Corrosion Behaviour of Nodular Cast Iron Used for Rotor Manufacturing in Different Wastewaters
Abstract
:1. Introduction
- The acidic pH of the water, which increases the content of active ions, and the chemical reactivity of the liquid is also influenced by the temperature at which the process takes place;
- Corrosion, given by sulfuric acid and sulphur dioxide dissolved in water, occurs as a result of the decomposition of substances of an organic origin;
- The accentuated corrosion due to the presence of dissolved oxygen in the transported liquid, which modifies the acidity of the water;
- Accelerated flow corrosion due to the removal of the protective oxide layer from the pump metal (especially for low-alloy steels and grey cast iron).
2. Materials and Methods
2.1. Material
2.2. Methods
3. Results and Discussion
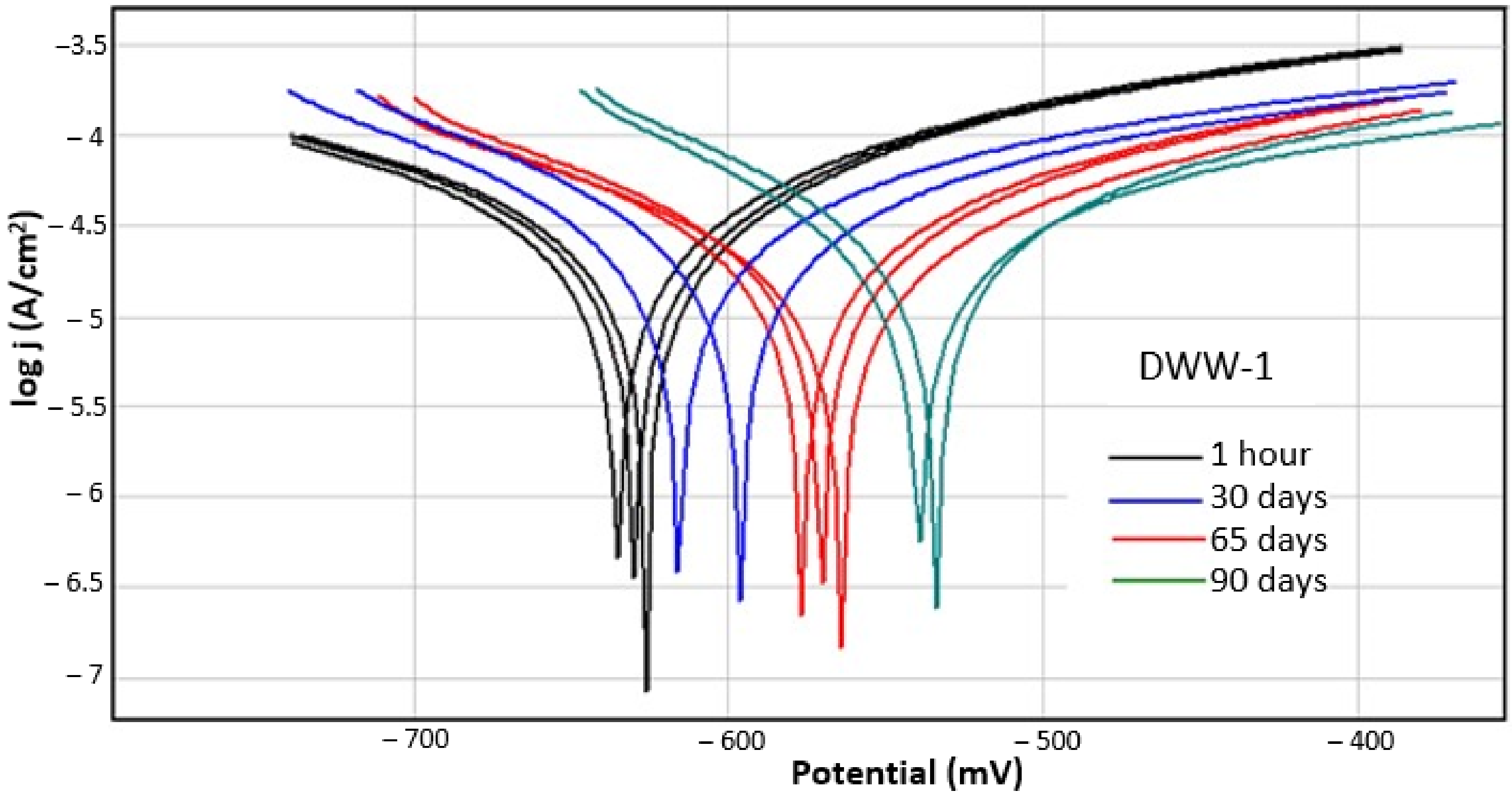
3.1. Instantaneous Corrosion Rate
- The data in the tables show very good reproducibility of the measurements, with 1%–2% arithmetic average errors being detected.
- In neutral and basic solutions (solutions DWW-1 and DWW-3), E (I = 0), which is the corrosion potential, moves to more positive values as the immersion time in the solution increases. As this quantity expresses the thermodynamic probability of corrosion, its positivity over time expresses a tendency to passivate. In acidic solutions, however (respectively in DWW-2) E (I = 0) varies randomly: after 30 days of immersion, it is positive, but later, after 65 and 90 days, it reaches even higher negative values in absolute value than in the initial moment. This is probably a consequence of the fact that in the acid environment the corrosion process but also the evolution of the reaction product are more complex.
- In DWW-1 and DWW-3, the polarisation resistance increases with the immersion time, and consequently the current density and corrosion rate decrease accordingly. Quantitatively DWW-1, the corrosive agent with pH = 6.5, is much less corrosive than the DWW-3 solution, the polarisation resistance in DWW-1 is 3–4 times higher than in DWW-3. Under these conditions, the corrosion rate is 5–6 times lower in DWW-1 than in DWW-3.
- In the acid solution (DWW-2) the polarisation resistances and consequently the corrosion rates are of the same order of magnitude as in the basic solution (DWW-3) but, as in the case of the variation of the corrosion potential, the time dependence immersion does not follow a certain order.
