Single Dense Layer of PEO Coating on Aluminum Fabricated by “Chain-like” Discharges
Abstract
:1. Introduction
2. Materials and Methods
2.1. PEO Preparation
2.2. Characterization and Analysis
3. Results and Discussion
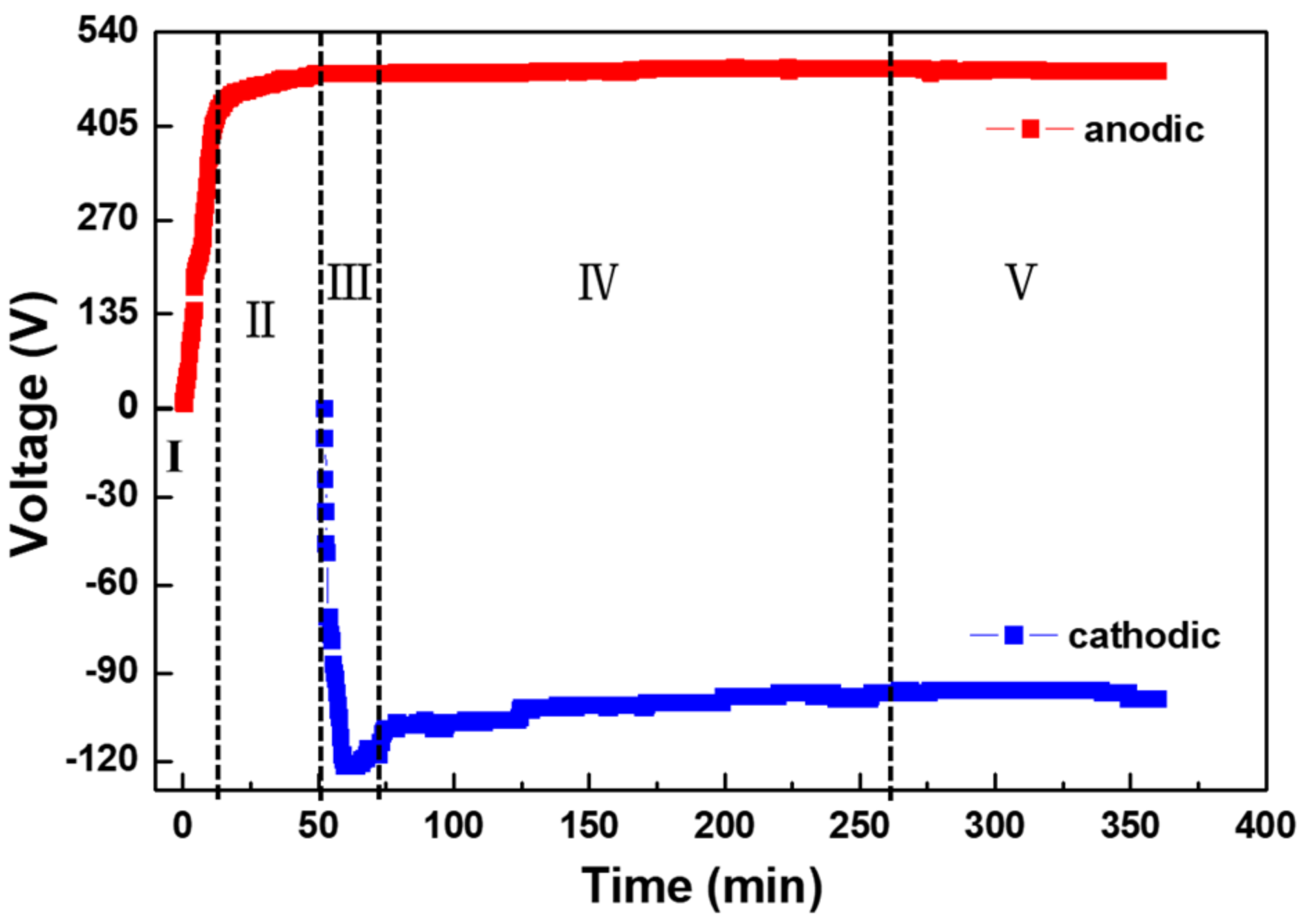
