Hormone Replacement Therapy and Outcomes for Women with Non-Small-Cell Lung Cancer: Can An Association be Confirmed?
Abstract
1. INTRODUCTION
2. PATIENTS AND METHODS
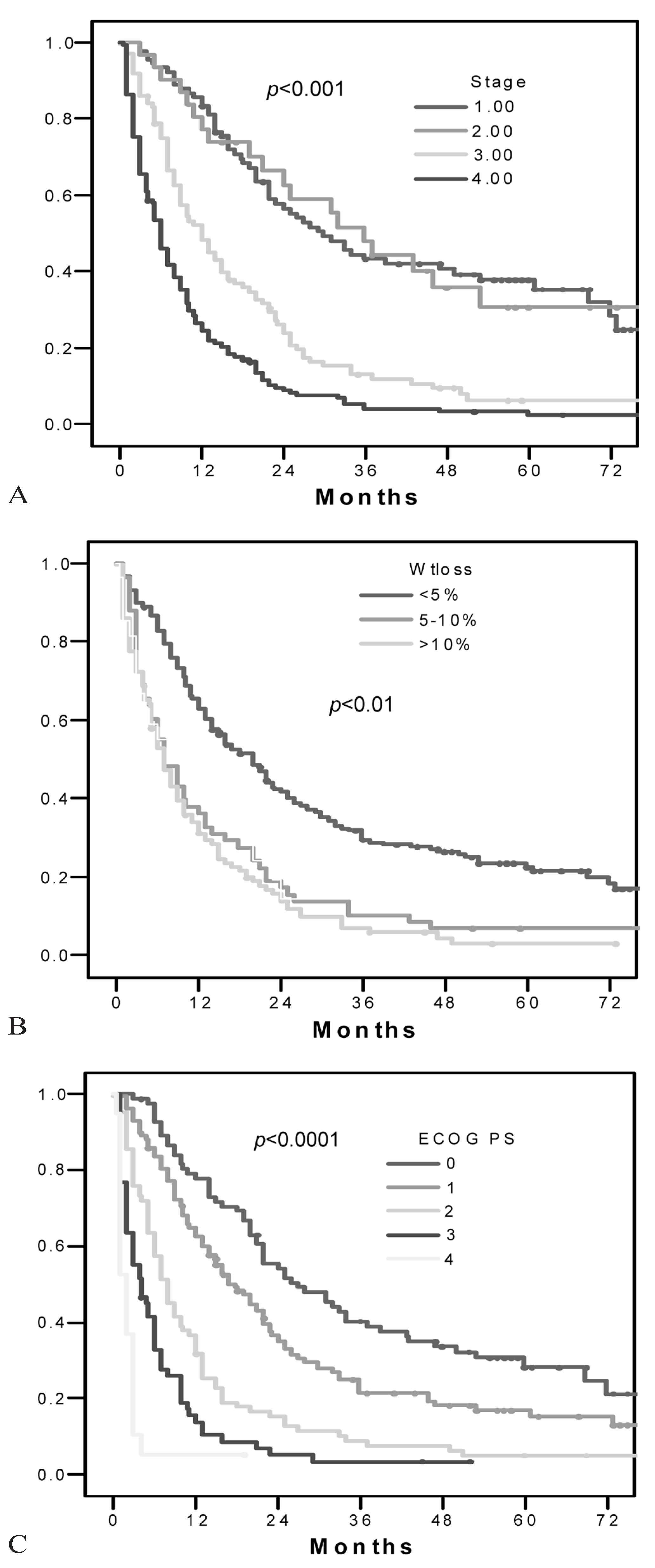
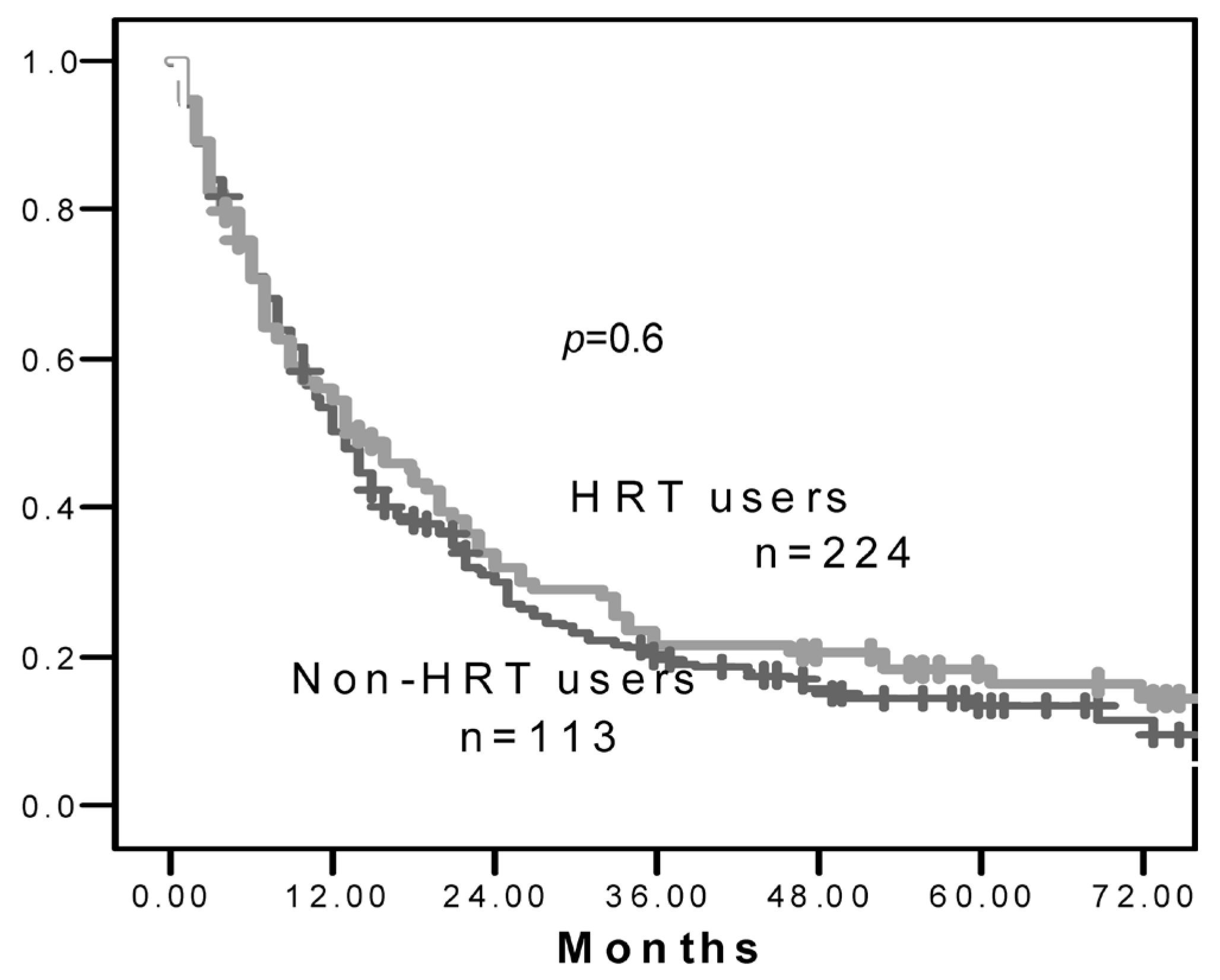
3. RESULTS
 |
4. DISCUSSION
Acknowledgments
Conflicts of Interest
References
- Thomas, L.; Doyle, L.A.; Edelman, M.J. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest 2005, 128, 370–81. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, E.; Glaz, P.; Roszkowski, K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20,561 cases. Ann Oncol 2002, 13, 1087–93. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.H.; Christiani, D.C.; Mark, E.J.; Wiencke, J.K.; Wain, J.C.; Kelsey, K.T. Implications and prognostic value of K-ras mutation for early-stage lung cancer in women. J Natl Cancer Inst 1999, 91, 2032–8. [Google Scholar] [CrossRef] [PubMed]
- Bain C, Feskanich D, Speizer FE, et al. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst 2004, 96, 826–34. [CrossRef]
- Henschke, C.I.; Miettinen, O.S. Women’s susceptibility to tobacco carcinogens. Lung Cancer 2004, 43, 1–5. [Google Scholar] [CrossRef]
- Ryberg, D.; Hewer, A.; Phillips, D.H.; Haugen, A. Different susceptibility to smoking-induced DNA damage among male and female lung cancer patients. Cancer Res 1994, 54, 5801–3. [Google Scholar] [PubMed]
- Lindell, R.M.; Hartman, T.E.; Swensen, S.J.; et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology 2007, 242, 555–62. [Google Scholar] [CrossRef]
- O’Connell, J.P.; Kris, M.G.; Gralla, R.J.; et al. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced non-small-cell lung cancer treated with combination chemotherapy. J Clin Oncol 1986, 4, 1604–14. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006, 355, 2542–50. [Google Scholar] [CrossRef]