- In DWW-2 and DWW-3 solutions, the absolute slope of the Tafel cathode is significantly higher than the anodic slope, which indicates that the overall corrosion process is anodically controlled and is limited only by concentration. In the DWW-1 solution (pH = 6.5), in the initial stages, the Tafel slopes are of the same order of magnitude, while at longer periods of immersion the anodic slope becomes larger than the cathodic slope, indicating that the metal tends to passivation.
- The anodic slope of the Tafel curves is much more accentuated compared to the cathodic slope, this aspect being observed also in the case of corrosion in water with neutral pH (drinking water) from nodular cast iron covered with cement (cement mortar lining), a phenomenon studied and confirmed by Yunhul et al. [30].
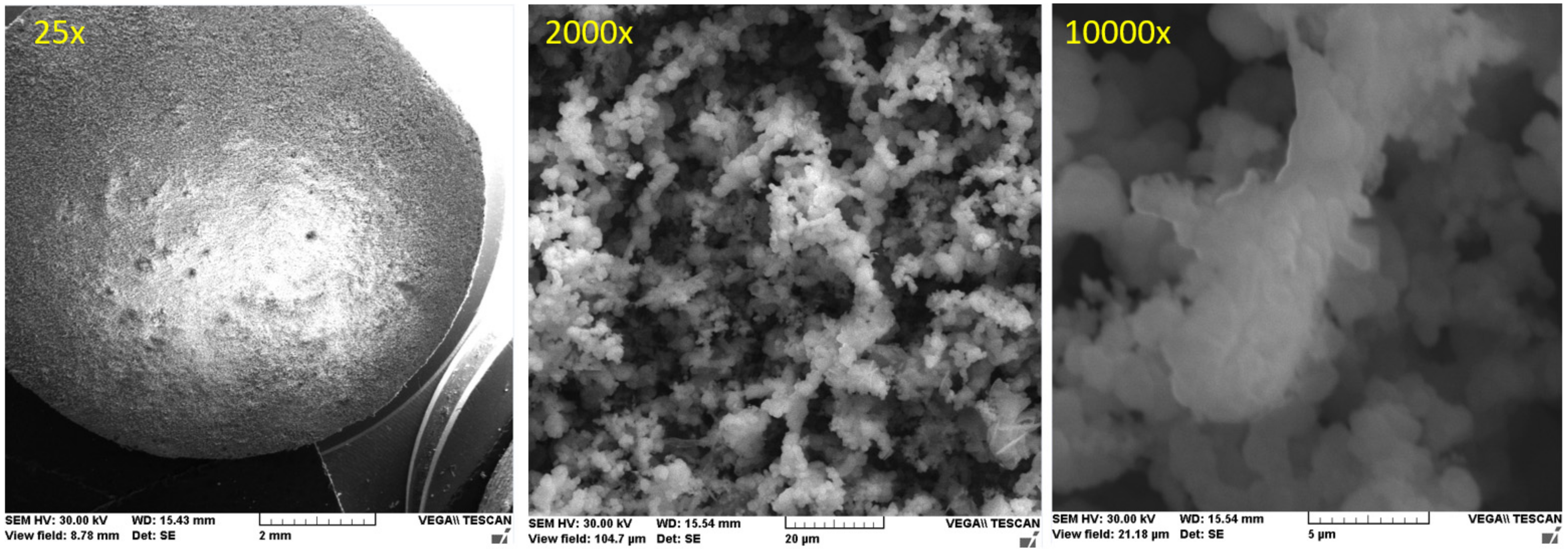
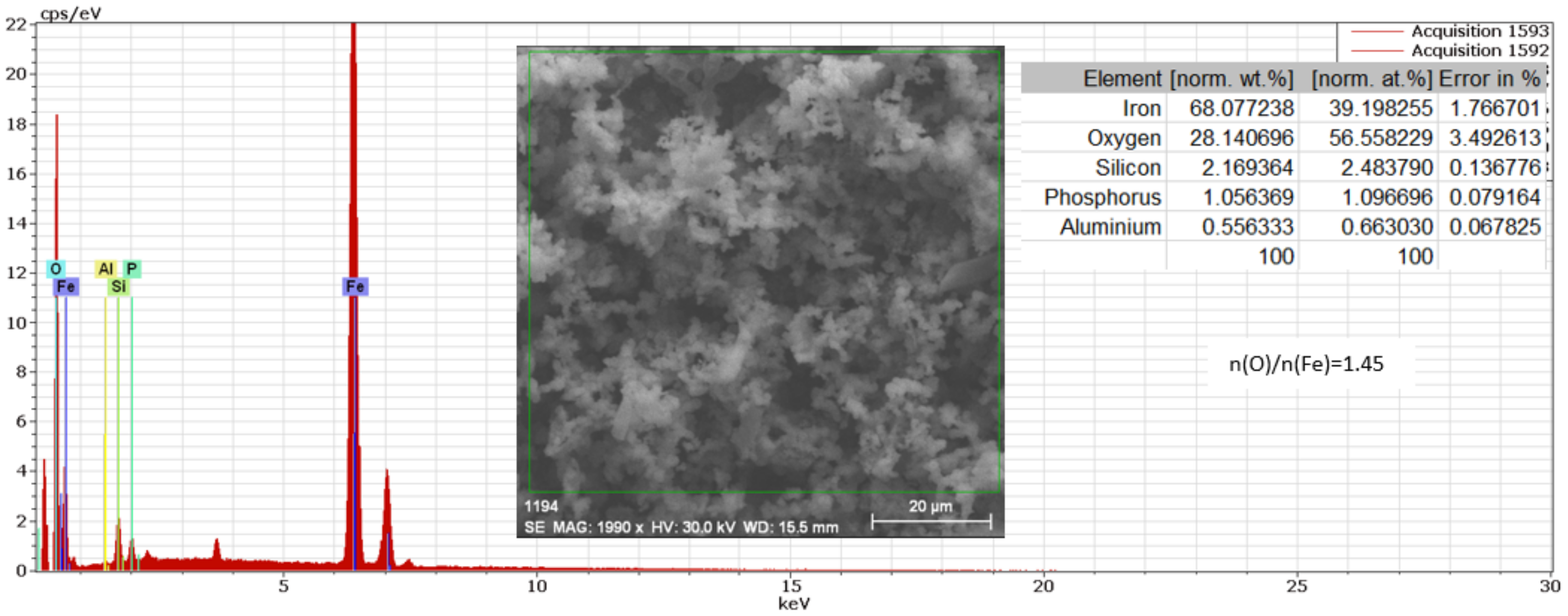
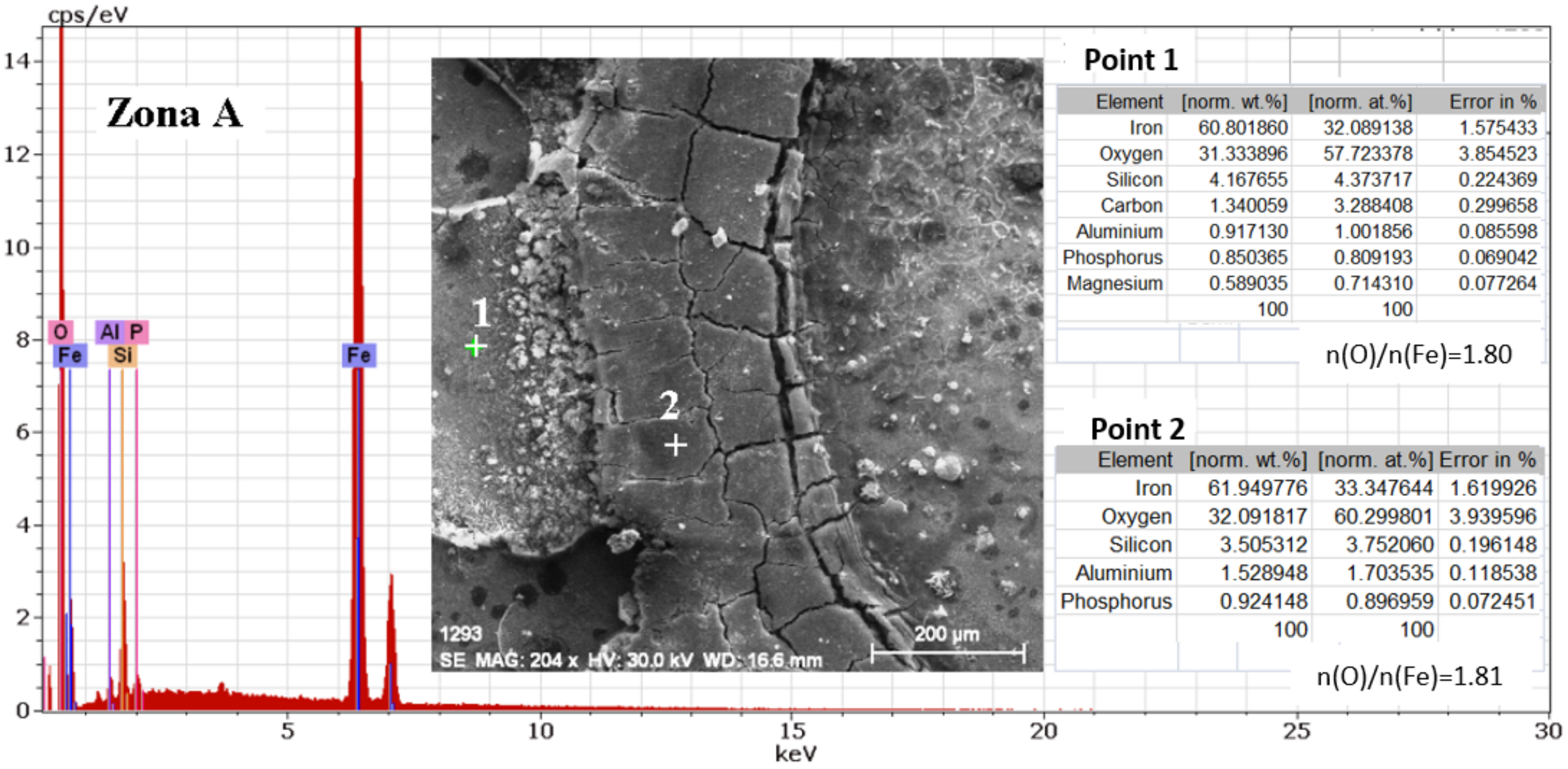
3.2. The Cast Iron Surface Layer Structure and Composition after Various Time Intervals of Immersion in Wastewaters with Different pHs
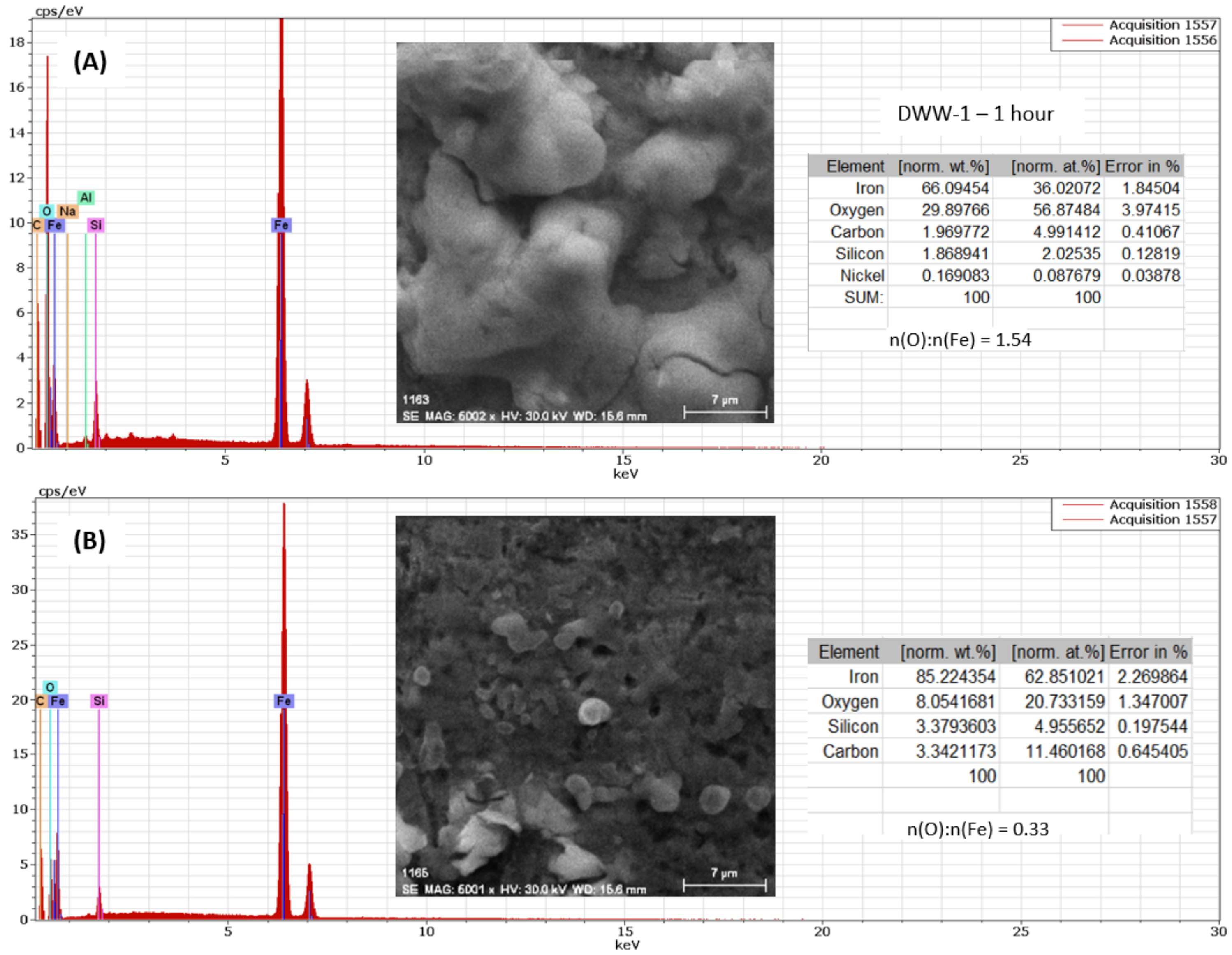
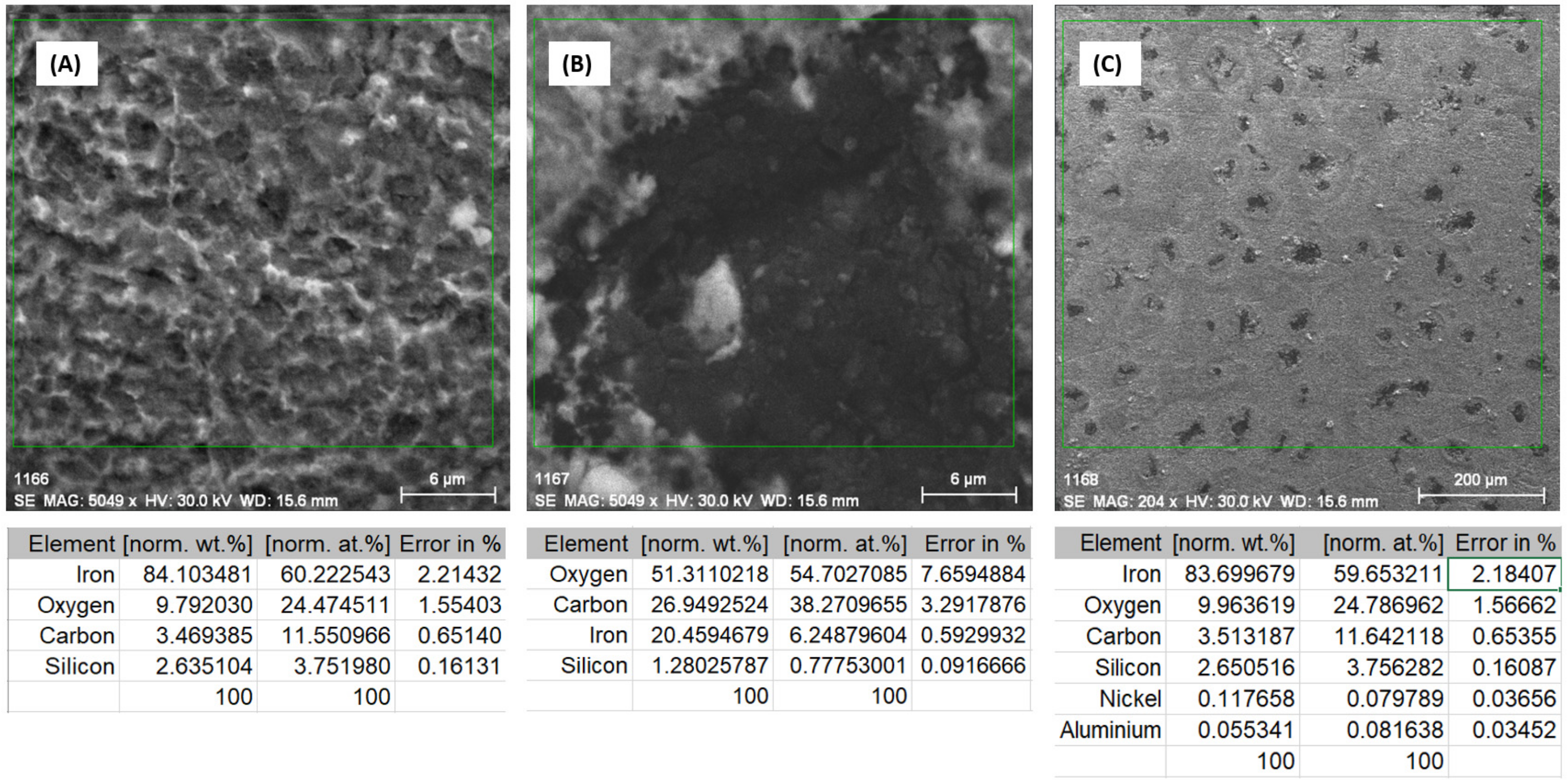
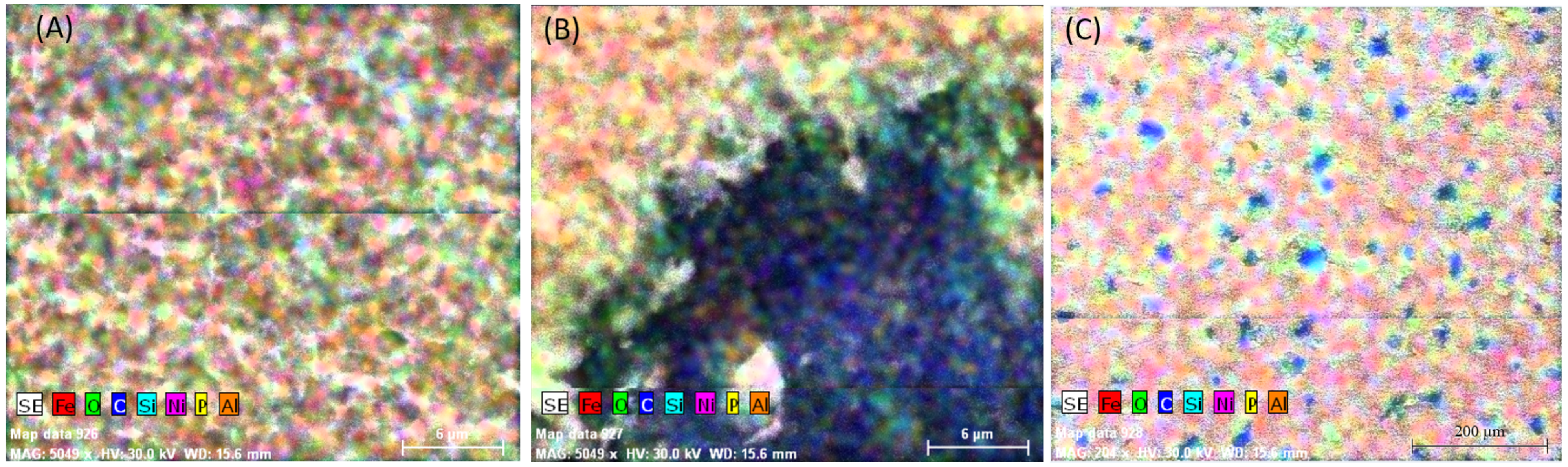
3.2.1. Study of Nodular Cast Iron Sample (Immersion Time 1 Hour)
3.2.2. Study of Nodular Cast Iron Samples Immersed for 30 Days in Solution
3.2.3. Study of Nodular Cast Iron Samples Immersed for 65 Days in Solution
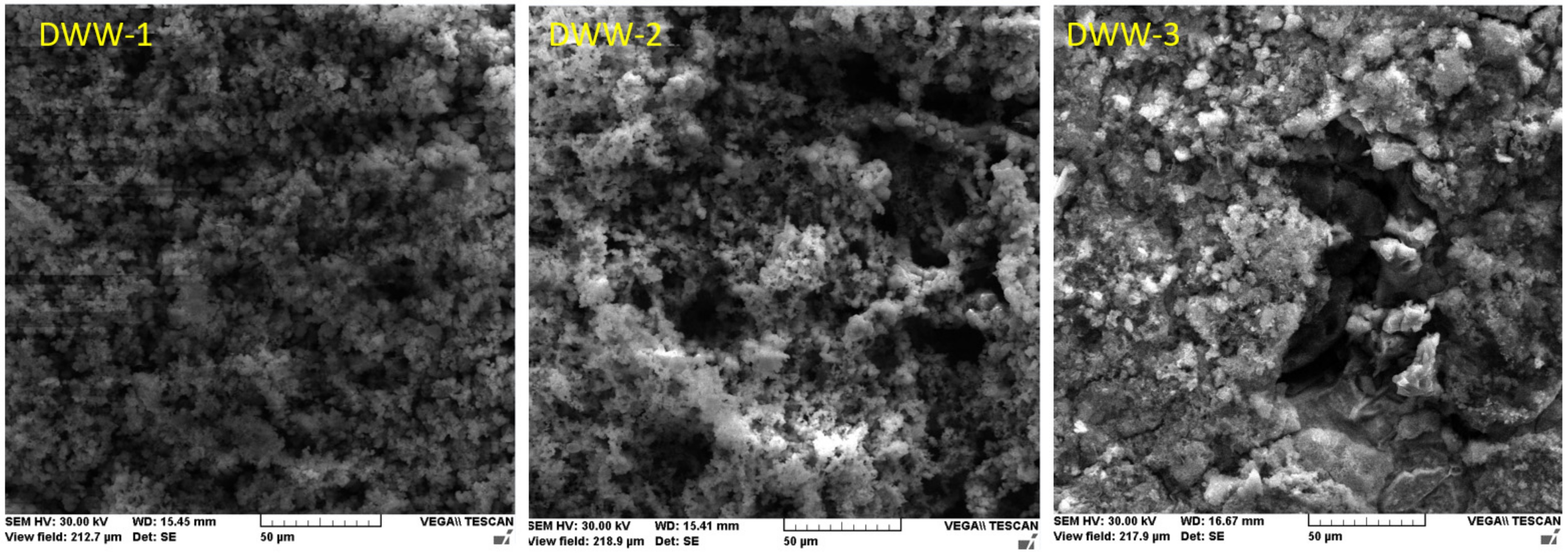
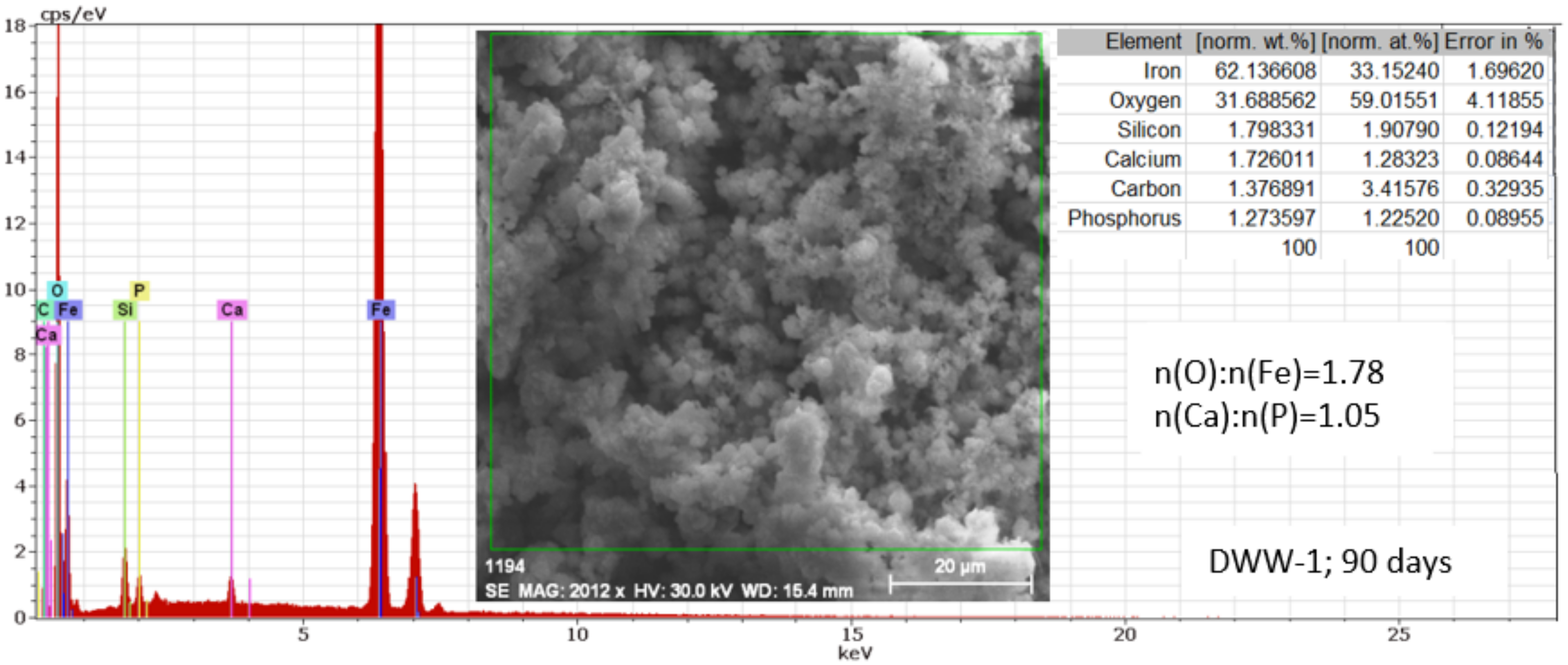
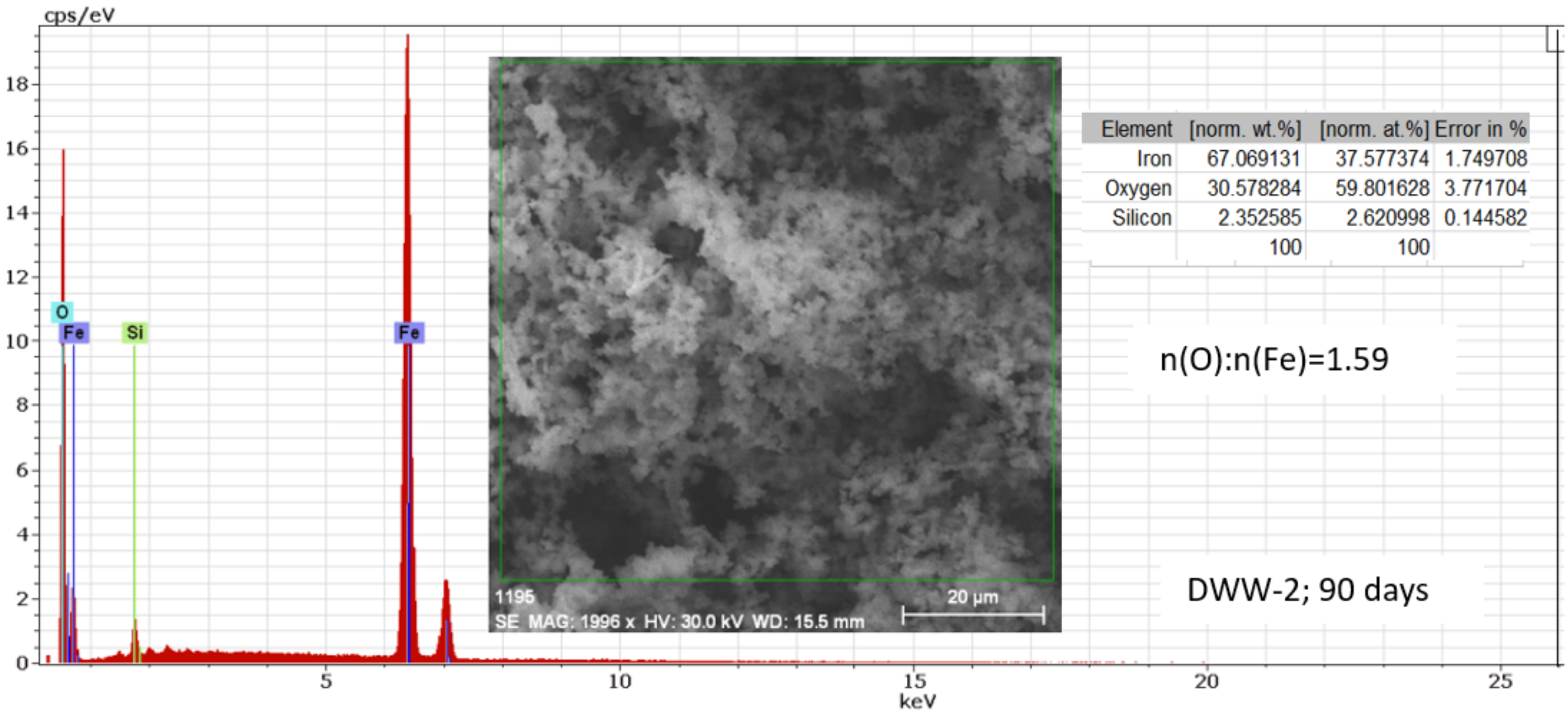
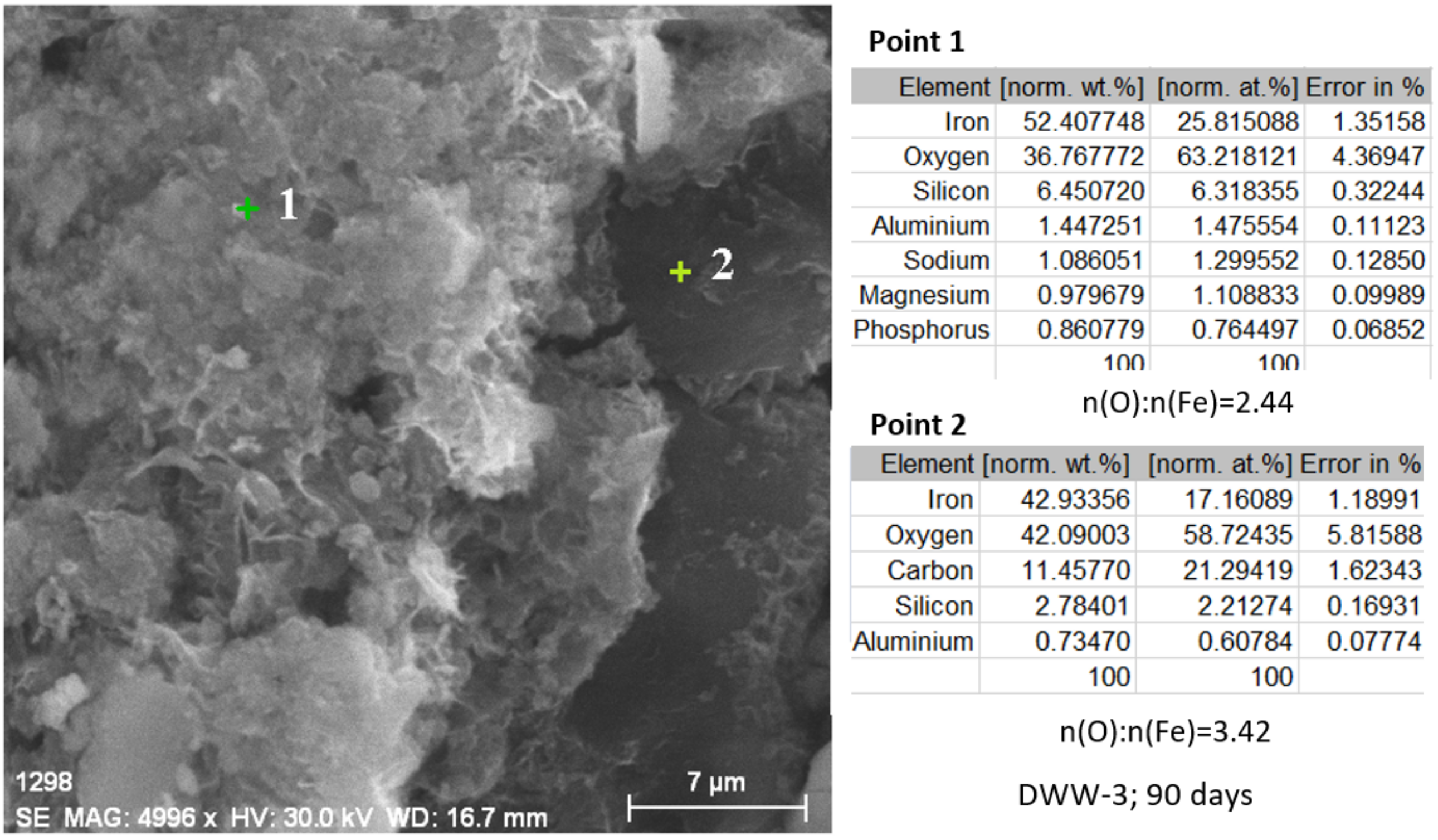
3.2.4. Study of Nodular Cast Iron Samples Immersed for 90 Days in Solution
4. Conclusions
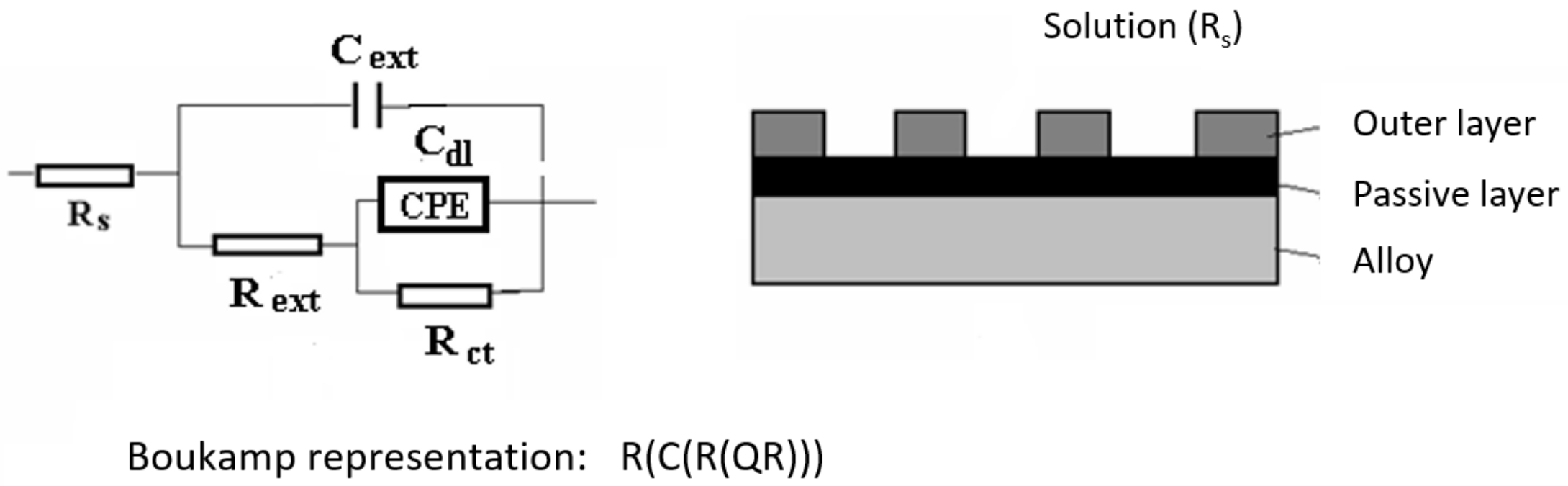
- Comparing the values of polarisation resistance (Rp), corrosion rate (vcor), and current density (jcor) of the samples immersed in wastewaters it can be seen that the value of the polarisation resistance for DWW-1 is four times higher compared to the other two. Also, the values of corrosion rate and current density for DWW-1 are four times lower, indicating that the pH-neutral solution is the least aggressive. This is also supported by the EIS results (the values of Rs, Rext, and Rct) for all the samples immersed in different periods in all three wastewaters.
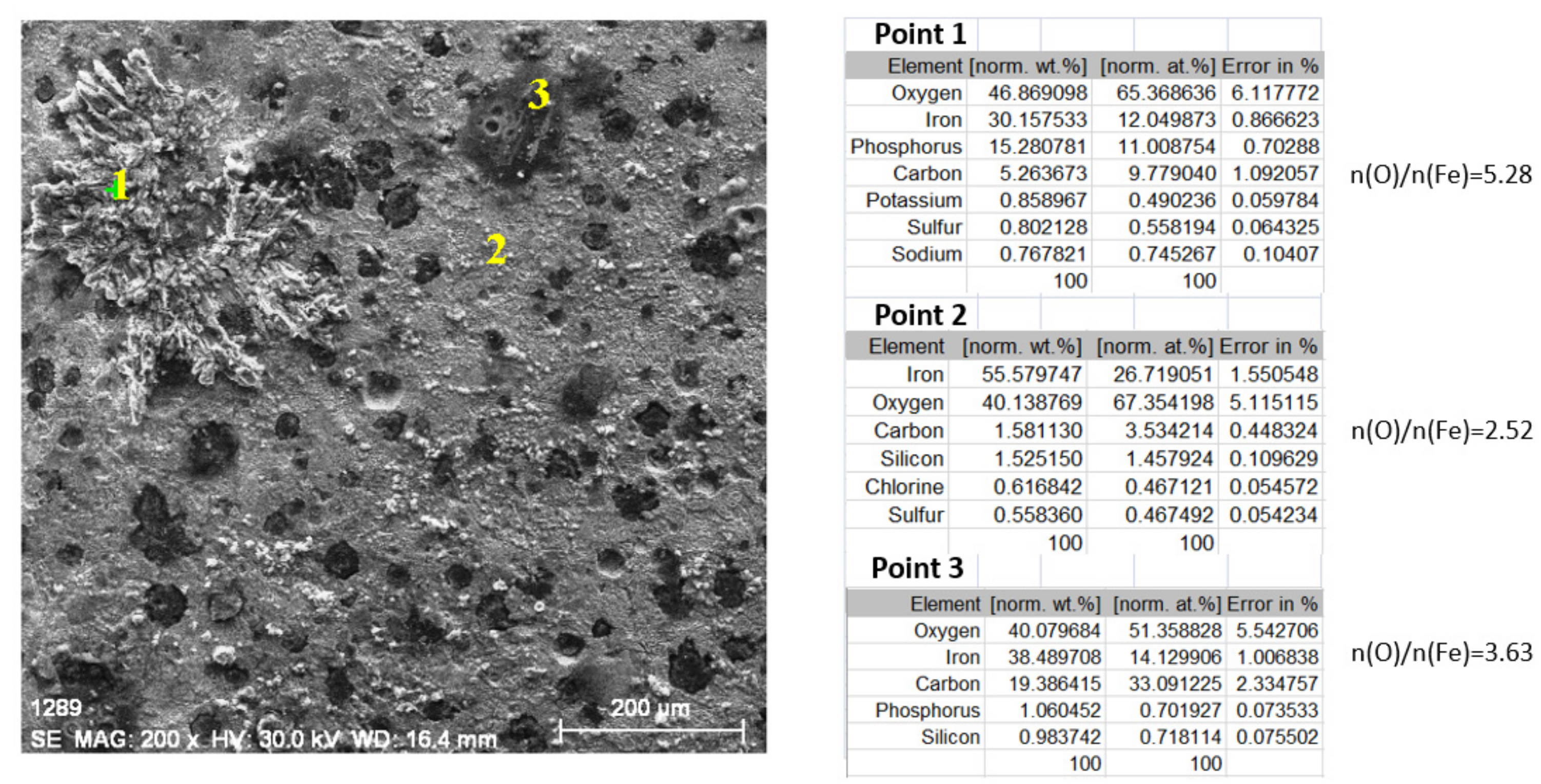
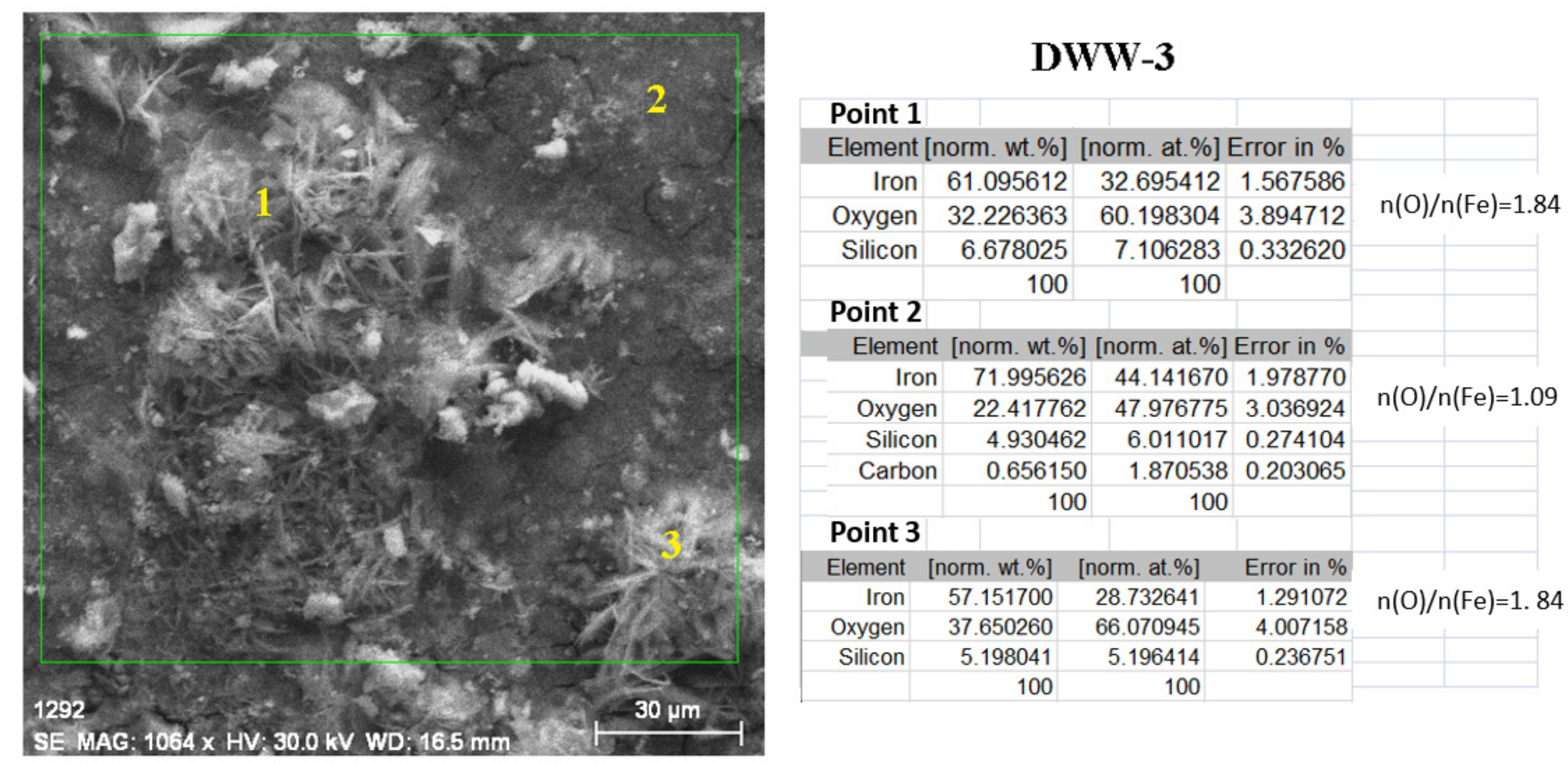
