Resistive-Based Gas Sensors Using Quantum Dots: A Review
Abstract
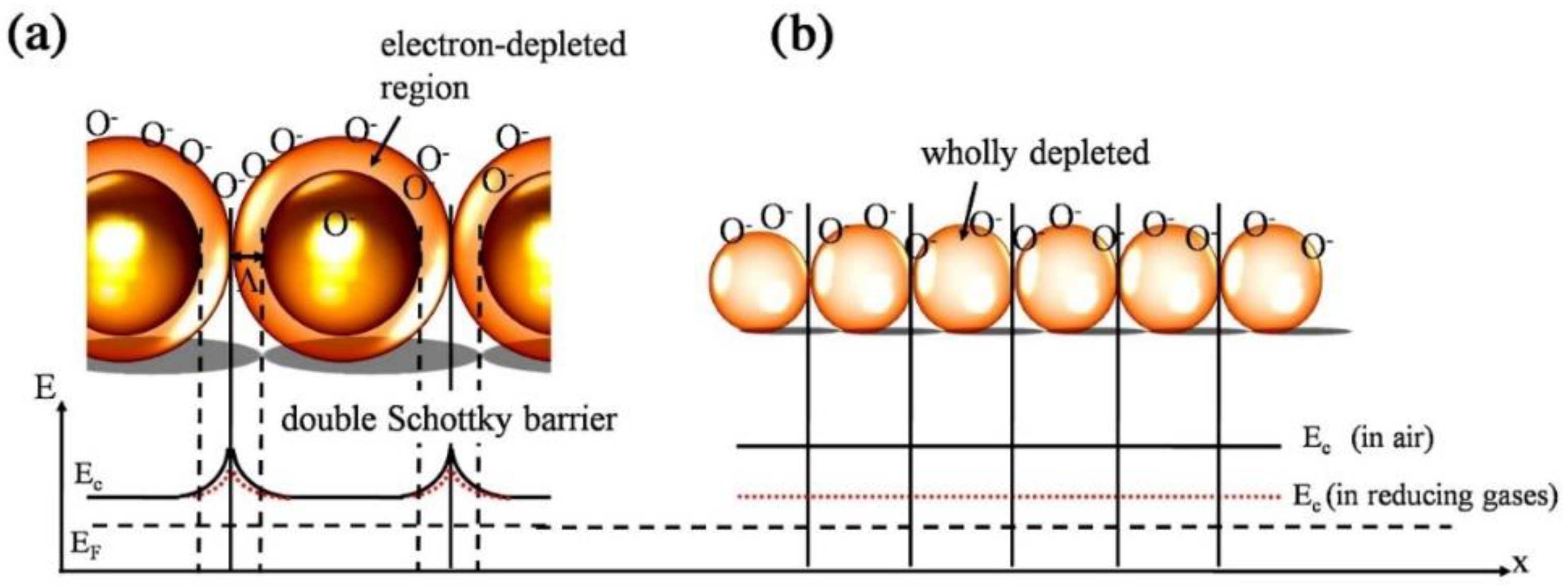
:1. Resistive-Based Gas Sensors: Basics
2. Quantum Dots: Definition and Applications
3. Resistive-Based Gas Sensors Based on QDs
3.1. Pristine Metal Oxide and Metal Sulfide Quantum Dot Gas Sensors
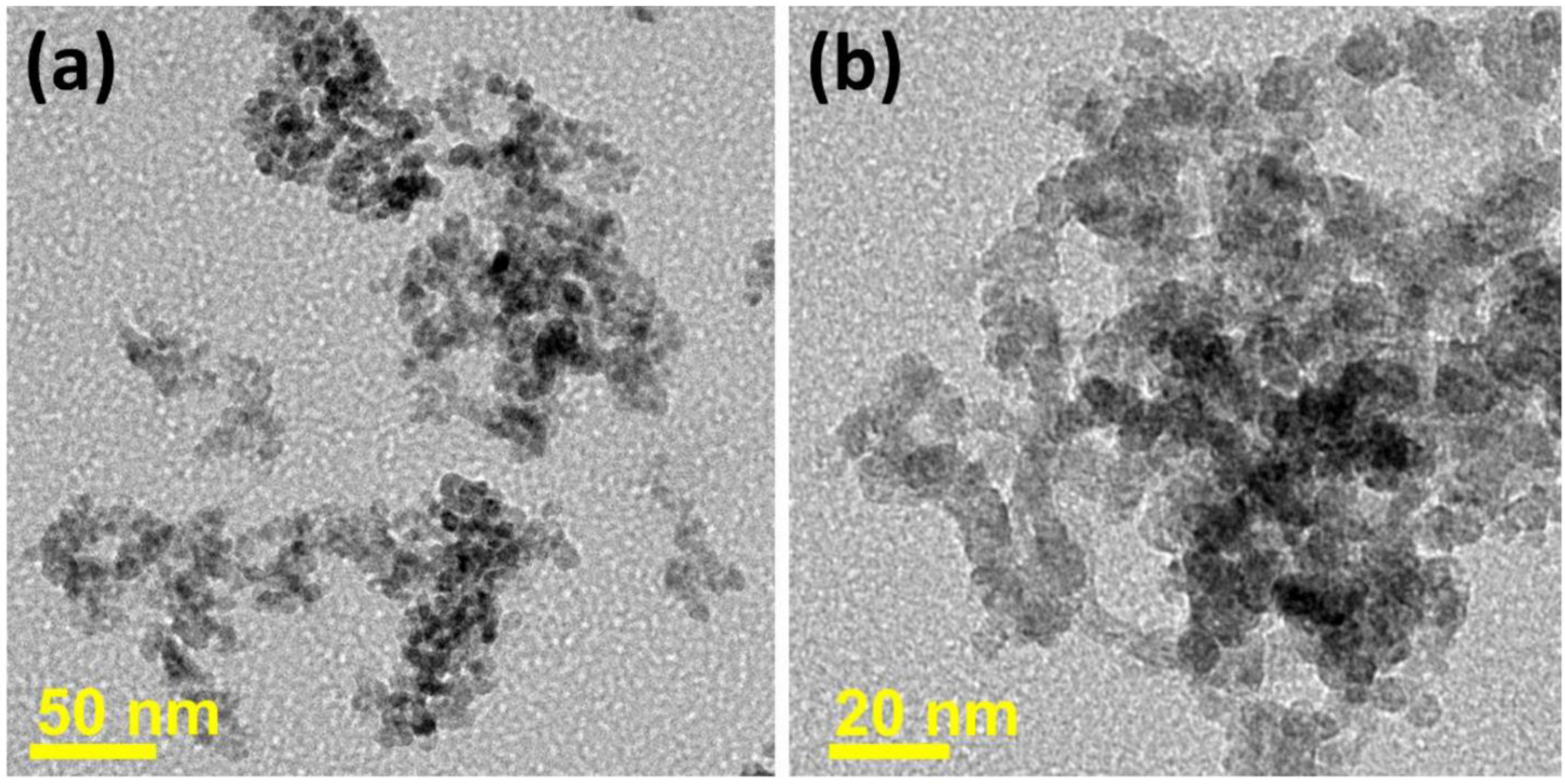
3.1.1. SnO2-Based Gas Sensors
3.1.2. ZnO QDs Gas Sensors
3.1.3. TiO2 QDs Gas Sensors
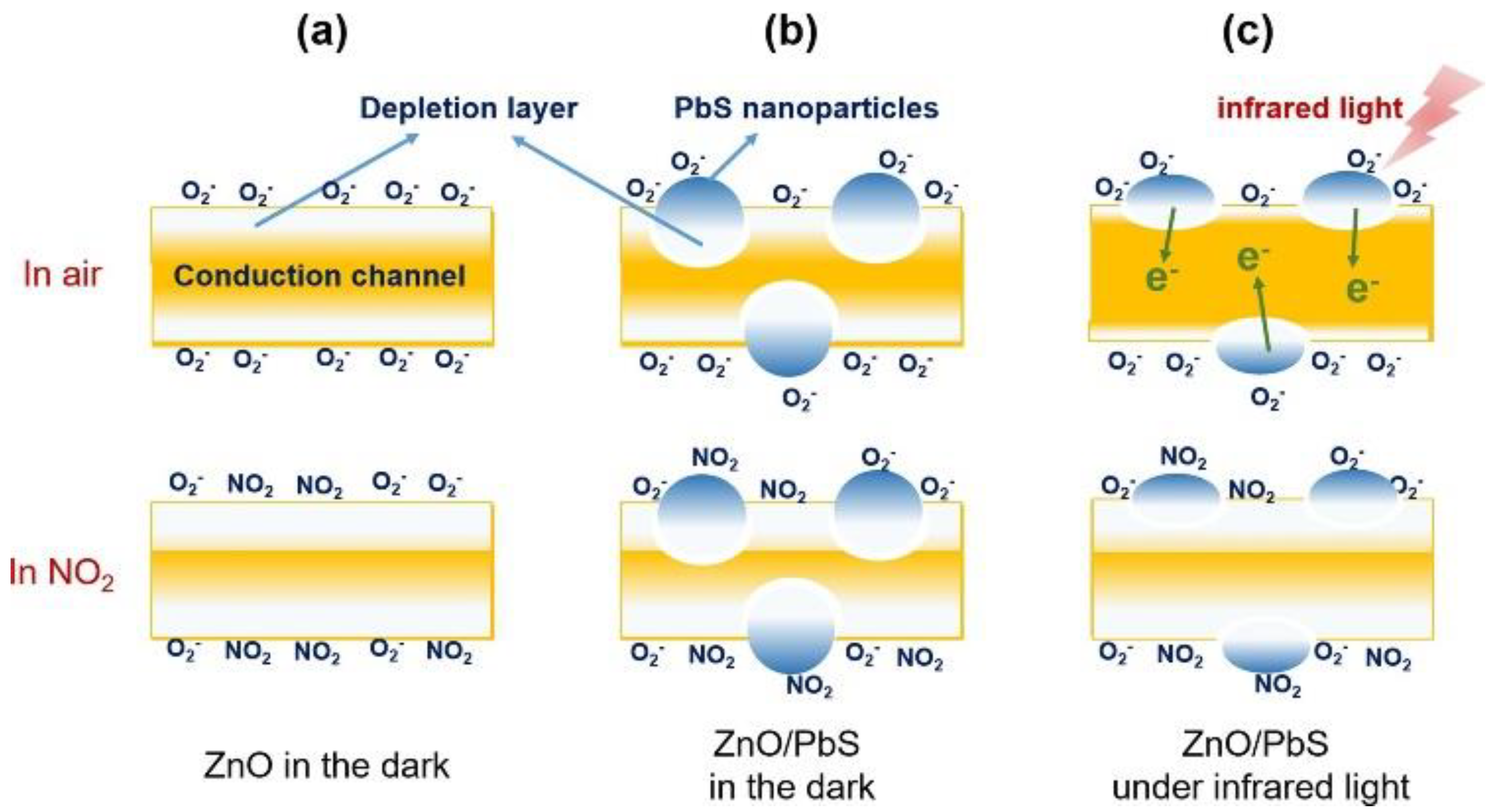
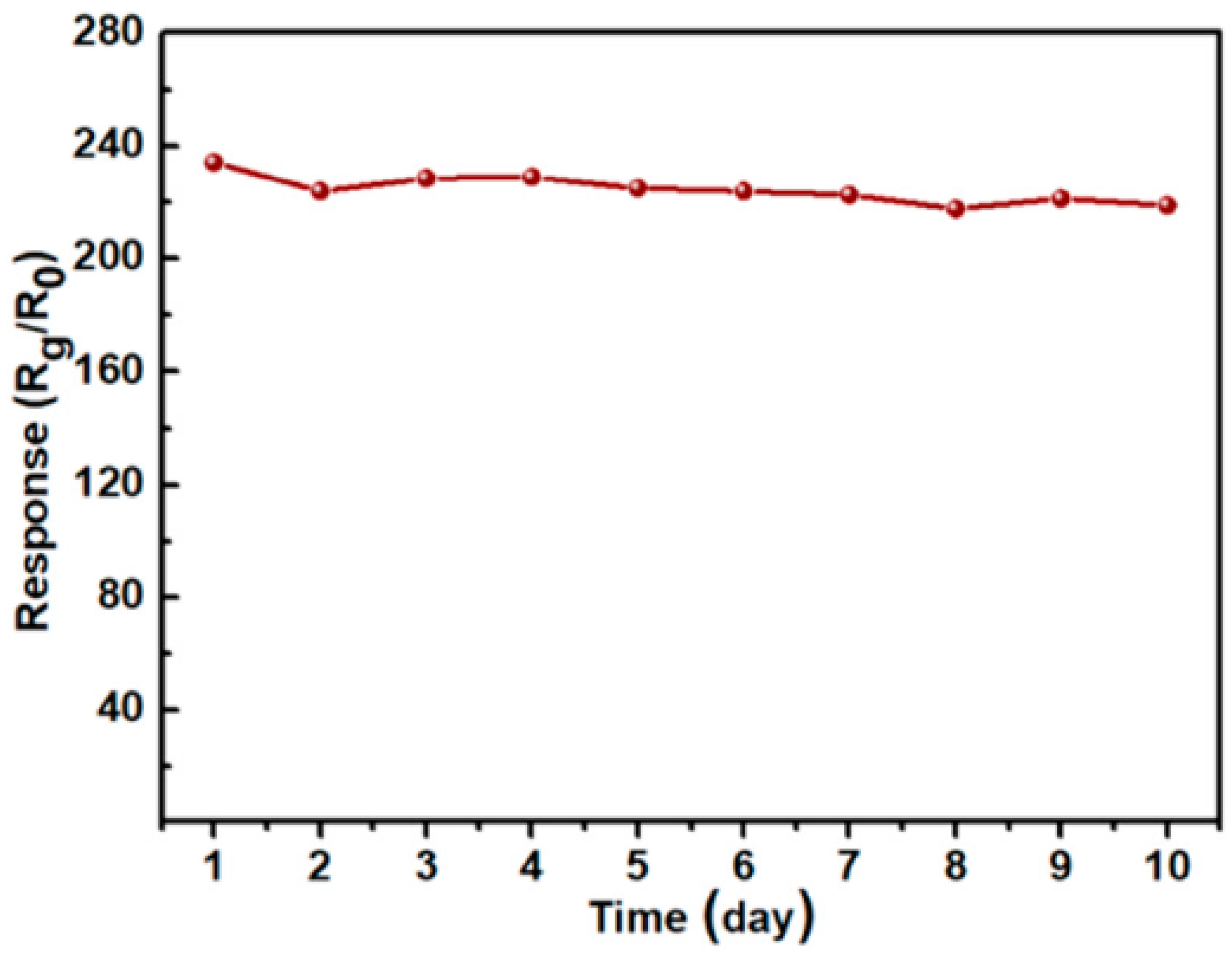
3.1.4. PbS QD Gas Sensors
3.1.5. ZnS QD Gas Sensor
