Effects of Physicochemical Parameters on Struvite Crystallization Based on Kinetics
Abstract
:1. Introduction
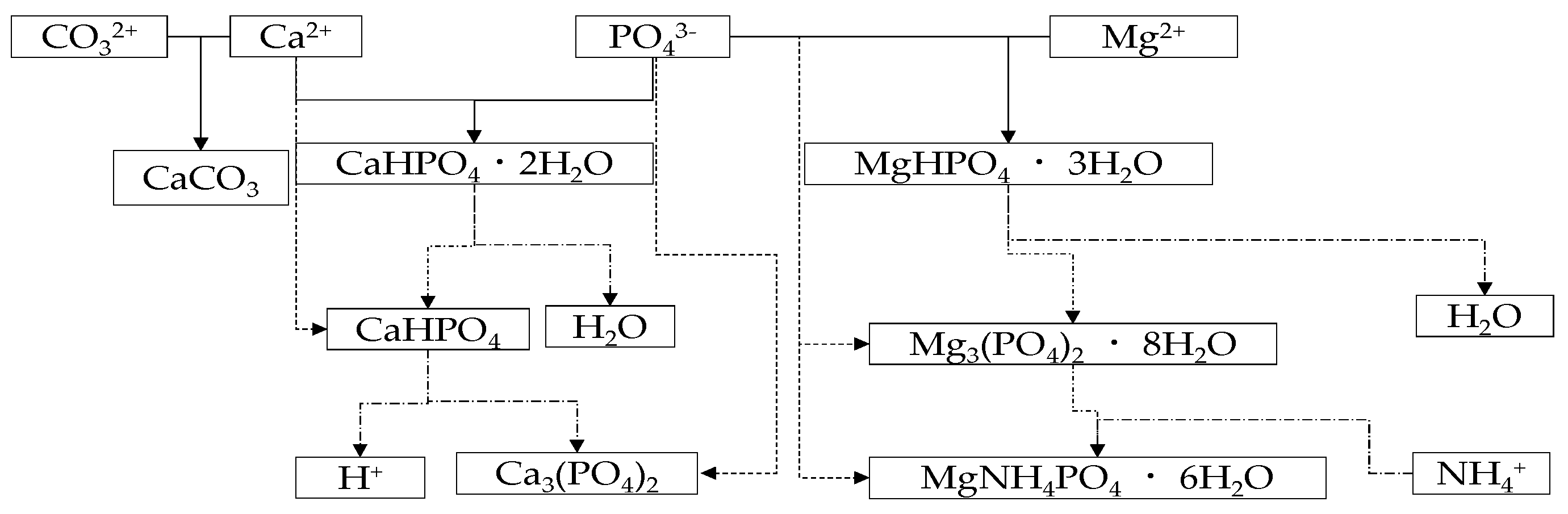
2. Model of Struvite Crystallization
3. The Parameters That Influence Struvite Crystallization
3.1. pH
3.2. Molar Ratio of Mg:N:P
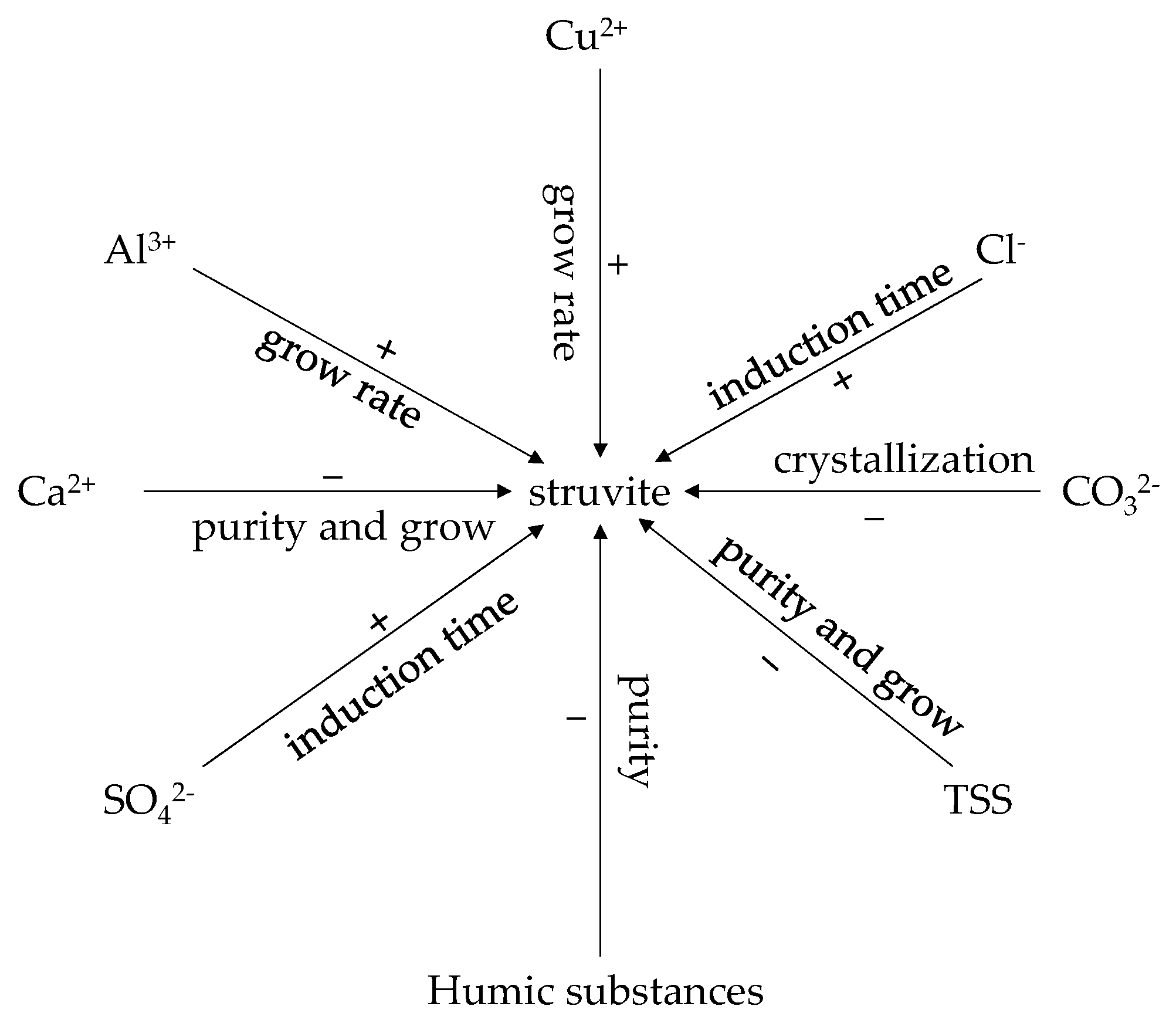
3.3. The Effects of Foreign Impurities on Struvite Crystallization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Wu, J.; He, Y.; Zhang, Y.; Yu, R.; Lu, X. Enhanced Nutrient Removal in A2N Effluent by Reclaimed Biochar Adsorption. Int. J. Environ. Res. Public Health 2022, 19, 4016. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Yu, S.I.; Im, K.; Shin, J.; Shin, S.G. Responses of Coagulant Type, Dosage and Process Conditions to Phosphate Removal Efficiency from Anaerobic Sludge. Int. J. Environ. Res. Public Health 2022, 19, 1693. [Google Scholar] [CrossRef] [PubMed]
- Nur, T.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Phosphate Adsorption from Membrane Bioreactor Effluent Using Dowex 21K XLT and Recovery as Struvite and Hydroxyapatite. Int. J. Environ. Res. Public Health 2016, 13, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaterell, M.R.; Gay, R.; Wilson, R.; Gochin, R.J.; Lester, J.N. An Economic and Environmental Evaluation of the Opportunities for Substituting Phosphorus Recovered from Wastewater Treatment Works in Existing UK Fertiliser Markets. Environ. Technol. Lett. 2017, 21, 1067–1084. [Google Scholar] [CrossRef]
- Li, B.; Huang, H.M.; Boiarkina, I.; Yu, W.; Young, B.R. Phosphorus recovery through struvite crystallisation: Recent developments in the understanding of operational factors. J. Environ. Manag. 2019, 248, 109254. [Google Scholar] [CrossRef]
- Farrow, C.; Crolla, A.; Kinsley, C.; Mcbean, E. Ammonia removal from poultry manure leachate via struvite precipitation: A strategy for more efficient anaerobic digestion. Int. J. Environ. Technol. Manag. 2017, 20, 87–100. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Benjannet, R.; Nyiraneza, J.; Khiari, L.; Cambouris, A.; Ziadi, N. Potato response to struvite in comparison with conventional phosphorus fertilizer in Eastern Canada. Agron. J. 2020, 112, 1360–1376. [Google Scholar] [CrossRef]
