Multisensory and Sensorimotor Integration in the Embodied Self: Relationship between Self-Body Recognition and the Mirror Neuron System
Abstract
:1. Introduction
2. Embodied Self
2.1. Sense of Body Ownership and Rubber Hand Illusion
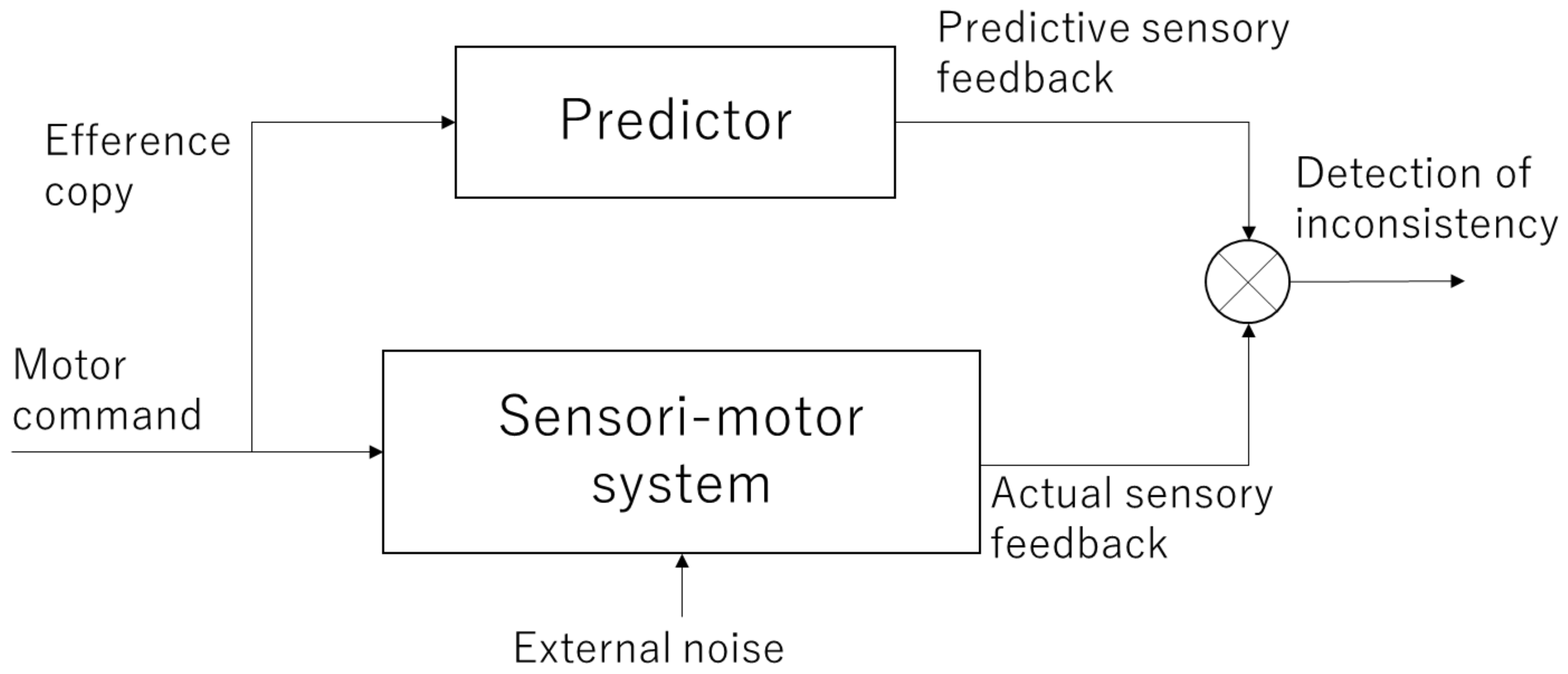
2.2. Sense of Agency and Forward Model
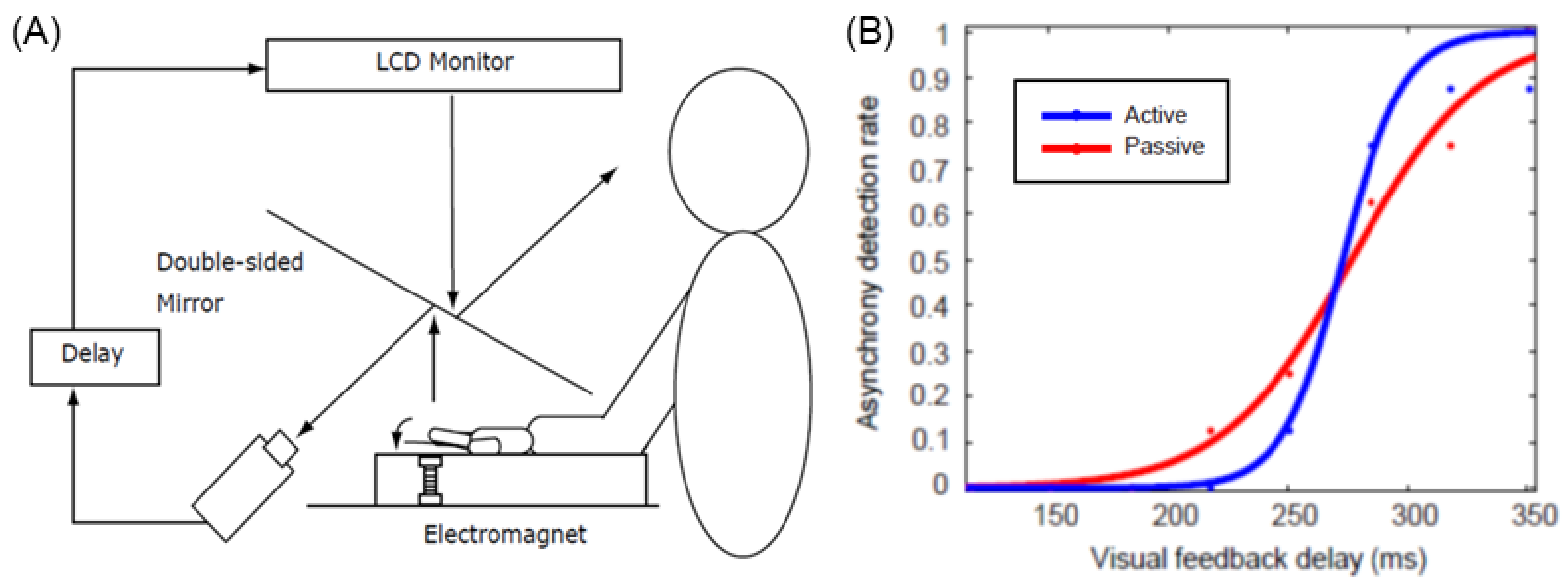
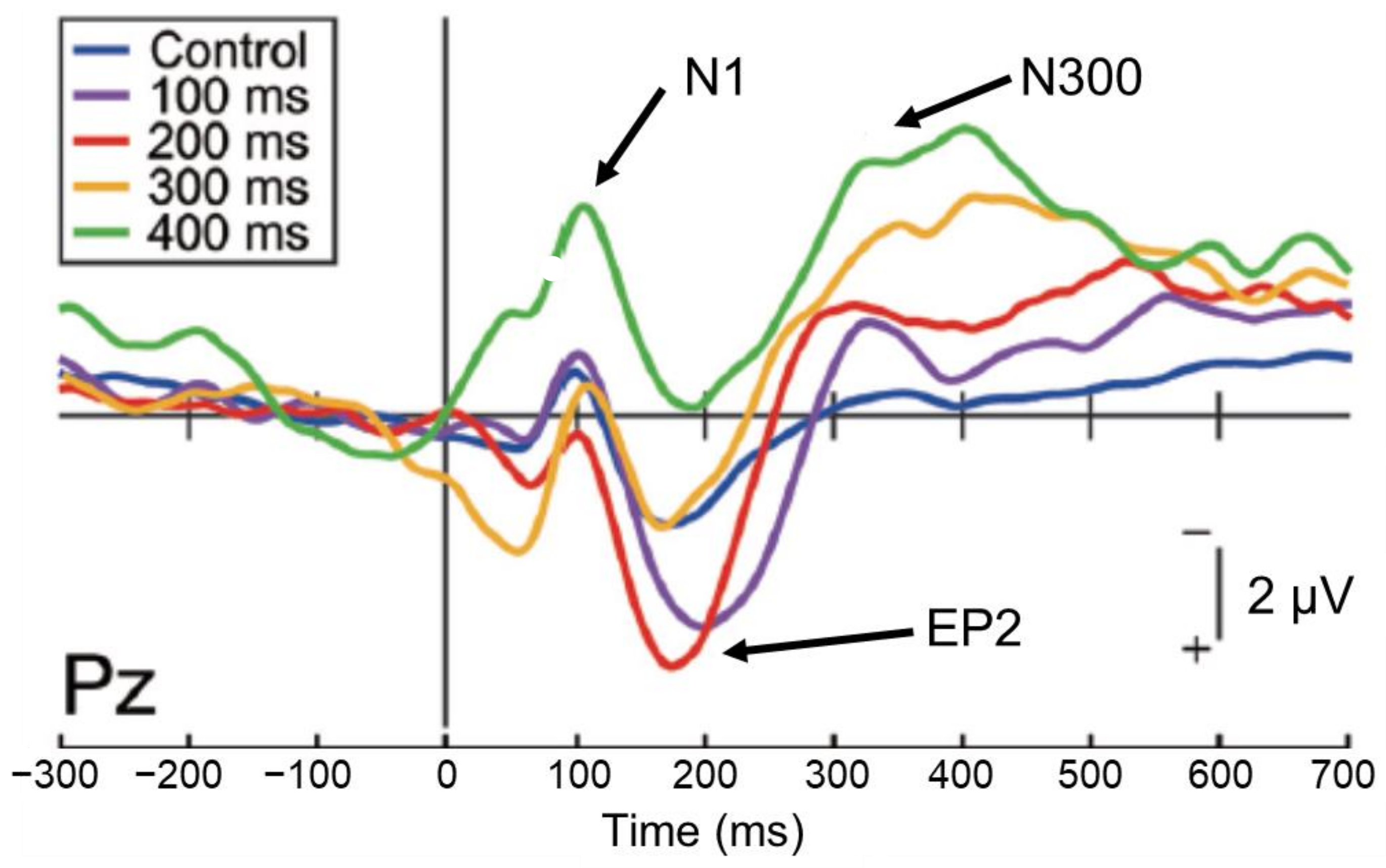
2.3. Implicit Measures of the Sense of Agency
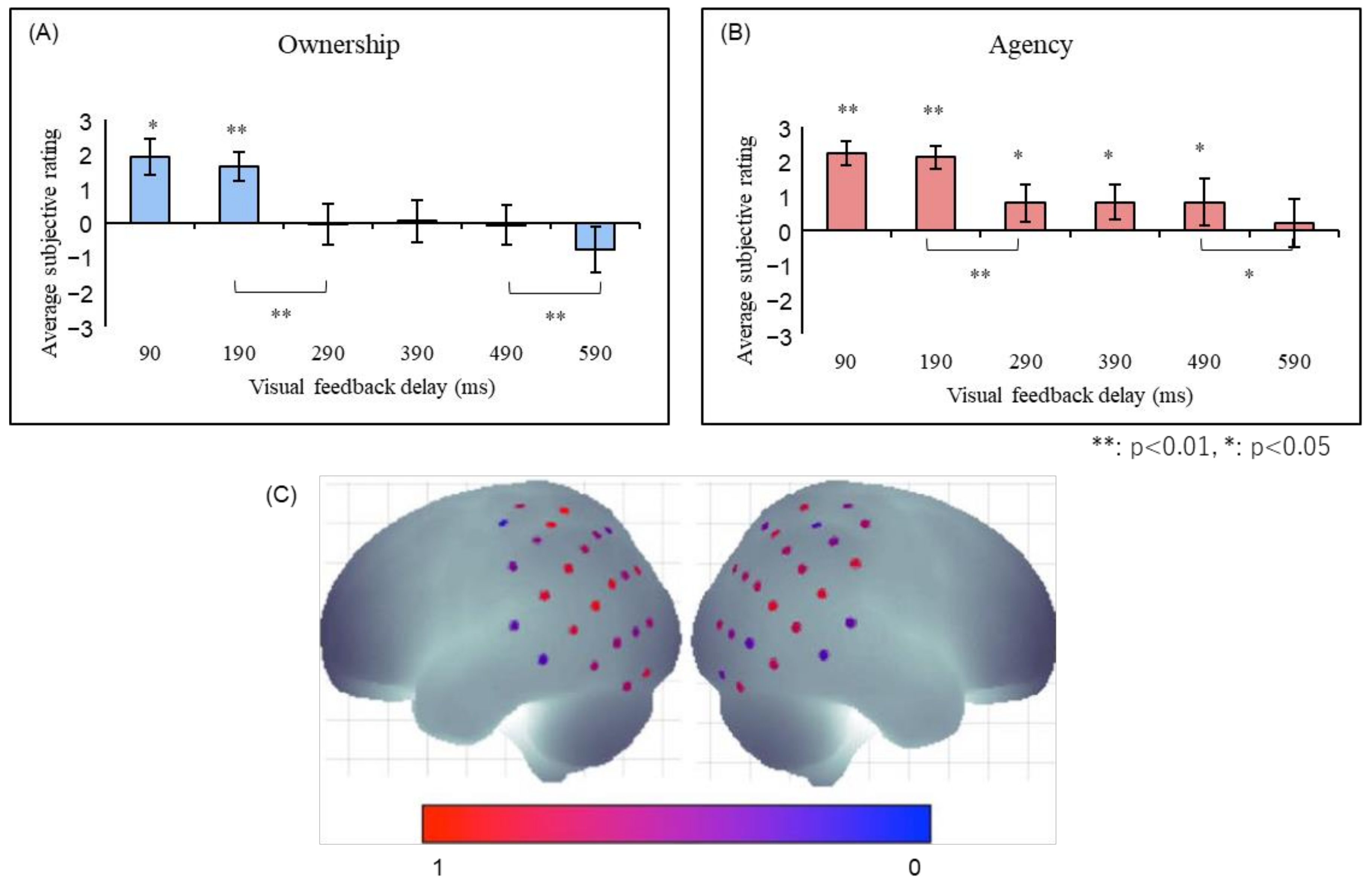
2.4. Robot Hand Illusion
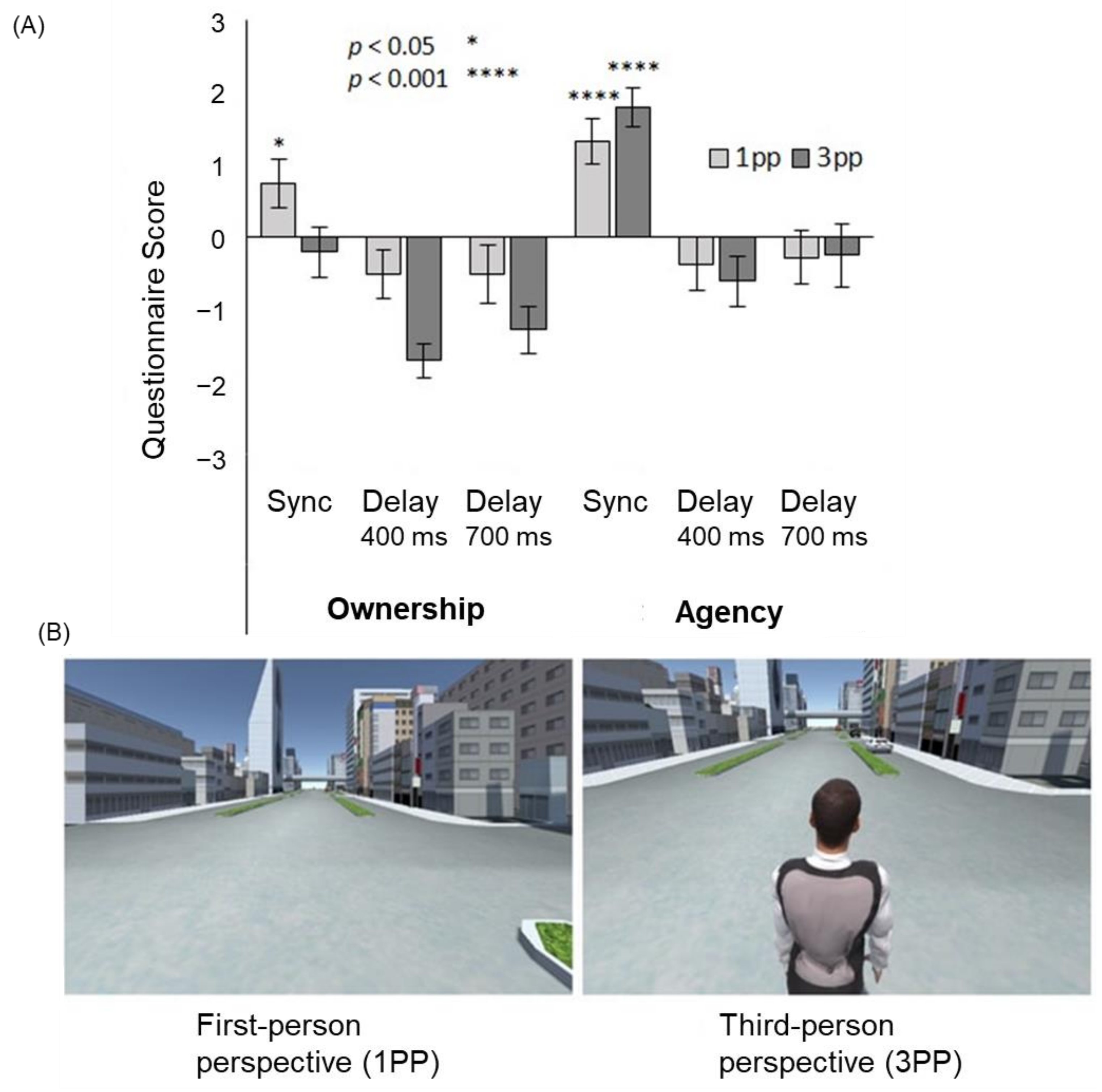
2.5. Full-Body Illusion
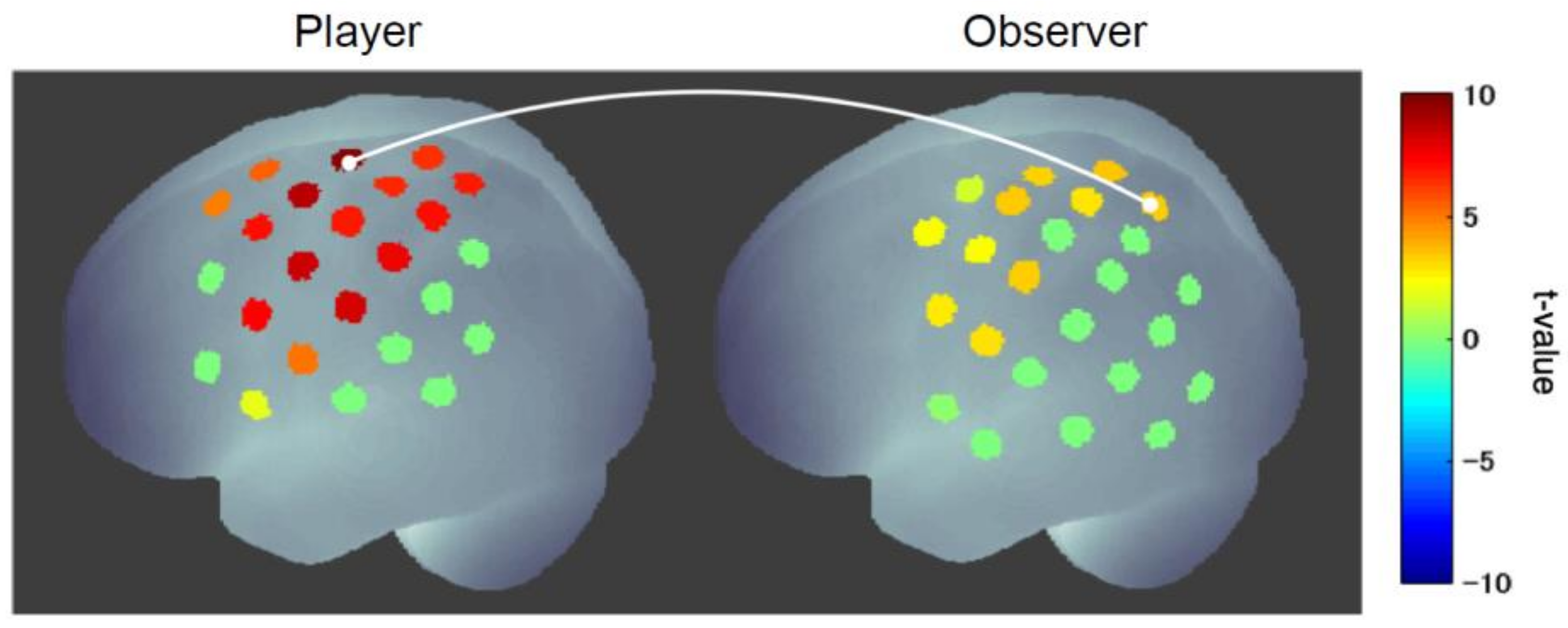
3. Back Projection and the Mirror Neuron System
3.1. Back Projection
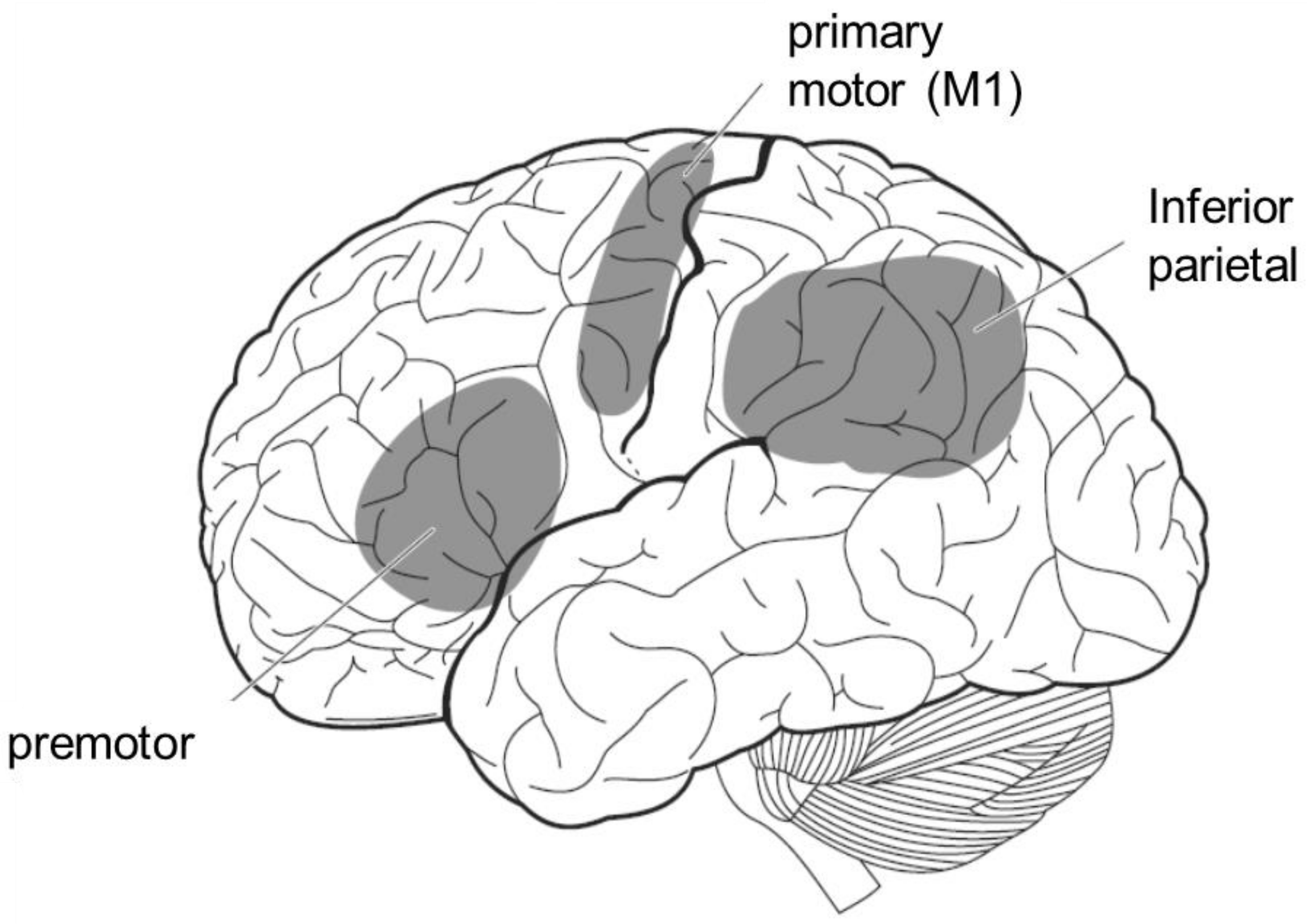
3.2. Mirror Neuron System and Body Image
3.3. Mirror Neuron System and Back-Projection
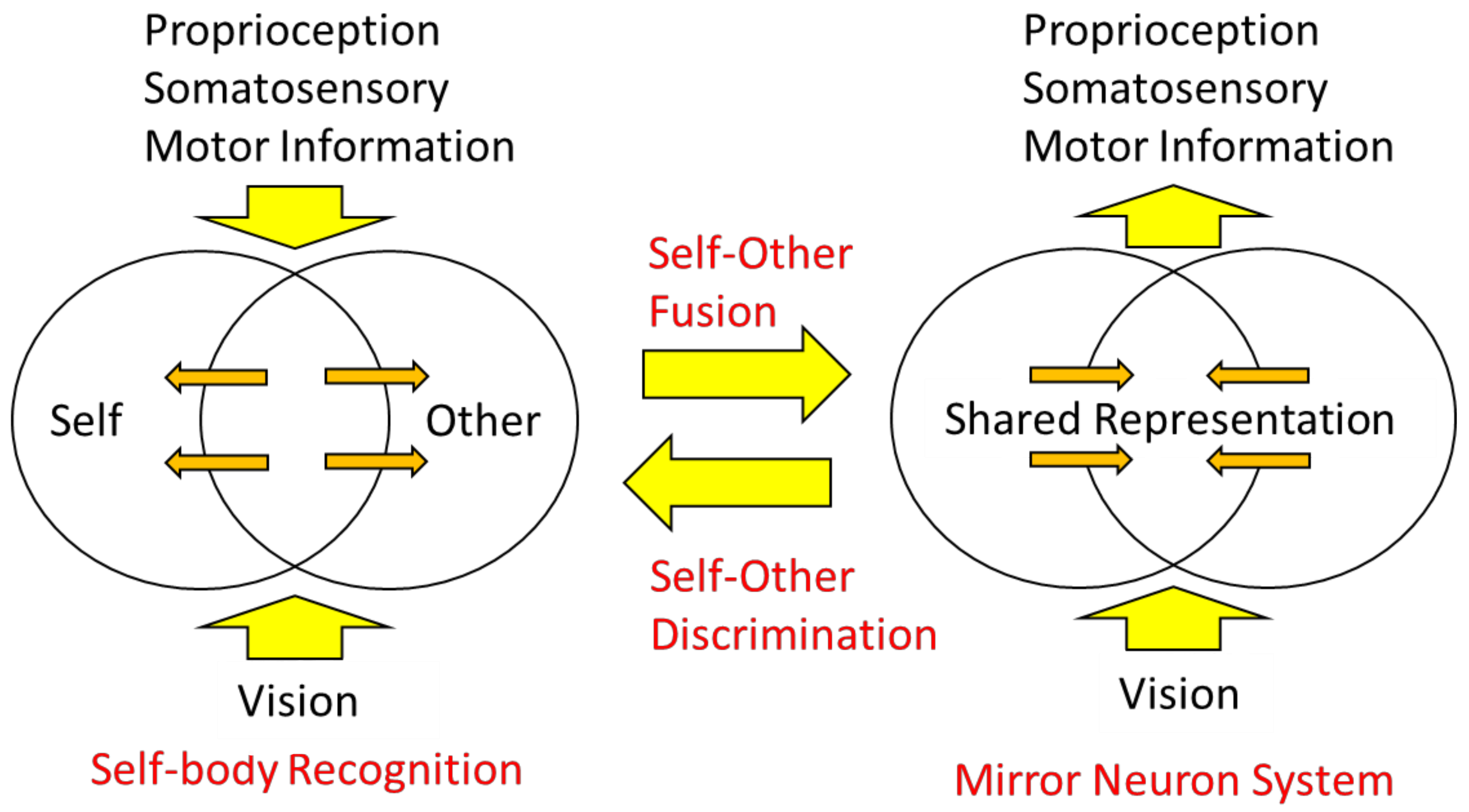
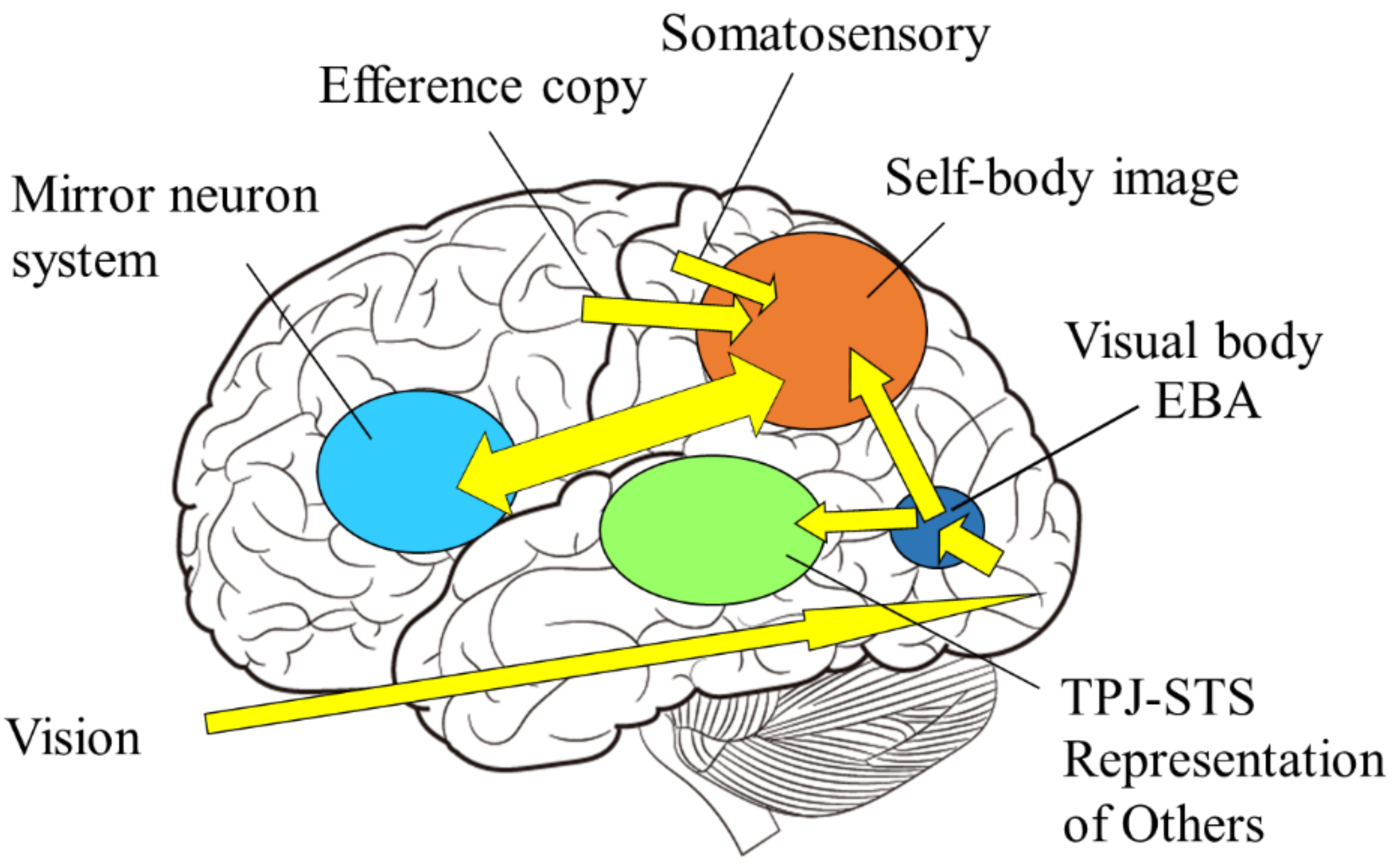
4. Multisensory and Sensorimotor Integration in the Embodied Self
Funding
Conflicts of Interest
References
- Gallagher, S. Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn. Sci. 2000, 4, 14–21. [Google Scholar] [CrossRef]
- Gallagher, S. How the Body Shapes the Mind; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Botvinick, M.; Cohen, J. Rubber hands ‘feel’ touch that eyes see. Nature 1998, 391, 756. [Google Scholar] [CrossRef] [PubMed]
- Armel, K.C.; Ramachandran, V.S. Projecting sensations to external objects: Evidence from skin conductance response. Proc. R. Soc. B Biol. Sci. 2003, 270, 1499–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrsson, H.H.; Spence, C.; Passingham, R.E. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 2004, 305, 875–877. [Google Scholar] [CrossRef] [Green Version]
- Shimada, S.; Fukuda, K.; Hiraki, K. Rubber hand illusion under delayed visual feedback. PLoS ONE 2009, 4, e6185. [Google Scholar] [CrossRef] [Green Version]
- Shimada, S.; Suzuki, T.; Yoda, N.; Hayashi, T. Relationship between sensitivity to visuotactile temporal discrepancy and the rubber hand illusion. Neurosci. Res. 2014, 85, 33–38. [Google Scholar] [CrossRef]