- Dubey, S.; Siegfried, J.M.; Traynor, A.M. Non-small-cell lung cancer and breast carcinoma: chemotherapy and beyond. Lancet Oncol 2006, 7, 416–24. [Google Scholar] [CrossRef]
- Di Nunno, L.; Larsson, L.G.; Rinehart, J.J.; Beissner, R.S. Estrogen and progesterone receptors in non-small cell lung cancer in 248 consecutive patients who underwent surgical resection. Arch Pathol Lab Med 2000, 124, 1467–70. [Google Scholar] [CrossRef] [PubMed]
- Ganti, A.K.; Sahmoun, A.E.; Panwalkar, A.W.; Tendulkar, K.K.; Potti, A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol 2006, 24, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Wu, X.; Vassilopoulou–Sellin, R.; Vaporciyan, A.A.; Spitz, M.R. Hormone replacement therapy and lung cancer risk: a case–control analysis. Clin Cancer Res 2004, 10, 113–23. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.M.; Travers–Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007, 85, 1586–91. [Google Scholar] [CrossRef]
- Greendale, G.A.; Gold, E.B. Lifestyle factors: are they related to vasomotor symptoms and do they modify the effectiveness or side effects of hormone therapy? Am J Med 2005, 118, 148–54. [Google Scholar] [CrossRef]
- Delaney, M.F. Strategies for the prevention and treatment of osteoporosis during early postmenopause. Am J Obstet Gynecol 2006, 194, S12–S23. [Google Scholar] [CrossRef]
- Bond, G.L.; Levine, A.J. A single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humans. Oncogene 2007, 26, 1317–23. [Google Scholar] [CrossRef]
- Márquez–Garbán, D.C.; Chen, H.W.; Fishbein, M.C.; Goodglick, L.; Pietras, R.J. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 2007, 72, 135–43. [Google Scholar] [CrossRef]
- Kawai H, Ishii A, Washiya K, et al. Combined overexpression of EGFR and estrogen receptor alpha correlates with a poor outcome in lung cancer. Anticancer Res 2005, 25, 4693–8.