3.1.6. SnS QD Gas Sensors
3.1.7. PbCdSe QD Gas Sensor
4. Resistive-Based Gas Sensors on Composite QDs
5. Resistive-Based Gas Sensors on Noble Metal Decorated QDs
6. Conclusions and Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Tian, X.; Zhao, Y.; Liu, L.; Li, Z.; Tao, L.; Wang, X.; Guo, X.; Luo, Y. Application of nonlinear land use regression models for ambient air pollutants and air quality index. Atmos. Pollut. Res. 2021, 12, 101186. [Google Scholar] [CrossRef]
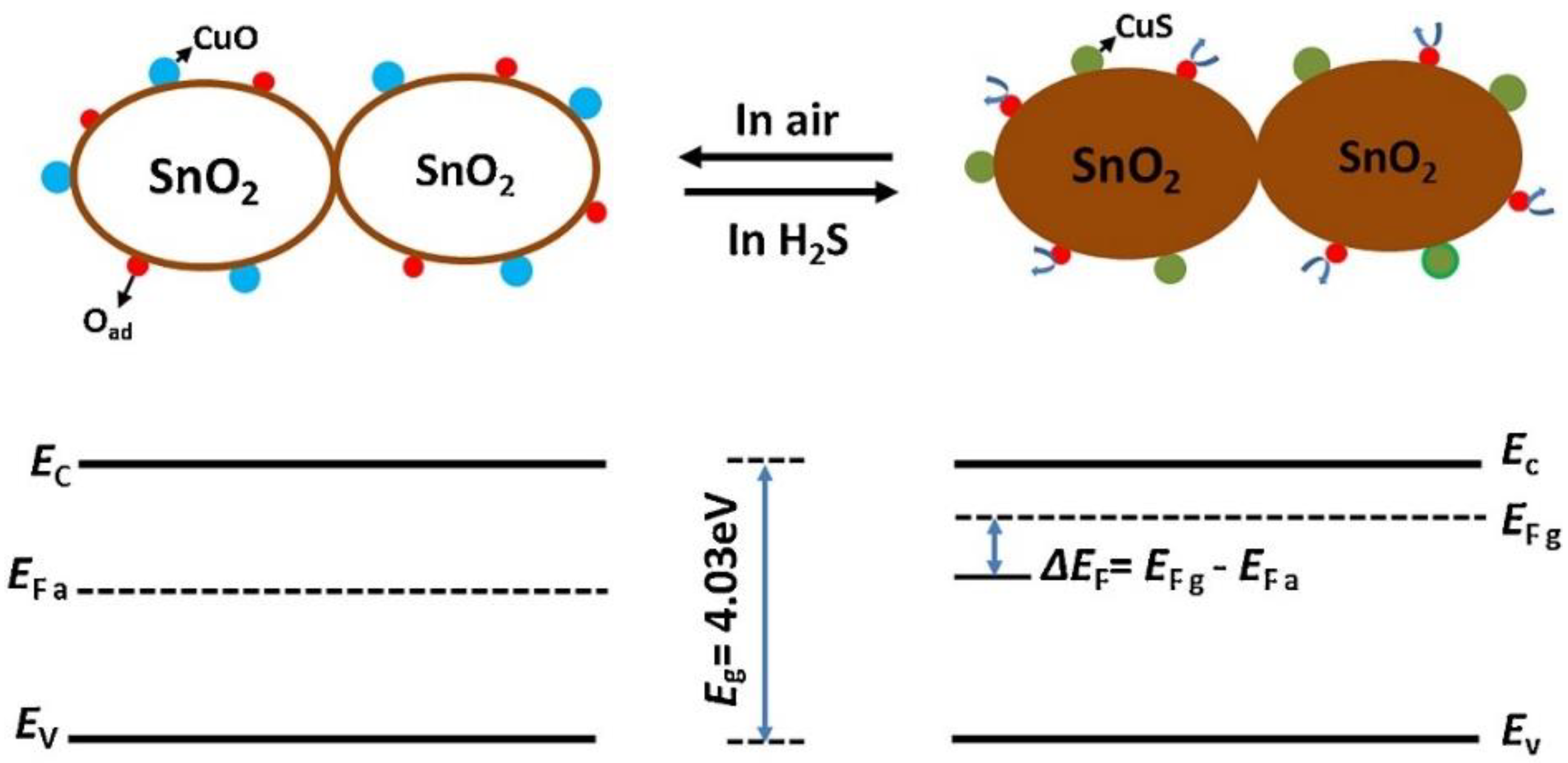