- In the initial stage, ferrous ion (Fe2+) is formed, and by the transfer of dissolved oxygen from the solution to the metal surface, ferrous oxide (FeO) is formed. It is further oxidised to ferro-ferric oxide Fe3O4 (Magnetite). A thin phase layer rich in Fe(II) is formed at the metal/solution interface. As corrosion continues, the thickness of the oxide monolayer increases to form a double-layer crust (oxide film and electric double layer) that reduces the flow of oxygen to the metal surface and thereby decreases the corrosion rate. The product layer is porous and easily removable, especially if the liquid is moving (as in pipes or pumps), so that under dynamic conditions the reaction rate does not decrease.
- In the intermediate stages of development, because the dissolved oxygen in the solution is an electron acceptor, in addition to the transformation of FeO to Fe2O3, the formation of α -FeOOH (Goethite) and γ-FeOOH (Lepidocrocyte) takes place. This forms a compact crust on the metal surface, further reducing the flow at the metal-crust interface and decreasing the corrosion rate.
- In the final stage by the oxidation of magnetite to FeOOH, which takes place in the initial layer, the thickness of the layer of ferric compounds (mainly FeOOH) increases, forming a compact crust. Thus, the final structure of the product layer is that of a tri-layer: the double-electric layer directly connected to the metal surface, an inner layer consisting of ferrous compounds and ferric compounds, and an outer layer, a compact crust formed of ferric compounds. Anions, cations, and non-dissociated compounds (food compounds) from corrosion environments are absorbed in both the outer and inner layers. As the thickness of the product crust increases, there is a slow decrease in the corrosion rate. The inner layer controls the diffusion of oxygen to the metal interface with the crust and thereby controls the reaction rate. The product crust is easily removable from both wet and dry metal.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nedeff, V.; Bejenariu, C.; Lazar, G.; Agop, M. Generalized lift force for complex fluid. Powder Technol. 2013, 235, 685–695. [Google Scholar] [CrossRef]
- Karassik, I.J.; Messina, J.P.; Cooper, P.; Heald, C.C.; San, N.Y.; Washington, F.; Auckland Bogotá, D.C.; Lisbon, C.; Madrid, L.; City, M.; et al. Pump Handbook; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Pantazopoulos, G.A. A Process-Based Approach in Failure Analysis. J. Fail. Anal. Prev. 2014, 14, 551–553. [Google Scholar] [CrossRef] [Green Version]
- Dargahi, A.; Barzoki, H.R.; Vosoughi, M.; Mokhtari, S.A. Enhanced electrocatalytic degradation of 2,4-Dinitrophenol (2,4-DNP) in three-dimensional sono-electrochemical (3D/SEC) process equipped with Fe/SBA-15 nanocomposite particle electrodes: Degradation pathway and application for real wastewater. Arab. J. Chem. 2022, 15, 103801. [Google Scholar] [CrossRef]