- Rohde, M.; Di Luca, M.; Ernst, M.O. The rubber hand illusion: Feeling of ownership and proprioceptive drift do not go hand in hand. PLoS ONE 2011, 6, e21659. [Google Scholar] [CrossRef] [Green Version]
- Gentile, G.; Guterstam, A.; Brozzoli, C.; Ehrsson, H.H. Disintegration of multisensory signals from the real hand reduces default limb self-attribution: An fMRI study. J. Neurosci. 2013, 33, 13350–13366. [Google Scholar] [CrossRef]
- Guterstam, A.; Collins, K.L.; Cronin, J.A.; Zeberg, H.; Darvas, F.; Weaver, K.E.; Ojemann, J.G.; Ehrsson, H.H. Direct electrophysiological correlates of body ownership in human cerebral cortex. Cereb. Cortex 2019, 29, 1328–1341. [Google Scholar] [CrossRef] [Green Version]
- Brozzoli, C.; Gentile, G.; Ehrsson, H.H. That’s near my hand! Parietal and premotor coding of hand-centered space contributes to localization and self-attribution of the hand. J. Neurosci. 2012, 32, 14573–14582. [Google Scholar] [CrossRef] [Green Version]
- Sperry, R.W. Neural basis of the spontaneous optokinetic response produced by visual inversion. J. Comp. Physiol. Psychol. 1950, 43, 482–489. [Google Scholar] [CrossRef]
- Miall, R.C.; Weir, D.J.; Wolpert, D.M.; Stein, J.F. Is the cerebellum a smith predictor? J. Mot. Behav. 1993, 25, 203–216. [Google Scholar] [CrossRef]
- Kawato, K. Internal models for motor control and trajectory planning. Curr. Opin. Neurobiol. 1999, 9, 718–727. [Google Scholar] [CrossRef]
- Daprati, E.; Franck, N.; Georgieff, N.; Proust, J.; Pacherie, E.; Dalery, J.; Jeannerod, M. Looking for the agent: An investigation into consciousness of action and self-consciousness in schizophrenic patients. Cognition 1997, 65, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Sirigu, A.; Daprati, E.; Pradat-Diehl, P.; Franck, N.; Jeannerod, M. Perception of self-generated movement following left pari-etal lesion. Brain 1999, 122, 1867–1874. [Google Scholar] [CrossRef]
- Tsakiris, M.; Haggard, P.; Franck, N.; Mainy, N.; Sirigu, A. A specific role for efferent information in self-recognition. Cognition 2005, 96, 215–231. [Google Scholar] [CrossRef]
- Shimada, S.; Qi, Y.; Hiraki, K. Detection of visual feedback delay in active and passive self-body movements. Exp. Brain Res. 2010, 201, 359–364. [Google Scholar] [CrossRef]