- Rawn, A.M.; Banta, A.P.; Pomeroy, R. Multiple-Stage Sewage Sludge Digestion. Am. Soc. Civ. Eng. 1939, 104, 93–119. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Gonzalez-Martinez, A.; Gonzalez-Lopez, J.; Martin-Ramos, D.; Martinez-Toledo, M.V.; Rivadeneyra, M.A. Precipitation of Phosphate Minerals by Microorganisms Isolated from a Fixed-Biofilm Reactor Used for the Treatment of Domestic Wastewater. Int. J. Environ. Res. Public Health 2014, 11, 3689–3704. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Jose, S.; Tyagi, M.; Jagadevan, S. A holistic and sustainable approach for recovery of phosphorus via struvite crystallization from synthetic distillery wastewater. J. Clean. Prod. 2020, 254, 120037. [Google Scholar] [CrossRef]
- Iaconi, C.D.; Pagano, M.; Ramadori, R.; Lopez, A. Nitrogen recovery from a stabilized municipal landfill leachate. Bioresour. Technol. 2010, 101, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Bayuseno, A.P.; Schmahl, W.W. Crystallization of struvite in a hydrothermal solution with and without calcium and carbonate ions. Chemosphere 2020, 250. [Google Scholar] [CrossRef] [PubMed]
- Ariyanto, E.; Ang, H.M.; Sen, T.K. Impact of various physico-chemical parameters on spontaneous nucleation of struvite (MgNH4PO4·6H2O) formation in a wastewater treatment plant: Kinetic and nucleation mechanism. Desalination Water Treat. 2014, 52, 6620–6631. [Google Scholar] [CrossRef]
- Almatouq, A.; Babatunde, A.O. Concurrent Phosphorus Recovery and Energy Generation in Mediator-Less Dual Chamber Microbial Fuel Cells: Mechanisms and Influencing Factors. Int. J. Environ. Res. Public Health 2016, 13, 375. [Google Scholar] [CrossRef] [Green Version]
- Burns, M.; Schneider, P.A.; Sheehan, M. Nucleation and crystal growth kinetic parameter optimization of a Continuous Poiseuille flow struvite crystallizer using a discretized population balance and dynamic fluid model. Chem. Eng. J. 2020, 405, 126607. [Google Scholar] [CrossRef]
- Hutnik, N.; Stanclik, A.; Piotrowski, K.; Mat Yn Ia, A. Kinetic conditions of struvite continuous reaction crystallisation from wastewater in presence of aluminium(III) and iron(III) ions. Int. J. Environ. Pollut. 2018, 64, 358. [Google Scholar] [CrossRef]
- Bhuiyan, M.; Mavinic, D.S.; Beckie, R.D. Nucleation and growth kinetics of struvite in a fluidized bed reactor. J. Cryst. Growth 2008, 310, 1187–1194. [Google Scholar] [CrossRef]
- Bayuseno, A.P.; Perwitasari, D.S.; Muryanto, S.; Tauviqirrahman, M.; Jamari, J. Kinetics and morphological characteristics of struvite (MgNH4PO4.6H2O) under the influence of maleic acid. Heliyon 2020, 6, e03533. [Google Scholar] [CrossRef]
- Tao, W.; Fattah, K.P.; Huchzermeier, M.P. Struvite recovery from anaerobically digested dairy manure: A review of application potential and hindrances. J. Env. Manag. 2016, 169, 46–57. [Google Scholar] [CrossRef]