3.1. Voltage–Time Curves and Discharge Evolution of Aluminum
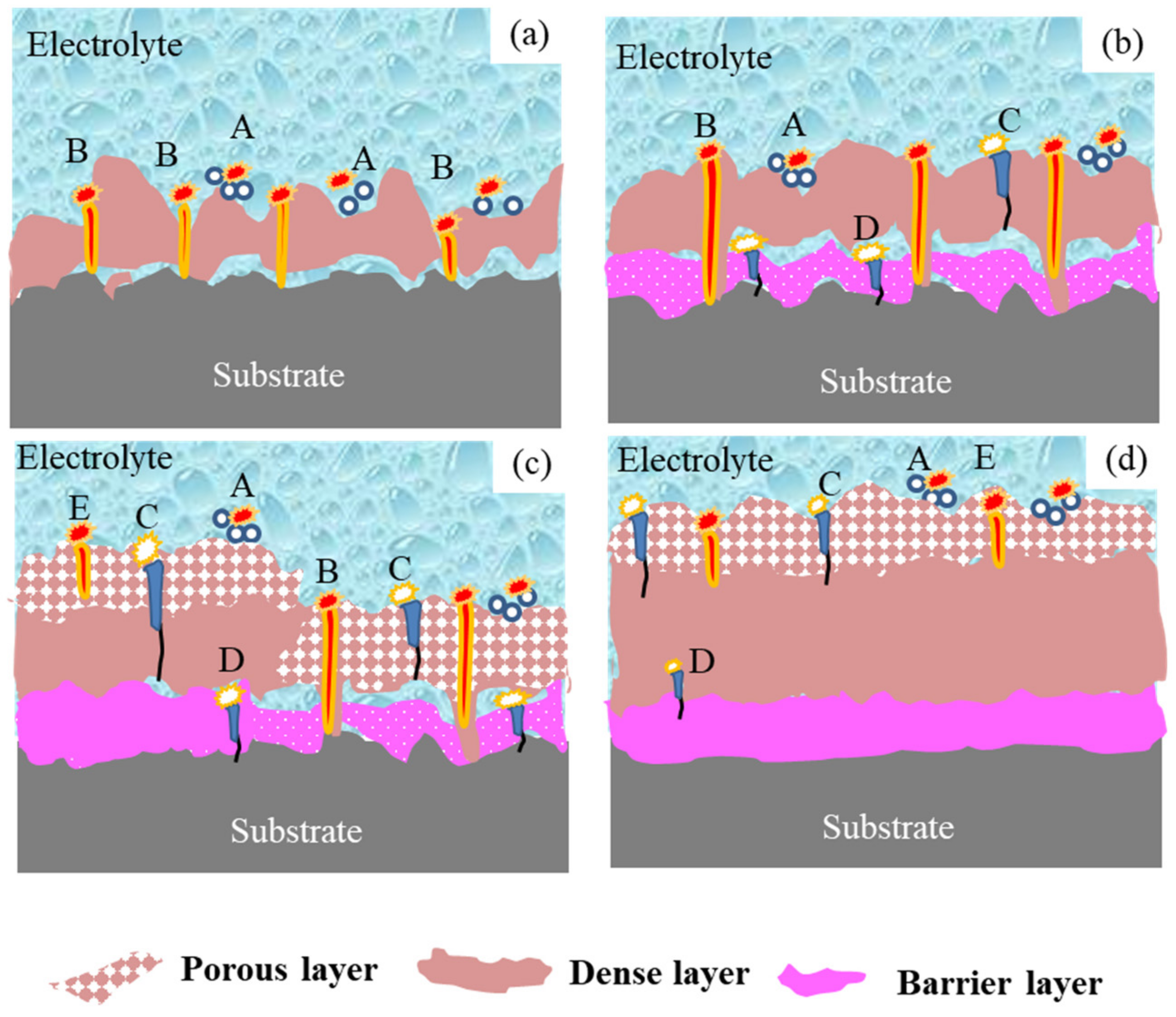
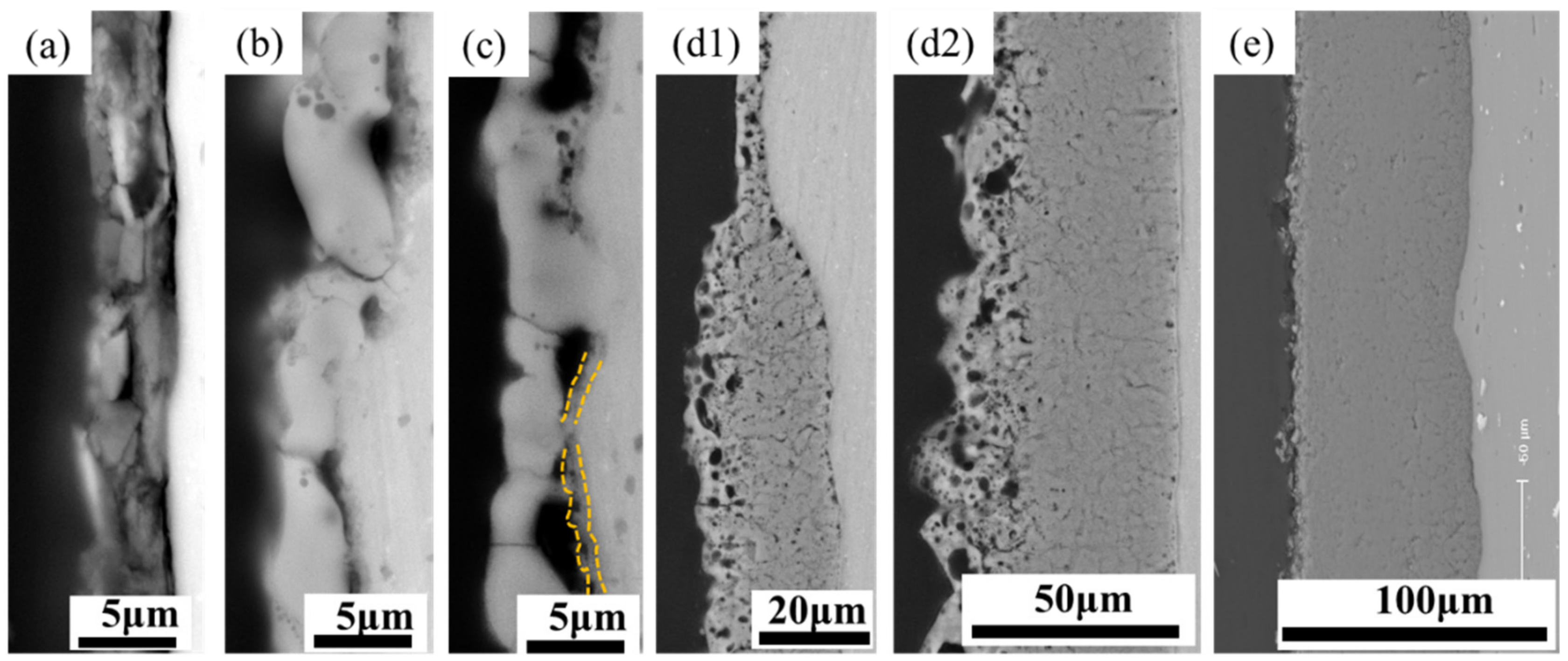
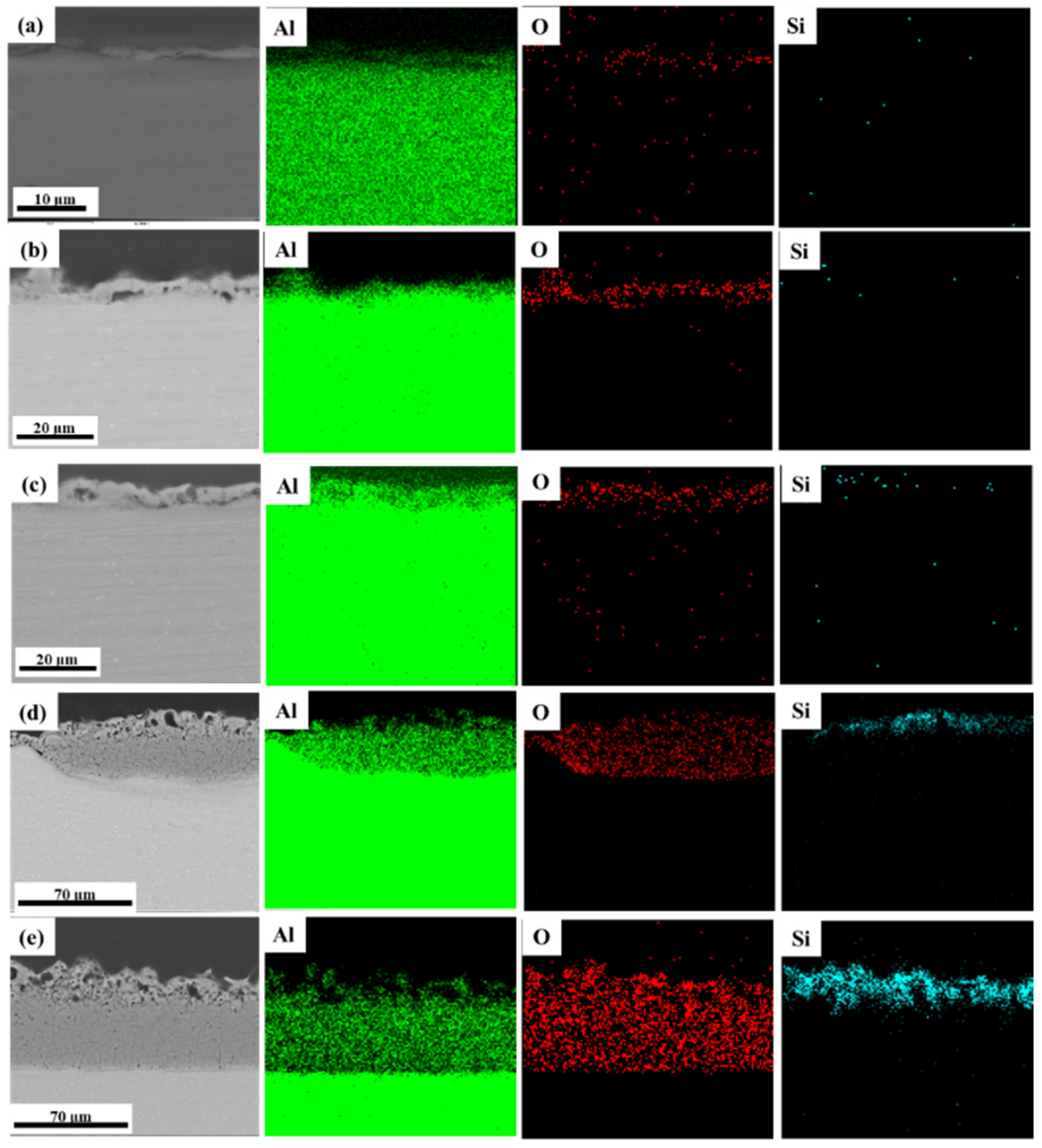
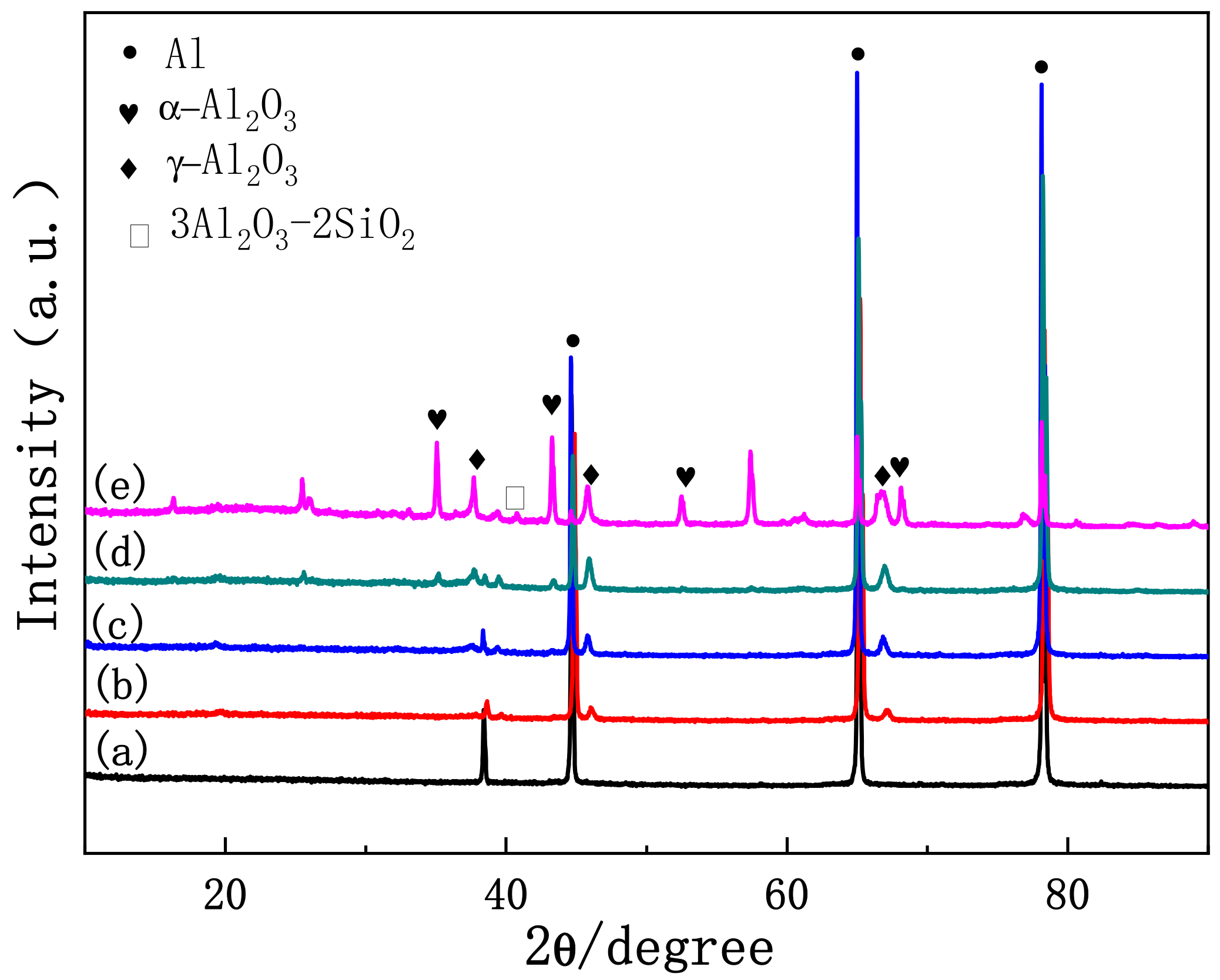