- Schafer, E.W.P.; Marcus, M.M. Self-stimulation alters human sensory brain responses. Science 1973, 181, 175–177. [Google Scholar] [CrossRef]
- Sato, A. Action observation modulates auditory perception of the consequence of others’ actions. Conscious. Cogn. 2008, 17, 1219–1227. [Google Scholar] [CrossRef]
- Blakemore, S.-J.; Frith, C.D.; Wolpert, D.M. Spatio-temporal prediction modulates the perception of self-produced stimuli. J. Cogn. Neurosci. 1999, 11, 551–559. [Google Scholar] [CrossRef]
- Beck, B.; Di Costa, S.; Haggard, P. Having control over the external world increases the implicit sense of agency. Cognition 2017, 162, 54–60. [Google Scholar] [CrossRef]
- Weller, L.; Schwarz, K.A.; Kunde, W.; Pfister, R. Was it me?—Filling the interval between action and effects increases agency but not sensory attenuation. Biol. Psychol. 2017, 123, 241–249. [Google Scholar] [CrossRef]
- Toida, K.; Ueno, K.; Shimada, S. Neural basis of the time window for subjective motor-auditory integration. Front. Hum. Neurosci. 2016, 9, 688. [Google Scholar] [CrossRef] [Green Version]
- Haggard, P.; Clark, S.; Kalogeras, J. Voluntary action and conscious awareness. Nat. Neurosci. 2002, 5, 382–385. [Google Scholar] [CrossRef]
- Haggard, P. Sense of agency in the human brain. Nat. Rev. Neurosci. 2017, 18, 196–207. [Google Scholar] [CrossRef]
- Spence, S.A.; Brooks, D.; Hirsch, S.R.; Liddle, P.F.; Meehan, J.; Grasby, P.M. A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control). Brain 1997, 120, 1997–2011. [Google Scholar] [CrossRef]
- Farrer, C.; Franck, N.; Georgieff, N.; Frith, C.D.; Decety, J.; Jeannerod, M.Z. Modulating the experience of agency: A positron emission tomography study. NeuroImage 2003, 18, 324–333. [Google Scholar] [CrossRef]
- Farrer, C.; Frey, S.H.; van Horn, J.D.; Tunik, E.; Turk, D.; Inati, S.; Grafton, S.T. The angular gyrus computes action awareness representations. Cereb. Cortex 2008, 18, 254–261. [Google Scholar] [CrossRef]