- Wei, L.; Hong, T.; Cui, K.; Chen, T.; Zhou, Y.; Zhao, Y.; Yin, Y.; Wang, J.; Zhang, Q. Probing the effect of humic acid on the nucleation and growth kinetics of struvite by constant composition technique. Chem. Eng. J. 2019, 378, 122130. [Google Scholar] [CrossRef]
- Corona, F.; Hidalgo, D.; Martín-Marroquín, J.M.; Antolín, G. Study of the influence of the reaction parameters on nutrients recovering from digestate by struvite crystallisation. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bouropoulos, N.C.; Koutsoukos, P.G. Spontaneous precipitation of struvite from aqueous solutions. J. Cryst. Growth 2000, 213, 381–388. [Google Scholar] [CrossRef]
- Fromberg, M.; Pawlik, M.; Mavinic, D.S. Induction time and zeta potential study of nucleating and growing struvite crystals for phosphorus recovery improvements within fluidized bed reactors. Powder Technol. 2019, 360, 715–730. [Google Scholar] [CrossRef]
- Mazienczuk, A.; Matynia, A.; Piotrowski, K.; Wierzbowska, B. Continuous Reaction Crystallization of Struvite in a DTM Type Crystallizer With Jet Pump of Ascending Suspension Flow in a Mixing Chamber–Kinetic Approach of the Process. J. Cryst. Process Technol. 2012, 02, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Hutnik, N.; Stanclik, A.; Piotrowski, K.; Matynia, A. Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions. Open Chem. 2020, 18, 196–206. [Google Scholar] [CrossRef]
- Mehta, C.M.; Batstone, D.J. Nucleation and growth kinetics of struvite crystallization. Water Res. 2013, 47, 2890–2900. [Google Scholar] [CrossRef]
- Srisanga, S.; Flood, A.E.; Galbraith, S.C.; Rugmai, S.; Soontaranon, S.; Ulrich, J. Crystal Growth Rate Dispersion versus Size-Dependent Crystal Growth: Appropriate Modeling for Crystallization Processes. Cryst. Growth Des. 2015, 15, 2330–2336. [Google Scholar] [CrossRef]
- Garside, J.; Jani, S.J. Prediction and measurement of crystal size distributions for size-dependent growth. Chem. Eng. Sci. 1978, 33, 1623–1630. [Google Scholar] [CrossRef]
- Jones, J. On the estimation of size-dependent crystal growth rate functions in MSMPR crystallizers. Chem. Eng. J. Biochem. Eng. J. 1993, 53, 125–135. [Google Scholar] [CrossRef]
- Mydlarz, J. An Exponential-Hyperbolic Crystal Growth Rate Model. Cryst. Res. Technol. 1995, 30, 747–761. [Google Scholar] [CrossRef]
- Rojkowski, Z. New empirical kinetic equation of size dependent crystal growth and its use. Krist. Und Tech. 1977, 12, 1121–1128. [Google Scholar] [CrossRef]