- Tabandeh, L.; Shams Khorramabadi, G.; Karami, A.; Atafar, Z.; Sharafi, H.; Dargahi, A.; Amirian, F. Evaluation of Heavy Metal Contamination and Scaling and Corrosion Potential in Drinking Water Resources in Nurabad City of Lorestan, Iran. Int. J. Pharm. Technol. 2016, 8, 13137–13154. [Google Scholar]
- Fuseini, M.; Zaghloul, M.M.Y.; Elkady, M.F.; El-Shazly, A.H. Evaluation of Synthesized Polyaniline Nano-fibres as Corrosion Protection Film Coating on Copper Substrate by Electrophoretic Deposition. J. Mater. Sci. 2022, 57, 6085–6101. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.Y.M.; Zaghloul, M.M.Y. Experimental and modeling analysis of mechanical-electrical behaviors of polypropylene composites filled with graphite and MWCNT fillers. Polym. Test. 2017, 63, 467–474. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Mohamed, Y.S.; El-Gamal, H. Fatigue and tensile behaviors of fiber-reinforced thermosetting composites embedded with nanoparticles. J. Compos. Mater. 2018, 53, 709–718. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Steel, K.; Veidt, M.; Heitzmann, M.T. Wear behaviour of polymeric materials reinforced with man-made fibres: A comprehensive review about fibre volume fraction influence on wear performance. J. Reinf. Plast. Compos. 2021, 41, 215–241. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.M. Mechanical properties of linear low-density polyethylene fire-retarded with melamine polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46770. [Google Scholar] [CrossRef]
- Zaghloul, M.Y.M.; Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Developments in polyester composite materials—An in-depth review on natural fibres and nano fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Akhtar, M.; Khan, M.; Khan, S.; Afzal, A.; Subbiah, R.; Ahmad, S.; Husain, M.; Butt, M.; Othman, A.; Bakar, E. Determination of Non-Recrystallization Temperature for Niobium Microalloyed Steel. Materials 2021, 14, 2639. [Google Scholar] [CrossRef]
- Sharath, B.; Venkatesh, C.; Afzal, A.; Aslfattahi, N.; Aabid, A.; Baig, M.; Saleh, B. Multi Ceramic Particles Inclusion in the Aluminium Matrix and Wear Characterization through Experimental and Response Surface-Artificial Neural Networks. Materials 2021, 14, 2895. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lee, J.R.; Teng, P.J. Effect of Thermal Conditions on Corrosion Behavior of Ductile Cast Iron. Int. J. Electrochem. Sci. 2019, 14, 10032–10042. [Google Scholar] [CrossRef]