- Yomogida, Y.; Sugiura, M.; Sassa, Y.; Wakusawa, K.; Sekiguchi, A.; Fukushima, A.; Takeuchi, H.; Horie, K.; Sato, S.; Kawashima, R. The neural basis of agency: An fMRI study. NeuroImage 2010, 50, 198–207. [Google Scholar] [CrossRef]
- Zito, G.A.; Wiest, R.; Aybek, S. Neural correlates of sense of agency in motor control: A neuroimaging meta-analysis. PLoS ONE 2020, 15, e0234321. [Google Scholar] [CrossRef]
- Balslev, D.; Nielsen, F.; Lund, T.E.; Law, I.; Paulson, O.B. Similar brain networks for detecting visuo-motor and visuo-proprioceptive synchrony. NeuroImage 2006, 31, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M.; Longo, M.R.; Haggard, P. Having a body versus moving your body: Neural signatures of agency and body-ownership. Neuropsychologia 2010, 48, 2740–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, S.; Hiraki, K.; Oda, I. The parietal role in the sense of self-ownership with temporal discrepancy between visual and proprioceptive feedbacks. NeuroImage 2005, 24, 1225–1232. [Google Scholar] [CrossRef]
- Damasio, A. Self Comes to Mind: Constructing the Conscious Brain; Pantheon/Random House: New York, NY, USA, 2010. [Google Scholar]
- Hackley, S.A.; Hirao, T.; Onoda, K.; Ogawa, K.; Masaki, H. Anterior insula activity and the effect of agency on the stimulus-preceding negativity. Psychophysiology 2019, 57, e13519. [Google Scholar] [CrossRef] [PubMed]
- Seghezzi, S.; Zirone, E.; Paulesu, E.; Zapparoli, L. The brain in (willed) actin: A meta-analytical comparison of imaging studies on motor intentionality and sense of agency. Front. Psychol. 2019, 10, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seghezzi, S.; Giannini, G.; Zapparoli, L. Neurofunctional correlates of body-ownership and sense of agency: A meta-analytical account of self-consciousness. Cortex 2019, 121, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ohata, R.; Asai, T.; Kadota, H.; Shigemasu, H.; Ogawa, K.; Imamizu, H. Sense of agency beyond sensorimotor process: De-coding self-other action attribution in the human brain. Cereb. Cortex 2020, 30, 4076–4091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspar, E.A.; Cleeremans, A.; Haggard, P. The relationship between human agency and embodiment. Conscious. Cogn. 2015, 33, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.A.F.; Shimada, S. ‘Robot’ hand illusion under delayed visual feedback: Relationship between the senses of ownership and agency. PLoS ONE 2016, 11, e0159619. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.A.F.; Shimada, S. Inferior parietal lobe activity in visuo-motor integration during robot hand illusion. Psychology 2018, 9, 2996–3006. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.A.F.; Shimada, S. Activity of the inferior parietal cortex is modulated by visual feedback delay in robot hand illusion. Sci. Rep. 2019, 9, 10030. [Google Scholar] [CrossRef]
- Ehrsson, H.H. The experimental induction of out-of-body experiences. Science 2007, 317, 1048. [Google Scholar] [CrossRef] [Green Version]
- Lenggenhager, B.; Tadi, T.; Metzinger, T.; Blanke, O. Video ergo sum: Manipulating bodily self-consciousness. Science 2007, 317, 1096–1099. [Google Scholar] [CrossRef] [Green Version]
- Ionta, S.; Heydrich, L.; Lenggenhager, B.; Mouthon, M.; Fornari, E.; Chapuis, D.; Gassert, R.; Blanke, O. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron 2011, 70, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Gentile, G.; Björnsdotter, M.; Petkova, V.I.; Abdulkarim, Z.; Ehrsson, H.H. Patterns of neural activity in the human ventral premotor cortex reflect a whole-body multisensory percept. NeuroImage 2015, 109, 328–340. [Google Scholar] [CrossRef] [Green Version]
- Blanke, O.; Metzinger, T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn. Sci. 2009, 13, 7–13. [Google Scholar] [CrossRef]
- Blanke, O.; Ortigue, S.; Landis, T.; Seeck, M. Stimulating illusory own-body perceptions. Nature 2002, 419, 269–270. [Google Scholar] [CrossRef]
- Zeev-Wolf, M.; Dor-Ziderman, Y.; Gldstein, A.; Bonne, O.; Abramowitz, E.G. Oscillatory brain mechanisms of the hypnotically-induced out-of-body experience. Cortex 2017, 96, 19–30. [Google Scholar] [CrossRef]
- Otsuka, K.; Shimada, S. Effects of delayed visual feedback on the full-body illusion in a VR environment. Intelligence and information. J. Jpn. Soc. Fuzzy Intell. Inf. 2021, 33, 657–662. (In Japanese) [Google Scholar]