3.2. Effect of Discharge Evolution on Microstructure of PEO Coating at Different Stages
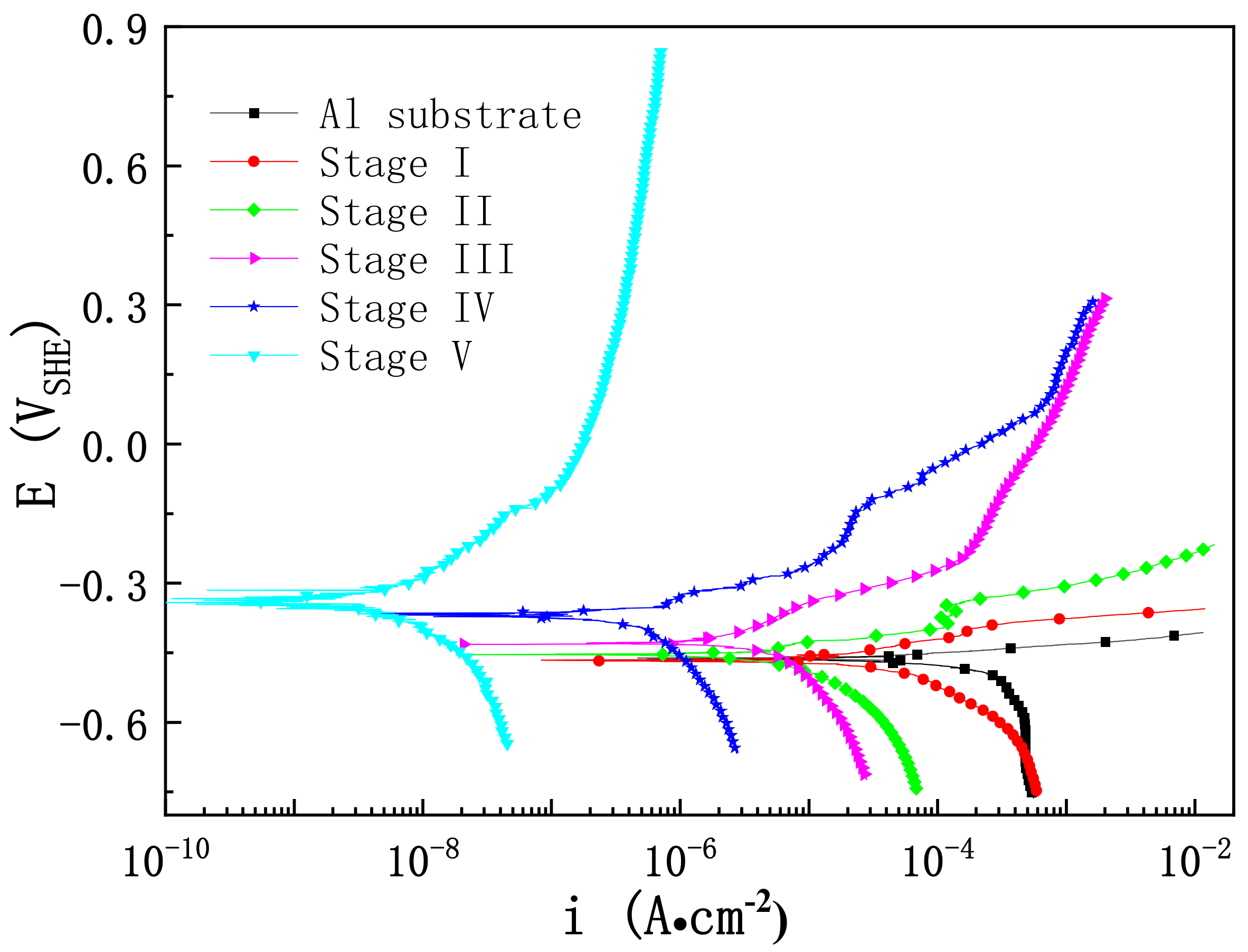
3.3. Impact of Microstructure on the Corrosion Resistance of PEO Coating at Different Stages
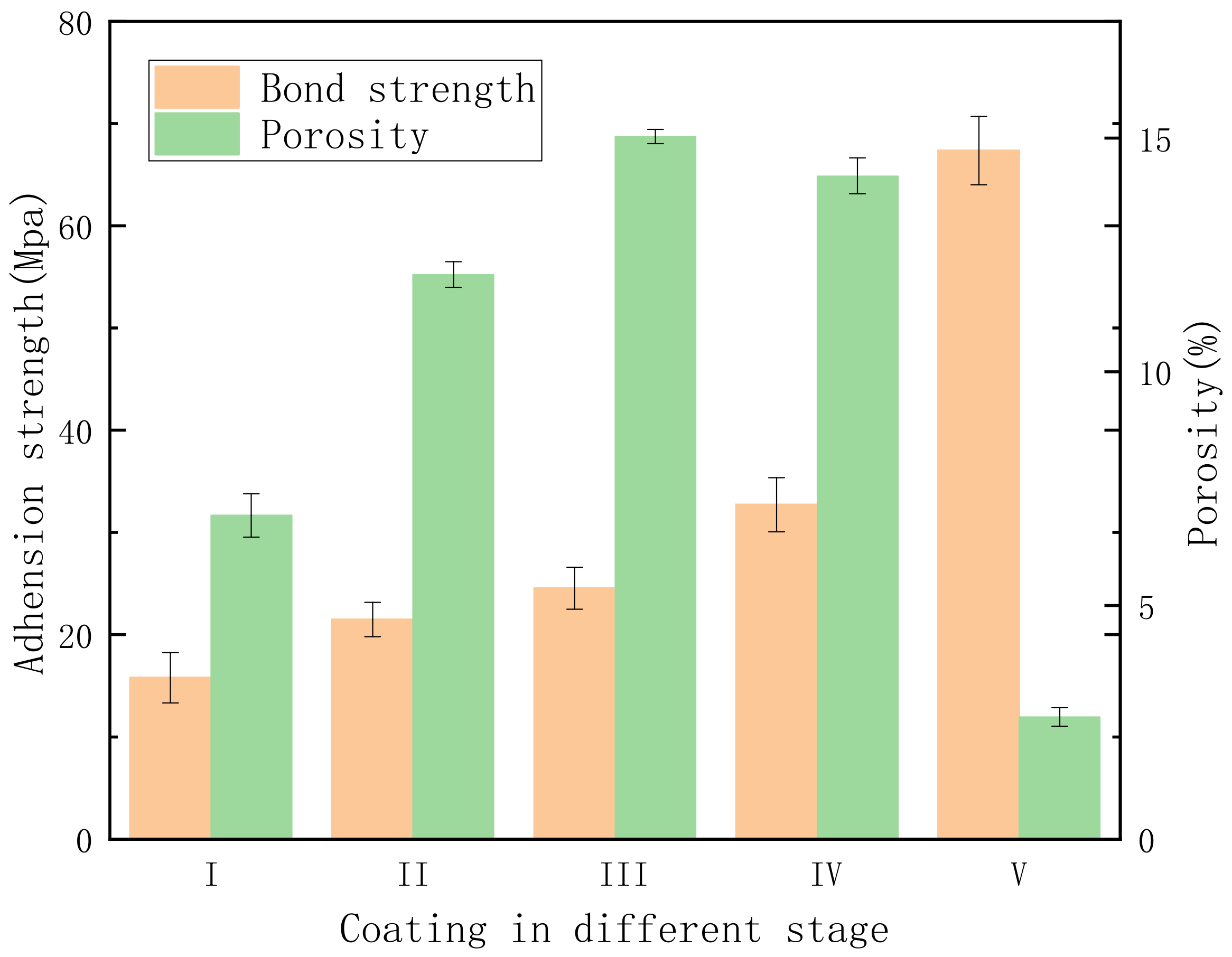
3.4. Effect of Microstructure and Phase Composition on the Mechanical Properties of PEO Coating at Different Stages
4. Conclusions
- (1)
- According to the voltage–time curve and the evolution of the plasma spark discharge state under the conditions of unipolar + bipolar (DC + AC) pulses, the PEO process comprises five stages: the anodic oxidation stage (I), the microarc discharge stage (II), the soft-spark stage (III), the transformation stage (IV), and the “chain-like” discharge stage (V).