- Akinribide, O.J.; Akinwamide, S.O.; Ajibola, O.O.; Obadele, B.A.; Olusunle, S.O.O.; Olubambi, P.A. Corrosion behavior of ductile and austempered ductile cast iron in 0.01M and 0.05M NaCl Environments. Procedia Manuf. 2019, 30, 167–172. [Google Scholar] [CrossRef]
- Masters, S.; Wang, H.; Pruden, A.; Edwards, M.A. Redox gradients in distribution systems influence water quality, corrosion, and microbial ecology. Water Res. 2015, 68, 140–149. [Google Scholar] [CrossRef]
- Wang, H.; Masters, S.; Falkinham, I.J.O.; Edwards, M.A.; Pruden, A. Distribution System Water Quality Affects Responses of Opportunistic Pathogen Gene Markers in Household Water Heaters. Environ. Sci. Technol. 2015, 49, 8416–8424. [Google Scholar] [CrossRef]
- Sancy, M.; Gourbeyre, Y.; Sutter, E.; Tribollet, B. Mechanism of corrosion of cast iron covered by aged corrosion products: Application of electrochemical impedance spectrometry. Corros. Sci. 2010, 52, 1222–1227. [Google Scholar] [CrossRef]
- Rios, J.; Calderón, J.; Nogueira, R. Electrochemical Behavior of Metals Used in Drinking Water Distribution Systems: A Rotating Cylinder Electrode’s Study. Corrosion 2013, 69, 875–885. [Google Scholar] [CrossRef]
- Fabbricino, M.; Korshin, G.V. Changes of the corrosion potential of iron in stagnation and flow conditions and their relationship with metal release. Water Res. 2014, 62, 136–146. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Zheng, Y.Y. Electrochemical Techniques for Determining Corrosion Rate of Rusted Steel in Seawater. Corros. Sci. 2011, 53, 208–216. [Google Scholar] [CrossRef]
- Sadeghi, A.; Moloodi, A.; Golestanipour, M.; Shahri, M.M. An investigation of abrasive wear and corrosion behavior of surface repair of gray cast iron by SMAW. J. Mater. Res. Technol. 2017, 6, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Tian, Y.; Zhang, R.; Liu, X.; Guo, H.; Song, Y.; Peng, S. Corrosion of Ductile Cast Iron Pipeline in a Water Supply Network Based on the Flow Field Changing. Int. J. Electrochem. Sci. 2018, 13, 9110–9130. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Zhang, H.; Liu, X.; Chen, Y.; Tian, Y.; Yin, J. Study on Growth of Corrosion Scale on Various Iron Based Materials (Grey cast iron/Carbon steel/Ductile Iron) in Water Distribution Systems. Int. J. Electrochem. Sci. 2020, 15, 8479–8497. [Google Scholar] [CrossRef]