- Osumi, M.; Imai, R.; Ueta, K.; Nobusako, S.; Morioka, S. Negative body image associated with changes in visual body appearance increases pain perception. PLoS ONE 2014, 9, e107376. [Google Scholar] [CrossRef] [Green Version]
- Kanaya, S.; Matsushima, Y.; Yokosawa, K. Does seeing ice really feel cold? Visual-thermal interaction under an illusory body-ownership. PLoS ONE 2012, 7, e47293. [Google Scholar] [CrossRef]
- Shibuya, S.; Unenaka, S.; Zama, T.; Shimada, S.; Ohki, Y. Spontaneous imitative movements induced by an illusory embodied fake hand. Neuropsychologia 2018, 111, 77–84. [Google Scholar] [CrossRef]
- Giroux, M.; Barra, J.; Zrelli, I.E.; Barraud, P.A.; Cian, C.; Guerraz, M. The respective contributions of visual and proprioceptive afferents to the mirror illusion in virtual reality. PLoS ONE 2018, 13, e0203086. [Google Scholar] [CrossRef] [Green Version]
- Metral, M.; Guerraz, M. The fake hand in movement: Visual motion cues from the rubber hand are processed for the purpose of kinsthesia. Conscious. Cogn. 2019, 73, 102761. [Google Scholar] [CrossRef]
- Barra, J.; Giroux, M.; Metral, M.; Cian, C.; Luyat, M.; Kavounoudias, A.; Guerraz, M. Functional properties of extended body representations in the context of kinesthesia. Neurophysiol. Clin. 2020, 50, 455–465. [Google Scholar] [CrossRef]
- Moseley, G.L.; Parsons, T.J.; Spence, C. Visual distortion of a limb modulates the pain and swelling evoked by movement. Curr. Biol. 2008, 18, R1047–R1048. [Google Scholar] [CrossRef] [Green Version]
- Matamala-Gomez, M.; Gonzalez, A.M.D.; Slater, M.; Sanchez-Vives, M.V. Decreasing pain ratings in chronic arm pain through changing a virtual body: Different strategies for different pain types. J. Pain 2019, 20, 685–697. [Google Scholar] [CrossRef]
- Preston, C.; Gilpin, H.R.; Newport, R. An exploratory investigation into the longevity of pain reduction following multi-sensory illusions designed to alter body perception. Musculoskelet. Sci. Pract. 2020, 45, 102080. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Brang, D. Sensations evoked in patients with amputation from watching an individual whose corresponding intact limb is being touched. Arch. Neurol. 