- (2)
- The plasma spark discharge state affects the microstructure and phase composition of the PEO coating. The morphology of the PEO coating changes across the entire PEO process: stage I comprises a single layer of stacked insulating film; a layer of molten oxide coating forms in stage II; during stage III, a barrier layer forms, and the coating presents a two-layer structure; the dense layer thickens considerably after the transformation stage (IV); and the “chain-like” discharge stage (V) produces a single dense layer with few defects. The diffraction peak intensities of the γ-Al2O3, α-Al2O3, and 3Al2O3 · 2SiO2 phases increases as the PEO treatment duration increases.
- (3)
- The type of plasma spark discharge affects the coating formation mechanism: type-A discharge represents gas discharge, which encourages the solution components to participate in oxidation reactions; type-B discharge demonstrates a high intensity, which effectively dissolves the matrix and releases a large amount of gas; type-C and -D discharge reduce the defects in the barrier layer and dense inner layer; and type-E discharge melts and condenses the loose external layer. Unlike traditional plasma spark discharge, “chain-like” discharge mainly cooperates with discharge types A, C, D, and E. The “chain-like” discharge moves continuously over the whole sample, which effectively reduces the defects and increases the uniformity of the coating, substantially improving the corrosion resistance and mechanical properties of the PEO coating.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, D.D.; Liu, X.T.; Wu, Y.K.; Han, H.P.; Yang, Z.; Su, Y.; Zhang, X.Z.; Wu, G.R.; Shen, D.J. Evolution process of the plasma electrolytic oxidation (PEO) coating formed on aluminum in an alkaline sodium hexametaphosphate ((NaPO3)6) electrolyte. J. Alloy. Compd. 2019, 798, 129–143. [Google Scholar] [CrossRef]
- Wang, H.B.; Fang, Z.G.; Jiang, B.L. Microarc Oxidation Technology and Its Application in Sea Environments; National Defense Industry Press: Beijing, China, 2010; pp. 1–316. [Google Scholar]
- Ogi, H.; Shimoike, G.; Hirao, M.; Takashima, K.; Higo, Y. Anisotropic elastic-stiffness coefficients of an amorphous ni–p film. J. Appl. Phycol. 2002, 91, 4857–4862. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chen, C.M.; Meng, L.; Jie, Y.F.; Sheng, J.H. pH’s effection to electroplating ni-sic plating on aluminum alloy. Adv. Mat. Res. 2011, 189–193, 355–358. [Google Scholar] [CrossRef]
- Terek, P.; Kovaevi, L.; Miletic, A.; Kori, B.; Drnovek, A. Metallurgical soldering of duplex CrN coating in contact with aluminum alloy. Coatings 2020, 10, 303. [Google Scholar] [CrossRef] [Green Version]
- Kai, Z.; Wu, J.; Chu, P.; Ge, Y.; Li, X. A novel CVD method for rapid fabrication of superhydrophobic surface on aluminum alloy coated nanostructured cerium-oxide and its corrosion resistance. Int. J. Electrochem. 2015, 10, 6257–6272. [Google Scholar]
- Liu, W.; Luo, Y.; Sun, L.; Wu, R.; Liu, Y. Fabrication of the superhydrophobic surface on aluminum alloy by anodizing and polymeric coating. Appl. Surf. Sci. 2013, 264, 872–878. [Google Scholar] [CrossRef]
- Egorkina, V.S.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Vyaliy, I.E.; Gnedenkov, A.S.; Chizhikov, R.G. Increasing thickness and protective properties of peo-coatings on aluminum alloy. Surf. Coat. Tech. 2018, 334, 29–42. [Google Scholar] [CrossRef]
- Qi, X.; Jiang, B.; Song, R. Effects of ageing treatment on corrosion behavior of 7075 aluminum alloy coated by micro arc oxidation (MAO). Corrs. Sci. 2022, 199, 110–164. [Google Scholar] [CrossRef]
- Alsrayheen, E.; Mcleod, E.; Rateick, R.; Molero, H.; Birss, V. Impact of ac/dc spark anodizing on the corrosion resistance of Al–Cu alloys. Electrochim. Acta 2011, 56, 6041–6048. [Google Scholar] [CrossRef]
- Cao, J.; Fang, Z.; Chen, J.; Chen, Z.; Yin, W.; Yang, Y.; Zhang, W. Preparation and properties of Micro-arc Oxide film with single dense layer on surface of 5083 aluminum alloy. J. Chin. Soc. Corr. Pro. 2020, 40, 251–258. [Google Scholar]