- Ogundare, O.; Babatope, B.; Adetunji, A.R.; Olusunle, S.O.O. Atmospheric Corrosion Studies of Ductile Iron and Austenitic Stainless Steel in an Extreme Marine Environment. J. Miner. Mater. Charact. Eng. 2012, 11, 914–918. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, G.; Chen, Y.; Zhao, P.; Tian, Y. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Roman, T.; Asavei, R.; Karkalos, N.E.; Roman, C.; Virlan, C.; Cimpoesu, N.; Istrate, B.; Zaharia, M.; Markopoulos, A.P.; Kordatos, K.; et al. Synthesis and adsorption properties of nanocrystalline ferrites for kinetic modeling development. Int. J. Appl. Ceram. Technol. 2018, 16, 693–705. [Google Scholar] [CrossRef]
- Zaharia, M.G.; Stanciu, S.; Cimpoesu, R.; Ionita, I.; Cimpoesu, N. Preliminary results on effect of H 2 S on P265GH commercial material for natural gases and petroleum transportation. Appl. Surf. Sci. 2018, 438, 20–32. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.-P.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Phosphate Surface Treatment for Improving the Corrosion Resistance of the C45 Carbon Steel Used in Carabiners Manufacturing. Materials 2020, 13, 3410. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Zhang, R.; Guo, H.; Zhao, W.; Huang, J. Corrosion behavior and mechanism of ductile iron with different degrees of deterioration of cement mortar lining in reclaimed water pipelines. RSC Adv. 2020, 10, 39627–39639. [Google Scholar] [CrossRef]
- Cottis, R.; Turgoose, S.; NACE International. Electrochemical Impedance and Noise; NACE International: Houston, TX, USA, 1999; ISBN 9781575900933. [Google Scholar]
- Zoltowski, P. On the electrical capacitance of interfaces exhibiting constant phase element behaviour. J. Electroanal. Chem. 1998, 443, 149–154. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.-P.; Vasilescu, G.D.; Burduhos-Nergis, D.-D.; Cimpoesu, R.; Bejinariu, C. Phosphate Coatings: EIS and SEM Applied to Evaluate the Corrosion Behavior of Steel in Fire Extinguishing Solution. Appl. Sci. 2021, 11, 7802. [Google Scholar] [CrossRef]
- De Marco, R.; Pejcic, B. Electrochemical Impedance Spectroscopy and X-ray Photoelectron Spectroscopy Study of the Response Mechanism of the Chalcogenide Glass Membrane Iron(III) Ion-Selective Electrode in Saline Media. Anal. Chem. 2000, 72, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Nofal, A.; Ahmed, A.S.; Ghanem, W.A.; Hussein, W.; Mohamed, N.K. Evaluation of Corrosion Behavior of Different Grades of Cast Iron Insodium Chloride Solutions. Key Eng. Mater. 2020, 835, 223–228. [Google Scholar] [CrossRef]
- Lagunas, C.; Gonzalez, S.; Velasco, M.; Muller, C. Evaluation of electrochemical impedance spectroscopy (EIS) for inline monitoring of internal corrosion in ductile iron water distribution pipes. Int. J. Emerg. Technol. Adv. Eng. 2014, 4, 413–419. [Google Scholar]