2009, 66, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzolatti, G.; Fogassi, L.; Gallese, V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001, 2, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Sinigaglia, C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 2010, 11, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemmerer, D. What modulates the mirror neuron system during action observation? Multiple factors involving the action, the actor, the observer, the resationship between actor and observer, and the context. Prog. Neurobiol. 2021, 205, 102128. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Merino, B.; Glaser, D.; Grèzes, J.; Passingham, R.; Haggard, P. Action observation and acquired motor skills: An fMRI study with expert dancers. Cereb. Cortex 2005, 15, 1243–1249. [Google Scholar] [CrossRef] [Green Version]
- Umiltà, M.A.; Kohler, E.; Gallese, V.; Fogassi, L.; Fadiga, L.; Keysers, C.; Rizzolatti, G. I know what you are doing. A neuro-physiological study. Neuron 2001, 31, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Iacoboni, M.; Molnar-Szakacs, I.; Gallese, V.; Buccino, G.; Mazziotta, J.C.; Rizzolatti, G. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 2005, 3, e79. [Google Scholar] [CrossRef] [Green Version]
- Umiltà, M.A.; Escola, L.; Intskirveli, I.; Grammont, F.; Rochat, M.; Caruana, F.; Jezzini, A.; Gallese, V.; Rizzolatti, G. When pliers become fingers in the monkey motor system. Proc. Natl. Acad. Sci. USA 2008, 105, 2209–2213. [Google Scholar] [CrossRef] [Green Version]
- Iacoboni, M.; Woods, R.P.; Brass, M.; Bekkering, H.; Mazziotta, J.C.; Rizzolatti, G. Cortical mechanisms of human imitation. Science 1999, 286, 2526–2528. [Google Scholar] [CrossRef] [Green Version]
- Vogt, S.; Buccino, G.; Wohlschläger, A.M.; Canessa, N.; Shah, N.J.; Zilles, K.; Eickhoff, S.B.; Freund, H.J.; Rizzolatti, G.; Fink, G.R. Pre-frontal involvement in imitation learning of hand actions: Effects of practice and expertise. NeuroImage 2007, 36, 1371–1383. [Google Scholar] [CrossRef] [Green Version]