- Liang, J.; Hu, L.; Hao, J. Characterization of microarc oxidation coatings formed on AM60B magnesium alloy in silicate and phosphate electrolytes. Appl. Surf. Sci. 2007, 253, 4490–4496. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Imshinetskiy, I.M.; Puz’, A.V. Plasma electrolytic oxidation of the magnesium alloy MA8 in electrolytes containing TiN nanoparticles. J. Mater. Sci. Technol. 2017, 33, 461–468. [Google Scholar] [CrossRef]
- Lee, K.M.; Ko, Y.G.; Shin, D.H. Incorporation of carbon nanotubes into micro-coatings film formed on aluminum alloy via plasma electrolytic oxidation. Mater. Lett. 2011, 65, 2269–2273. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Investigation of the growth processes of coatings formed by AC plasma electrolytic oxidation of aluminium. Electrochim. Acta 2009, 54, 6767–6778. [Google Scholar] [CrossRef]
- Du, K.Q.; Guo, X.H.; Guo, Q.Z.; Wang, F.H.; Tian, Y. A monolayer PEO coating on 2024 al alloy by transient self-feedback control mode. Mater. Lett. 2013, 91, 45–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Yekang, W.; Chena, D.; Wang, R.; Li, D.; Guo, C.; Jiang, G.; Shen, D.; Yu, S.; Nash, P. Micro-structures and growth mechanisms of plasma electrolytic oxidation coatings on aluminium at different current densities. Surf. Coat. Technol. 2017, 321, 236–246. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behaviour and aluminum oxide coating microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Dunleavy, C.S.; Golosnoy, I.O.; Curran, J.A.; Clyne, T.W. Characterisation of discharge events during plasma electrolytic oxidation. Surf. Coat. Technol. 2009, 203, 3410–3419. [Google Scholar] [CrossRef] [Green Version]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E.; Wood, P. Plasma electrolytic oxidation of a zirconium alloy under ac conditions. Surf. Coat. Technol. 2010, 204, 2142–2151. [Google Scholar] [CrossRef]
- Jaspard-Mécuson, F.; Czerwiec, T.; Henrion, G.; Belmonte, T.; Dujardin, L.; Viola, A.; Beauvir, J. Tailored aluminium oxide layers by bipolar current adjustment in the plasma electrolytic oxidation (PEO) process. Surf. Coat. Technol. 2007, 201, 8677–8682. [Google Scholar] [CrossRef]
- Li, Q.; Liang, J.; Liu, B.; Peng, Z.; Wang, Q. Effects of cathodic voltages on structure and wear resistance of plasma electrolytic oxidation coatings formed on aluminium alloy. Appl. Surf. Sci. 2014, 297, 176–181. [Google Scholar] [CrossRef]
- Guan, Y.; Xia, Y.; Li, G. Growth mechanism and corrosion behavior of ceramic coatings on aluminum produced by autocontrol ac pulse PEO. Surf. Coat. Technol. 2008, 202, 4602–4612. [Google Scholar] [CrossRef]
- Kai, W.; Koo, B.H.; Chan, G.L.; Kim, Y.J.; Lee, S.; Byon, E. Effects of hybrid voltages on oxide formation on 6061 al-alloys during plasma electrolytic oxidation. Chin. J. Aeronaut. 2009, 22, 564–568. [Google Scholar]
- Melhem, A.; Henrion, G.; Czerwiec, T.; Briancon, J.L.; Belmonte, T. Changes induced by process parameters in oxide layers grown by the PEO process on Al alloys. Surf. Coat. Technol. 2011, 205, S133–S136. [Google Scholar] [CrossRef]
- Golabadi, M.; Aliofkhazraei, M.; Toorani, M.; Rouhaghdam, A.S. Evaluation of La containing PEO pretreatment on protective performance of epoxy coating on magnesium. Prog. Org. Coat. 2017, 105, 258–266. [Google Scholar] [CrossRef]
- Rogov, A.B.; Shayapov, V.R. The role of cathodic current in PEO of aluminum: Influence of cationic electrolyte composition on the transient current-voltage curves and the discharges optical emission spectra. Appl. Surf. Sci. 2017, 394, 323–332. [Google Scholar] [CrossRef]
- Monfort, F.; Berkani, A.; Matykina, E.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K. Development of anodic coatings on aluminium under sparking conditions in silicate electrolyte. Corros. Sci. 2007, 49, 672–693. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203–105216. [Google Scholar] [CrossRef]