| Element | Wt, % | Characteristics | Values |
|---|---|---|---|
| Carbon (C) | 4.50 | Hardness | 160–210 N/mm3 |
| Silicone (Si) | 2.28 | ||
| Nickel (Ni) | 0.12 | ||
| Magnesium (Mg) | 0.10 | ||
| Manganese (Mn) | 0.09 | Thermal Expansion Coefficient | 0.0564%/°C |
| Phosphorus (P) | 0.05 | ||
| Sulphur (S) | 0.04 | ||
| Chrome (Cr) | 0.02 | ||
| Titanium (Ti) | 0.02 | Tensile strength | 440 MPa |
| Aluminium (Al) | 0.01 | ||
| Copper (Cu) | 0.01 | Tensile Elongation | 8% |
| Iron (Fe) | balance |
| Chemical Components | mg/L | Food Components | mg/L | Metal Traces | Mg Metal/L |
|---|---|---|---|---|---|
| NH4Cl | 15 | Powdered milk | 118 | Cr(NO3)3·9H2O | 0.10 |
| CH3COONa·3H2O | 142 | Yeast | 54 | CuCl2·2H2O | 0.20 |
| MgSO4·7H2O | 32 | Starch | 122 | MnCl2 | 0.05 |
| CaHPO4 | 20 | Soybean oil | 15 | NiSO4·7H2O | 0.08 |
| K2HPO4·3H2O | 56 | PbCl2 | 0.07 | ||
| FeSO4·7H2O | 14 | ||||
| Urea | 15 |
| Parameter | 1 h | 30 Days | 65 Days | 90 Days |
|---|---|---|---|---|
| E(I = 0), mV | −(633 ± 3) | −(575 ± 5) | −(544 ± 2) | −(522 ± 22) |
| Rp, kΩ·cm2 | 1.053 ± 0.5 | 1.36 ± 0.11 | 1.12 ± 0.07 | 1.13 ± 0.03 |
| Jcor, µA/cm2 | 30.47 ± 0.57 | 21.19 ± 087 | 20.71 ± 1.56 | 14.38 ± 0.52 |
| vcor, µm/an | 356.4 ± 6.8 | 247.8 ± 20.3 | 242.0 ± 1.5 | 185.8 ± 6.2 |
| ba, mV/decade | 179 ± 4 | 178 ± 7 | 166 ± 1 | 186 ± 4 |
| bc, mV/decade | −(215 ± 6) | −(170 ± 7) | −(119 ± 4) | −(152 ± 8) |
| Parameter | 1 h | 30 Days | 65 Days | 90 Days |
|---|---|---|---|---|
| E(I = 0), mV | −(545 ± 4) | −(536 ± 1) | −(591 ± 4) | −(572 ± 1) |
| Rp, kΩ·cm2 | 261.4 ± 1.7 | 297 ± 12 | 209.4 ± 3.2 | 372 ± 9.62 |
| Jcor, µA/cm2 | 125.0 ± 2 | 131.3 ± 2.3 | 177.33 ± 2.45 | 96.19 ± 2.95 |
| vcor, µm/an | 1440 ± 40 | 1540 ± 30 | 2042 ± 30 | 1127 ± 370 |
| ba, mV/decade | 144 ± 16 | 205 ± 7 | 214 ± 1 | 211 ± 5 |
| bc, mV/decade | −(243 ± 8) | −(339 ± 6) | −(271 ± 1) | −(245 ± 17) |
| Parameter | 1 h | 30 Days | 65 Days | 90 Days |
|---|---|---|---|---|
| E(I = 0), mV | −(657 ± 6) | −(638 ± 6) | −(622 ± 5) | −(607 ± 7) |
| Rp, kΩ·cm2 | 295.8 ± 2.3 | 284.6 ± 0.1 | 306.9 ± 8 | 341.9 ± 11.6 |
| Jcor, µA/cm2 | 149.1 ± 1.76 | 126.8 ± 3.9 | 114.7 ± 4.7 | 105.9 ± 3.0 |
| vcor, µm/an | 1743 ± 17 | 1483 ± 27 | 1319 ± 15 | 1238 ± 35 |
| ba, mV/decade | 196 ± 2 | 199 ± 6 | 192 ± 2 | 197 ± 2 |
| bc, mV/decade | −(762 ± 36) | −(319 ± 3) | −(300 ± 7) | −(300 ± 12) |
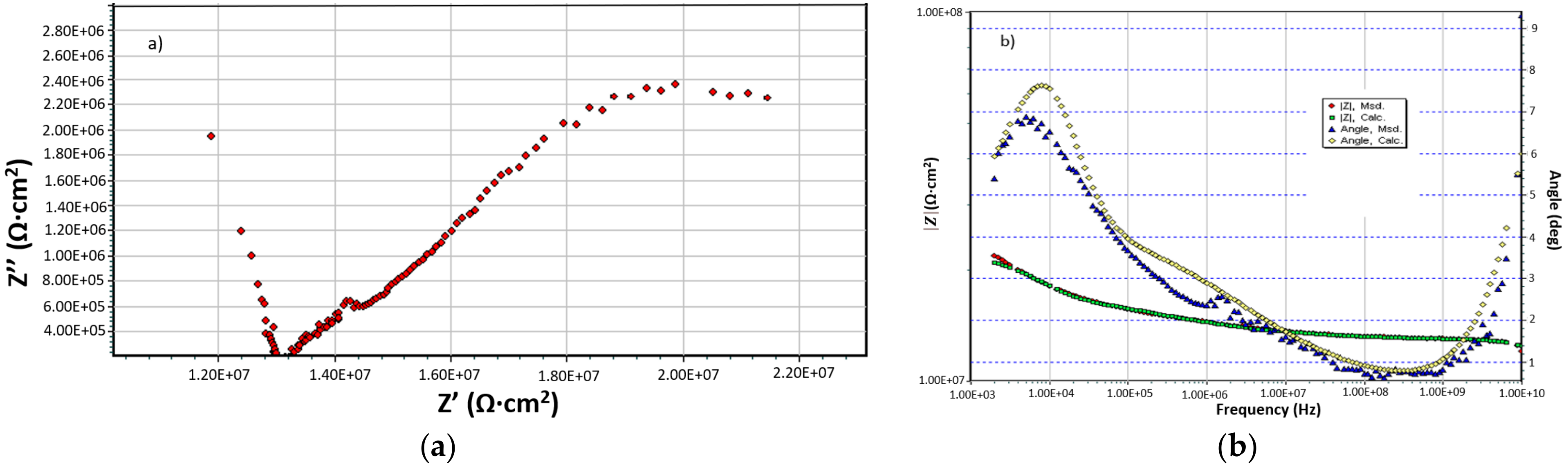
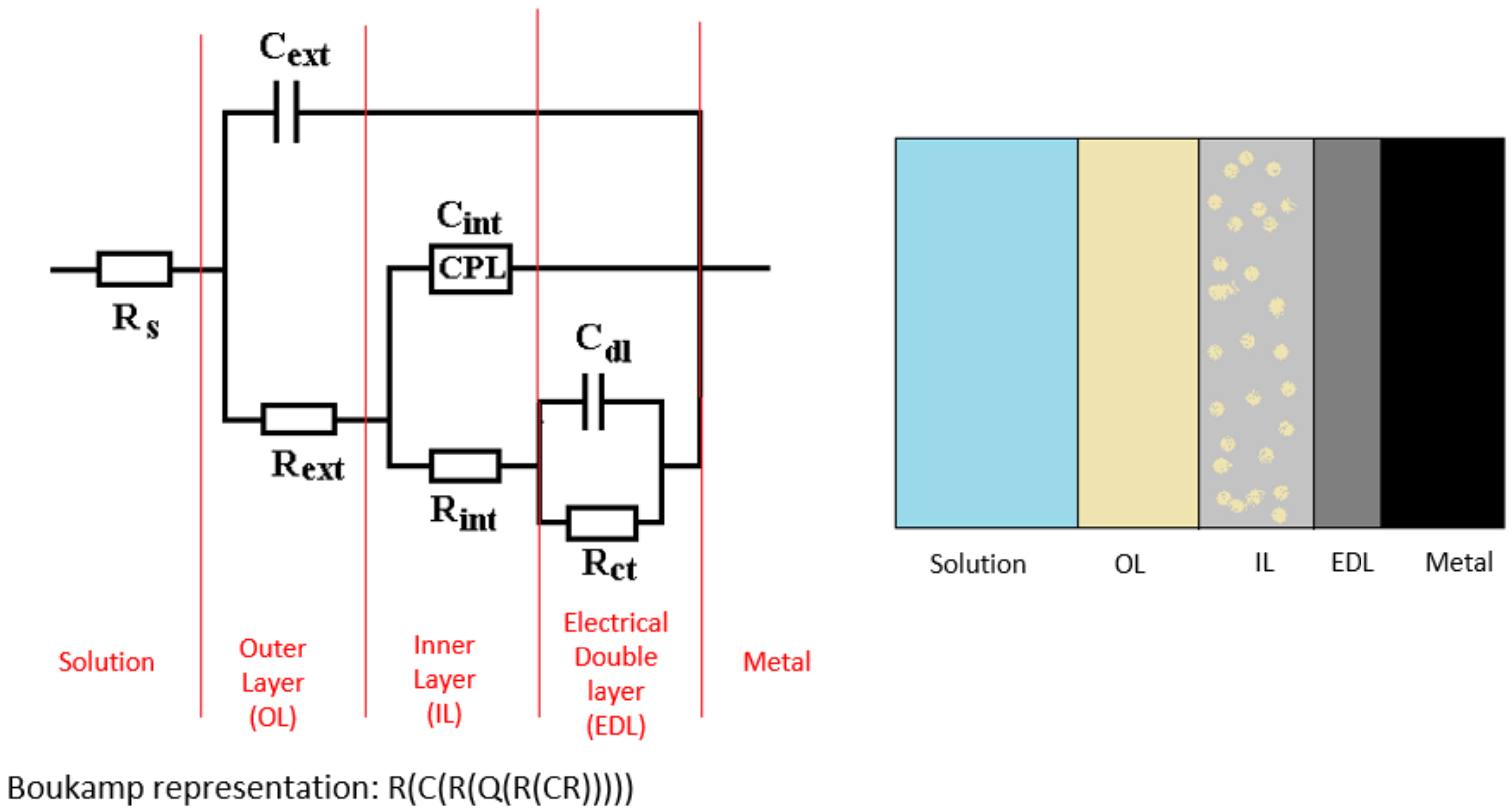
| Sample | Rs Ω·cm2 | Cext nF/cm2 | Rext Ω·cm2 | CPE | Rct Ω·cm2 | ε(Z) % | 104·χ2 | |
