 (0–42) |
| Number of comorbidities | 1 (0–2) |
| Material deprivation index score † | 2 (1–4) |
| Social deprivation index score † | 4 (2–5) |
| Primary care interval time, d | 35 (9–100.7) |
| Referral source, n (%) | |
| Family physician | 18 (36) |
| Emergency department | 26 (52) |
| Non-respiratory specialist | 6 (12) |
| Presenting symptoms, n (%) | |
| Cough | 21 (42) |
| Shortness of breath | 11 (22) |
| Hemoptysis | 3 (6) |
| Chest pain | 3 (6) |
| Back pain | 4 (8) |
| Other ‡ | 8 (16) |
| Stage of disease, n (%) | |
| Early | 8 (16) |
| Locoregional | 12 (24) |
| Advanced | 30 (60) |
| Latent Class Models | AIC |
|---|---|
| One-class | 334.5149 |
| Two-class | 323.4714 |
| Three-class | 322.1228 |
| Four-class | 329.7299 |
| Characteristic | FP-Centric; Median (IQR) of Patients, * n = 34 | ED-Centric; Median (IQR) of Patients, * n = 16 |
|---|---|---|
| Age at diagnosis, yr | 65 (58.5–76.7) | 67.5 (56.5–72) |
| Sex, female, n (%) | 18 (52.9) | 10 (62.5) |
| Smoking history, pack-yr | 20 (0.4–35) | 27.5 (0–49.2) |
| Number of comorbidities | 1 (0.2–2) | 0 (0–2) |
| Material deprivation index score † | 2 (1–3.7) | 1 (1–3.5) |
| Social deprivation index score † | 4 (2–5) | 4 (1.5–5) |
| Primary care interval time, d | 45 (11.7–111.2) | 22 (4.7–69.5) |
| Referral source, n (%) | ||
| Family physician | 17 (50) | 1 (6.2) |
| Emergency department | 13 (38.2) | 13 (81.2) |
| Non-respiratory specialist | 4 (11.6) | 2 (12.4) |
| Presenting symptoms, n (%) | ||
| Cough | 16 (47.1) | 5 (31.2) |
| Shortness of breath | 8 (23.5) | 3 (18.8) |
| Hemoptysis | 2 (5.9) | 1 (6.2) |
| Chest pain | 2 (5.9) | 1 (6.2) |
| Back pain | 3 (8.8) | 1 (6.2) |
| Other ‡ | 3 (8.8) | 5 (31.2) |
| Stage of disease, n (%) | ||
| Early | 5 (14.7) | 3 (18.8) |
| Locoregional | 7 (20.6) | 5 (31.2) |
| Advanced | 22 (64.7) | 8 (50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khare, S.R.; Madathil, S.A.; Batist, G.; Peter Brojde Lung Cancer Group; Vedel, I. Lung Cancer Pre-Diagnostic Pathways from First Presentation to Specialist Referral. Curr. Oncol. 2021, 28, 378-389. https://doi.org/10.3390/curroncol28010040
Khare SR, Madathil SA, Batist G, Peter Brojde Lung Cancer Group, Vedel I. Lung Cancer Pre-Diagnostic Pathways from First Presentation to Specialist Referral. Current Oncology. 2021; 28(1):378-389. https://doi.org/10.3390/curroncol28010040
Chicago/Turabian StyleKhare, Satya Rashi, Sreenath Arekunnath Madathil, Gerald Batist, Peter Brojde Lung Cancer Group, and Isabelle Vedel. 2021. "Lung Cancer Pre-Diagnostic Pathways from First Presentation to Specialist Referral" Current Oncology 28, no. 1: 378-389. https://doi.org/10.3390/curroncol28010040
APA StyleKhare, S. R., Madathil, S. A., Batist, G., Peter Brojde Lung Cancer Group, & Vedel, I. (2021). Lung Cancer Pre-Diagnostic Pathways from First Presentation to Specialist Referral. Current Oncology, 28(1), 378-389. https://doi.org/10.3390/curroncol28010040