- Stanclik, A.; Hutnik, N.; Piotrowski, K.; Matynia, A. Struvite nucleation and crystal growth kinetics from cattle liquid manure. Chem. Pap. 2019, 73, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Perwitasari, D.S.; Muryanto, S.; Jamari, J.; Bayuseno, A.P. Kinetics and morphology analysis of struvite precipitated from aqueous solution under the influence of heavy metals: Cu2+, Pb2+, Zn2+. J. Environ. Chem. Eng. 2018, 6, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Quintana, M.; Sánchez, E.; Colmenarejo, M.F.; Barrera, J.; García, G.; Borja, R. Kinetics of phosphorus removal and struvite formation by the utilization of by-product of magnesium oxide production. Chem. Eng. J. 2005, 111, 45–52. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Ellis, N.; Mavinic, D.S. Effects of various process parameters on struvite precipitation kinetics and subsequent determination of rate constants. Water Sci. Technol. 2008, 57, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Nelson, N.O.; Mikkelsen, R.L.; Hesterberg, D.L. Struvite precipitation in anaerobic swine lagoon liquid: Effect of pH and Mg:P ratio and determination of rate constant. Bioresour. Technol. 2003, 89, 229–236. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Kinetics of Struvite Precipitation: Effect of the Magnesium Dose on Induction Times and Precipitation Rates. Environ. Technol. 2007, 28, 1317–1324. [Google Scholar] [CrossRef] [Green Version]
- Crutchik, D.; Garrido, J.M. Kinetics of the reversible reaction of struvite crystallisation. Chemosphere 2016, 154, 567–572. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Z.; Wang, H.; Yan, Q. Optimization and reaction kinetics analysis for phosphorus removal in struvite precipitation process. Water Environ. Res. 2020, 92, 1162–1172. [Google Scholar] [CrossRef]
- Ge, K.; Ji, Y.; Tang, S. Crystallization Kinetics and Mechanism of Magnesium Ammonium Phosphate Hexahydrate: Experimental Investigation and Chemical Potential Gradient Model Analysis and Prediction. Ind. Eng. Chem. Res. 2020, 59, 13799–13809. [Google Scholar] [CrossRef]
- Bhuiyan, M.; Mavinic, D.S.; Beckie, R.D. Dissolution kinetics of struvite pellets grown in a pilot-scale crystallizer. Can. J. Civ. Eng. 2009, 36, 550–568. [Google Scholar] [CrossRef]
- Harrison, M.L.; Johns, M.R.; White, E.T.; Mehta, C.M. Growth Rate Kinetics for Struvite Crystallisation. Chem. Eng. Trans. 2011, 25, 309–314. [Google Scholar]