- Slonova, A.I.; Terleeva, O.P. Morphology, structure, and phase composition of microplasma coatings formed on Al–Cu–Mg alloy. Prot. Met. 2008, 44, 65–75. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Hashimoto, T.; Skeldon, P.; Thompson, G.E. Characterization of AC PEO coatings on magnesium alloys. Surf. Coat. Technol. 2009, 203, 2207–2220. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Mohamed, A.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of pre-anodized aluminium. Corros. Sci. 2009, 51, 2897–2905. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Optimisation of the plasma electrolytic oxidation process efficiency on aluminium. Surf. Interface Anal. 2010, 42, 221–226. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Xue, Z.G.; Wang, Q.; Wu, X.Q.; Matykina, E.; Skeldon, P.; Thompson, G.E. New findings on properties of plasma electrolytic oxidation coatings from study of an Al–Cu–Li alloy. Electrochim. Acta 2013, 107, 358–378. [Google Scholar] [CrossRef]
- Sah, S.P.; Tsuji, E.; Aoki, Y.; Habazaki, H. Cathodic pulse breakdown of anodic films on aluminium in alkaline silicate electrolyte-Understanding the role of cathodic half-cycle in AC plasma electrolytic oxidation. Corros. Sci. 2012, 55, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Cui, Y.; Li, Y.; Zhang, T.; Wang, F.H. Failure behavior of nano-SiO2 fillers epoxy coating under hydrostatic pressure. Elect. Acta 2012, 62, 42–50. [Google Scholar] [CrossRef]
- Ko, Y.G.; Namgung, S.; Shin, D.H. Correlation between KOH concentration and surface properties of AZ91 magnesium alloy coated by plasma electrolytic oxidation. Surf. Coat. Technol. 2010, 205, 2525–2531. [Google Scholar] [CrossRef]
- Hosseini, R.M.; Sarabi, A.; Mohammadloo, H.E.; Sarayloo, M. The performance improvement of Zr conversion coating through Mn incorporation: With and without organic coating. Surf. Coat. Technol. 2014, 258, 437–446. [Google Scholar] [CrossRef]




| Element | Al | Si | Cu | Mg | Zn | Mn | Ti | V | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Percentage | 99.60 | 0.25 | 0.05 | 0.03 | 0.05 | 0.03 | 0.03 | 0.05 | 0.350 |
| Reagent Name | Molecular Formula | Reagent Purity | Manufacturer |
|---|---|---|---|
| Sodium dihydrogen Phosphate dodecahydrate | NaH2PO4·12H2O | Analytically pure | Sinopharm Chemical ReagentCo., Ltd. Shenyang, China |
| Oxalic acid dihydrate | H2C2O4·2H2O | Analytically pure | Sinopharm Chemical ReagentCo., Ltd. Shenyang, China |
| Sodium silicate nonahydrate | Na2SiO3·9H2O | Analytically pure | Sinopharm Chemical ReagentCo., Ltd. Shenyang, China |
| Stage | Rout (Ω·cm2) | Qout (Sn·Ω−1·cm−2) | n | Rin (Ω·cm2) | Qin (Sn·Ω−1·cm−2) | n | Rct (Ω·cm2) | Qdl (Sn·Ω−1·cm−2) | n | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| II | 6.74 × 105 | 1.20 × 10−7 | 0.89 | 7.05 × 105 | 3.21 × 10−5 | 0.80 | 1.42 × 10−3 | |||
| III | 1.27 × 104 | 3.74 × 10−8 | 0.92 | 7.09 × 105 | 2.77 × 10−8 | 0.88 | 7.17 × 105 | 1.73 × 10−5 | 0.93 | 1.30 × 10−3 |
| IV | 3.56 × 105 | 2.29 × 10−5 | 0.88 | 7.17 × 105 | 1.54 × 10−6 | 0.95 | 1.50 × 106 | 5.75 × 10−8 | 0.94 | 5.68 × 10−4 |
| V | 1.98 × 108 | 2.42 × 10−8 | 0.85 | 1.56 × 109 | 4.96 × 10−8 | 0.87 | - | - | - | 8.32 × 10−4 |
| Stage | Ecorr (V SHE) | icorr (A∙cm−2) |
|---|---|---|
| Al | −0.457 ± 0.013 | 1.629 ± 3.804 × 10−4 |
| Stage I | −0.460 ± 0.011 | 2.886 ± 2.325 × 10−5 |
| Stage II | −0.438 ± 0.023 | 5.282 ± 1.384× 10−6 |
| Stage III | −0.426 ± 0.018 | 1.896 ± 1.278 × 10−6 |
| Stage IV | −0.372 ± 0.034 | 4.714 ± 4.143 × 10−7 |
| Stage V | −0.335 ± 0.027 | 8.564 ± 5.763 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhang, W.; Liu, H.; Liu, L.; Wang, F.; Qiao, Z. Single Dense Layer of PEO Coating on Aluminum Fabricated by “Chain-like” Discharges. Materials 2022, 15, 4635. https://doi.org/10.3390/ma15134635
Zhu L, Zhang W, Liu H, Liu L, Wang F, Qiao Z. Single Dense Layer of PEO Coating on Aluminum Fabricated by “Chain-like” Discharges. Materials. 2022; 15(13):4635. https://doi.org/10.3390/ma15134635
Chicago/Turabian StyleZhu, Liye, Wei Zhang, Haitao Liu, Lei Liu, Fuhui Wang, and Ziping Qiao. 2022. "Single Dense Layer of PEO Coating on Aluminum Fabricated by “Chain-like” Discharges" Materials 15, no. 13: 4635. https://doi.org/10.3390/ma15134635
APA StyleZhu, L., Zhang, W., Liu, H., Liu, L., Wang, F., & Qiao, Z. (2022). Single Dense Layer of PEO Coating on Aluminum Fabricated by “Chain-like” Discharges. Materials, 15(13), 4635. https://doi.org/10.3390/ma15134635