- Shimada, S.; Abe, R. Modulation of the motor area activity during observation of a competitive game. NeuroReport 2009, 20, 979–983. [Google Scholar] [CrossRef]
- Shimada, S.; Abe, R. Outcome and view of the player modulate motor area activity during observation of a competitive game. Neuropsychologia 2010, 48, 1930–1934. [Google Scholar] [CrossRef]
- Shimada, S.; Matsumoto, M.; Takahashi, H.; Yomogida, Y.; Matsumoto, K. Coordinated activation of premotor and ventromedial prefrontal cortices during vicarious reward. Soc. Cogn. Affect. Neurosci. 2016, 11, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Koide, T.; Shimada, S. Cheering enhances inter-brain synchronization between sensorimotor areas of player and observer. Jpn. Psychol. Res. 2018, 60, 265–275. [Google Scholar] [CrossRef]
- Downing, P.E.; Jiang, Y.; Shuman, M.; Kanwisher, N. A cortical area selective for visual processing of the human body. Science 2001, 293, 2470–2473. [Google Scholar] [CrossRef]
| Experiment | Function | Brain Region |
|---|---|---|
| RHI | SoO | premotor [5,9,10,11] |
| Proprioceptive drift | IPL [11] | |
| SoA | Sensory attenuation | sensory (auditory) cortex [19,20,24] |
| Comparator | IPL, TPJ [28,29,30,31,32,33] | |
| RoHI | SoO & SoA | IPL [42,43] |
| FBI | Out-of-body experience | TPJ [48,49,50] |
| MNS | Action observation | IPL, SPL, premotor, M1 [62,63,64,65,66,67,68,69,70,71,72,73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, S. Multisensory and Sensorimotor Integration in the Embodied Self: Relationship between Self-Body Recognition and the Mirror Neuron System. Sensors 2022, 22, 5059. https://doi.org/10.3390/s22135059
Shimada S. Multisensory and Sensorimotor Integration in the Embodied Self: Relationship between Self-Body Recognition and the Mirror Neuron System. Sensors. 2022; 22(13):5059. https://doi.org/10.3390/s22135059
Chicago/Turabian StyleShimada, Sotaro. 2022. "Multisensory and Sensorimotor Integration in the Embodied Self: Relationship between Self-Body Recognition and the Mirror Neuron System" Sensors 22, no. 13: 5059. https://doi.org/10.3390/s22135059