- Li, X.Z.; Zhao, Q.L.; Hao, X.D. Ammonium removal from landfill leachate by chemical precipitation. Int. J. Innov. Res. Dev. 2014, 19, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Ariyanto, E.; Ang, H.M.; Sen, T.K. Effect of initial solution pH on solubility morphology of and struvite crystal. In Proceedings of the CHEMECA Conference, Sydney, NSW, Australia, 18–21 September 2011. [Google Scholar]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Ariyanto, E.; Sen, T.K.; Ang, H.M. The influence of various physico-chemical process parameters on kinetics and growth mechanism of struvite crystallisation. Adv. Powder Technol. 2014, 25, 682–694. [Google Scholar] [CrossRef]
- Prywer, J.; Sadowski, R.R.; Torzewska, A. Aggregation of Struvite, Carbonate Apatite, and Proteus mirabilis as a Key Factor of Infectious Urinary Stone Formation. Cryst. Growth Des. 2015, 15, 1446–1451. [Google Scholar] [CrossRef]
- Matynia, A.; Koralewska, J.; Wierzbowska, B.; Piotrowski, K. The influence of process parameters on struvite continuous crystallization kinetics. Chem. Eng. Commun. 2006, 193, 160–176. [Google Scholar] [CrossRef]
- Kozik, A.; Hutnik, N.; Piotrowski, K.; Mazienczuk, A.; Matynia, A. Precipitation and Crystallization of Struvite from Synthetic Wastewater under Stoichiometric Conditions. Adv. Chem. Eng. Sci. 2013, 3, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, M.; Grasel, P.; Martin, L.; Tawfiq, K.; Chen, G. Ammonia removal from landfill leachate by struvite precipitation/coated silica sand filtration. Environ. Waste Manag. 2015, 15, 201. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, P.; Pal, P. Turning hazardous waste into value-added products: Production and characterization of struvite from ammoniacal waste with new approaches. J. Clean. Prod. 2013, 43, 59–70. [Google Scholar] [CrossRef]
- Gong, W.; Li, Y.; Luo, L.; Luo, X.; Cheng, X.; Liang, H. Application of Struvite-MAP Crystallization Reactor for Treating Cattle Manure Anaerobic Digested Slurry: Nitrogen and Phosphorus Recovery and Crystal Fertilizer Efficiency in Plant Trials. Int. J. Environ. Res. Public Health 2018, 15, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siciliano, A.; Siciliano, A.; Siciliano, A. Assessment of fertilizer potential of the struvite produced from the treatment of methanogenic landfill leachate using low-cost reagents. Env. Sci. Pollut. Res. Int. 2016, 23, 5949–5959. [Google Scholar] [CrossRef] [PubMed]
- AHutnik, N.; Kozik, A.; Mazienczuk, A.; Piotrowski, K.; Wierzbowska, B.; Matynia, A. Phosphates (V) recovery from phosphorus mineral fertilizers industry wastewater by continuous struvite reaction crystallization process. Water Res. 2013, 47, 3635–3643. [Google Scholar] [CrossRef]
- Giesen, A.; Giesen, A.; Giesen, A. Crystallisation Process Enables Environmental Friendly Phosphate Removal at Low Costs. Environ. Technol. 2010, 20, 769–775. [Google Scholar] [CrossRef]