- Dahm, A.E.; Iversen, N.; Birkenes, B.; Ree, A.H.; Sandset, P.M. Estrogens, selective estrogen receptor modulators, and a selective estrogen receptor down-regulator inhibit endothelial production of tissue factor pathway inhibitor 1. BMC Cardiovasc Disord 2006, 6, 40. [Google Scholar] [CrossRef][Green Version]
- Rollin, J.; Iochmann, S.; Bléchet, C.; et al. Expression and methylation status of tissue factor pathway inhibitor-2 gene in nonsmall-cell lung cancer. Br J Cancer 2005, 92, 775–83. [Google Scholar] [CrossRef] [PubMed]
- Healton, C.G.; Gritz, E.R.; Davis, K.C.; et al. Women’s knowledge of the leading causes of cancer death. Nicotine Tob Res 2007, 9, 761–8. [Google Scholar] [CrossRef] [PubMed]
- Buist, D.S.; Newton, K.M.; Miglioretti, D.L.; et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol 2004, 104, 1042–50. [Google Scholar] [CrossRef] [PubMed]
- Samant, R.S.; Tucker, T.L. Analysis of cigarette smoking habits of cancer patients referred to the Northeastern Ontario Regional Cancer Centre. J Cancer Educ 2003, 18, 157–60. [Google Scholar] [CrossRef]
- Palomares, M.R.; Sayre, J.W.; Shekar, K.C.; Lillington, L.M.; Chlebowski, R.T. Gender influence on weight-loss pattern and survival of nonsmall cell lung carcinoma patients. Cancer 1996, 78, 2119–26. [Google Scholar] [CrossRef]
- Daly, E.; Vessey, M.P.; Hawkins, M.M.; Carson, J.L.; Gough, P.; Marsh, S. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996, 348, 977–80. [Google Scholar] [CrossRef]
- Fu, J.B.; Kau, T.Y.; Severson, R.K.; Kalemkerian, G.P. Lung cancer in women: analysis of the national Surveillance, Epidemiology, and End Results database. Chest 2005, 127, 768–77. [Google Scholar] [CrossRef]
- Canadian Cancer Society and the National Cancer Institute of Canada. Canadian Cancer Statistics 2008. Toronto: Canadian Cancer Society; 2008. Available online: www.cancer.ca/ Ontario/About%20cancer/Cancer%20statistics/~/media/CCS/Canada%20wide/Files%20List/English%20files%20 heading/pdf%20not%20in%20publications%20section/Canadian%20Cancer%20Society%20Statistics%20PDF%20 2008_614137951.ashx (accessed on 17 March 2009).
© 2009 by the author. Multimed Inc.
Share and Cite
Ayeni, O.; Robinson, A. Hormone Replacement Therapy and Outcomes for Women with Non-Small-Cell Lung Cancer: Can An Association be Confirmed? Curr. Oncol. 2009, 16, 21-25. https://doi.org/10.3747/co.v16i3.302
Ayeni O, Robinson A. Hormone Replacement Therapy and Outcomes for Women with Non-Small-Cell Lung Cancer: Can An Association be Confirmed? Current Oncology. 2009; 16(3):21-25. https://doi.org/10.3747/co.v16i3.302
Chicago/Turabian StyleAyeni, O., and Andrew Robinson. 2009. "Hormone Replacement Therapy and Outcomes for Women with Non-Small-Cell Lung Cancer: Can An Association be Confirmed?" Current Oncology 16, no. 3: 21-25. https://doi.org/10.3747/co.v16i3.302
APA StyleAyeni, O., & Robinson, A. (2009). Hormone Replacement Therapy and Outcomes for Women with Non-Small-Cell Lung Cancer: Can An Association be Confirmed? Current Oncology, 16(3), 21-25. https://doi.org/10.3747/co.v16i3.302