|---|---|---|---|---|---|---|---|---|
| Qdl S·sn/cm2 | n | |||||||
| DWW-1 | 326 | 0.114 | 1016 | 1.72 × 10−4 | 0.814 | 1182 | 1.81 | 3.29 |
| DWW-2 | 158 | 6.14 | 20.44 | 3.61 × 10−4 | 0.753 | 284 | 2.16 | 4.67 |
| DWW-3 | 205 | 4.18 | 31.96 | 8.56 × 10−4 | 0.740 | 584 | 1.72 | 2.96 |
| Sample | Rs Ω·cm2 | Cext nF/cm2 | Rext Ω·cm2 | CPE | Rct Ω·cm2 | ε(Z) % | 104·χ2 | |
|---|---|---|---|---|---|---|---|---|
| Qdl S·sn/cm2 | n | |||||||
| DWW-1 | 347 | 0.370 | 325 | 1.42 × 10−4 | 0.816 | 1825 | 1.81 | 2.58 |
| DWW-2 | 117 | 5110 | 32.46 | 9.65 × 10−3 | 0.549 | 300 | 1.90 | 3.63 |
| DWW-3 | 205 | 4.27 | 31.76 | 8.28 × 10−4 | 0.743 | 572 | 1.72 | 2.52 |
| Sample | Rs Ω·cm2 | Cext nF/cm2 | Rext Ω·cm2 | CPE | Rint Ω·cm2 | Cdl µF/cm2 | Rct Ω·cm2 | ε(Z) % | 104·χ2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 102·Q S·sn/cm2 | n | |||||||||
| DWW-1 | 329 | 0.346 | 351 | 2.63 | 0.53 | 934 | 195 | 33 | 0.96 | 1.44 |
| DWW-2 | 95.1 | 2.037 | 34.0 | 1.17 | 0.38 | 62.6 | 2520 | 61 | 1.23 | 1.52 |
| DWW-3 | 132 | 2.761 | 33.1 | 6.77 | 0.52 | 164 | 114 | 93 | 1.51 | 1.12 |
| Sample | Rs Ω·cm2 | Cext nF/cm2 | Rext Ω·cm2 | W S·s1/2/cm2 | Rint Ω·cm2 | Cdl µF/cm2 | Rct Ω·cm2 | ε(Z) % | 104·χ2 |
|---|---|---|---|---|---|---|---|---|---|
| DWW-1 | 308 | 0.311 | 347 | 0.170 | 98.2 | 2390 | 213 | 1.08 | 1.15 |
| DWW-2 | 128 | 4.012 | 22.0 | 0.027 | 11.4 | 10.04 | 226 | 2.03 | 4.14 |
| DWW-3 | 148 | 4.855 | 31.1 | 0.081 | 164 | 247 | 113 | 1.46 | 2.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nejneru, C.; Burduhos-Nergis, D.-P.; Axinte, M.; Perju, M.C.; Bejinariu, C. Corrosion Behaviour of Nodular Cast Iron Used for Rotor Manufacturing in Different Wastewaters. Coatings 2022, 12, 911. https://doi.org/10.3390/coatings12070911
Nejneru C, Burduhos-Nergis D-P, Axinte M, Perju MC, Bejinariu C. Corrosion Behaviour of Nodular Cast Iron Used for Rotor Manufacturing in Different Wastewaters. Coatings. 2022; 12(7):911. https://doi.org/10.3390/coatings12070911
Chicago/Turabian StyleNejneru, Carmen, Diana-Petronela Burduhos-Nergis, Mihai Axinte, Manuela Cristina Perju, and Costica Bejinariu. 2022. "Corrosion Behaviour of Nodular Cast Iron Used for Rotor Manufacturing in Different Wastewaters" Coatings 12, no. 7: 911. https://doi.org/10.3390/coatings12070911
APA StyleNejneru, C., Burduhos-Nergis, D.-P., Axinte, M., Perju, M. C., & Bejinariu, C. (2022). Corrosion Behaviour of Nodular Cast Iron Used for Rotor Manufacturing in Different Wastewaters. Coatings, 12(7), 911. https://doi.org/10.3390/coatings12070911