- Capdevielle, A.; Sýkorová, E.; Biscans, B.; Béline, F.; Daumer, M.-L. Optimization of struvite precipitation in synthetic biologically treated swine wastewater—Determination of the optimal process parameters. J. Hazard. Mater. 2013, 244–245, 357–369. [Google Scholar] [CrossRef] [Green Version]
- Doyoung, K.; Ch Ara, O.; Mccoy, C.P.; Irwin, N.J.; Rimer, J.D. Time-Resolved Dynamics of Struvite Crystallization: Insights from the Macroscopic to Molecular Scale. Chemistry 2020, 26, 3555–3563. [Google Scholar] [CrossRef]
- Galbraith, S.C.; Schneider, P.A.; Flood, A.E. Model-driven experimental evaluation of struvite nucleation, growth and aggregation kinetics. Water Res. 2014, 56C, 122–132. [Google Scholar] [CrossRef]
- Koralewska, J.; Piotrowski, K.; Wierzbowska, B.; Matynia, A. Reaction—Crystallization of Struvite in a Continuous Liquid Jet—Pump DTM MSMPR Crystallizer with Upward Circulation of Suspension in a Mixing Chamber—An SDG Kinetic Approach. Chem. Eng. Technol. 2010, 30, 1576–1583. [Google Scholar] [CrossRef]
- Hakimi, M.H.; Jegatheesan, V.; Navaratna, D. The potential of adopting struvite precipitation as a strategy for the removal of nutrients from pre-AnMBR treated abattoir wastewater. J. Environ. Manag. 2020, 259, 109783. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef] [Green Version]
- Yaakoubi, M.; Kinoshita, S.; Liu, B.; Ha, N.T.; Van, L. Modelling Multiple Mineral Precipitation in Anaerobic Digestion Process. In Proceedings of the 7th IWA-ASPIRE Conference and Exhibition, Kuala Lumpur, Malaysia, 13–16 September 2017. [Google Scholar]
- Hutnik, N.; Stanclik, A.; Piotrowski, K.; Matynia, A. Effect of Copper and Zinc Ions on Struvite Nucleation and Crystal Growth Kinetics in Various Process Environments. Pol. J. Environ. Stud. 2020, 29, 2225–2233. [Google Scholar] [CrossRef]
- Hutnik, N.; Stanclik, A.; Piotrowski, K.; Matynia, A. Size-dependent growth kinetics in continuous struvite reaction crystallization in wastewaters and cattle liquid manure with potassium ions. Przem. Chem. 2019, 98, 644–649. [Google Scholar] [CrossRef]
- Ping, Q.; Li, Y.; Wu, X.; Yang, L.; Wang, L. Characterization of morphology and component of struvite pellets crystallized from sludge dewatering liquor: Effects of total suspended solid and phosphate concentrations. J. Hazard. Mater. 2016, 310, 261–269. [Google Scholar] [CrossRef]
- Li, S.; Zeng, W.; Xu, H.; Jia, Z.; Peng, Y. Performance investigation of struvite high-efficiency precipitation from wastewater using silicon-doped magnesium oxide. Environ. Sci. Pollut. Res. 2020, 27, 15463–15474. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dai, Y.; Hu, Q.; Yu, X.; Qian, F. Effects of three kinds of organic acids on phosphorus recovery by magnesium ammonium phosphate (MAP) crystallization from synthetic swine wastewater. Chemosphere 2014, 101, 41–48. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, S.; Ye, X.; Xiao, W. Effects of organic substances on struvite crystallization and recovery. Desalination Water Treat. 2015, 57, 10924–10933. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, D.; Ren, W.; Zhao, Y.; Jiang, L.M.; Wang, L. Effect of humic substances on phosphorus removal by struvite precipitation. Chemosphere 2015, 141, 94–99. [Google Scholar] [CrossRef]
| References | ||
|---|---|---|
b < 1 | [29] | |
a > 0, c ≠ 0 | [30] | |
| [31] | ||
| [32] |
| Research Object | pH (−) | Temperature | Molar Ratio (Mg:p) | k (min−1) | R2 (−) | References |
|---|---|---|---|---|---|---|
| Phosphate concentration | 7.5 | 22–25 | 1.5 | 0.039 | >0.92 | [35] |
| Phosphate concentration | 8.51 | 20 | 1.6 | 0.045 | 0.97 | [36] |
| Phosphate concentration | 8.4 | 22–24 | 1.2 | 0.061 | 0.96 | [37] |
| Magnesium concentration | 9.0 | 30 | 1.0 | 0.109 | 0.99 | [19] |
| Magnesium concentration | 9.0 | 20 | 0.5 | 0.156 | >0.92 | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Li, Y.; Xu, B.; Li, M.; Wang, J.; Shao, Y.; Chen, F.; Sun, M.; Liu, B. Effects of Physicochemical Parameters on Struvite Crystallization Based on Kinetics. Int. J. Environ. Res. Public Health 2022, 19, 7204. https://doi.org/10.3390/ijerph19127204
Wu J, Li Y, Xu B, Li M, Wang J, Shao Y, Chen F, Sun M, Liu B. Effects of Physicochemical Parameters on Struvite Crystallization Based on Kinetics. International Journal of Environmental Research and Public Health. 2022; 19(12):7204. https://doi.org/10.3390/ijerph19127204
Chicago/Turabian StyleWu, Jinzhu, Yifan Li, Baojian Xu, Mei Li, Jing Wang, Yuanyuan Shao, Feiyong Chen, Meng Sun, and Bing Liu. 2022. "Effects of Physicochemical Parameters on Struvite Crystallization Based on Kinetics" International Journal of Environmental Research and Public Health 19, no. 12: 7204. https://doi.org/10.3390/ijerph19127204
APA StyleWu, J., Li, Y., Xu, B., Li, M., Wang, J., Shao, Y., Chen, F., Sun, M., & Liu, B. (2022). Effects of Physicochemical Parameters on Struvite Crystallization Based on Kinetics. International Journal of Environmental Research and Public Health, 19(12), 7204. https://doi.org/10.3390/ijerph19127204