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.C.; Yu, J.B.; Lee, H.W.; Huh, J.S.; Lim, J.O. Investigation of exhaled breath samples from patients with Alzheimer’s disease using gas chromatography-mass spectrometry and an exhaled breath sensor system. Sensors 2017, 17, 1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalski, R.; Pecyna-Utylska, P.; Kernert, J. Determination of ammonium and biogenic amines by ion chromatography. A review. J. Chromatogr. A 2021, 1651, 462319. [Google Scholar] [CrossRef]
- Patial, P.; Deshwal, M.J.T.O.E.; Materials, E. Systematic review on design and development of efficient semiconductor based surface acoustic wave gas sensor. Transcation Electical Electron. Mater. 2021, 22, 1–9. [Google Scholar]
- Oprea, A.; Weimar, U.J.A.; Gas sensors based on mass-sensitive transducers. Part 1: Transducers and receptors—basic understanding. Anal. Bioanal. Chem. 2019, 411, 1761–1787. [Google Scholar]
- Popa, D.; Udrea, F.J.S. Towards integrated mid-infrared gas sensors. Sensors 2019, 19, 2076. [Google Scholar] [CrossRef] [Green Version]
- Hodgkinson, J.; Tatam, R.P.J.M.S. Optical gas sensing: A review. Meas. Sci. Technol. 2012, 24, 012004. [Google Scholar] [CrossRef] [Green Version]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-D.; Rothschild, A.; Tuller, H.L. Advances and new directions in gas-sensing devices. Acta Mater. 2013, 61, 974–1000. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Brattain, W.H.; Bardeen, J. Surface properties of germanium. Bell Syst. Tech. J. 1953, 32, 1–41. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Taguchi, N. A Metal Oxide Gas Sensor. Japanese Patent 4,538,200, 1962. [Google Scholar]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
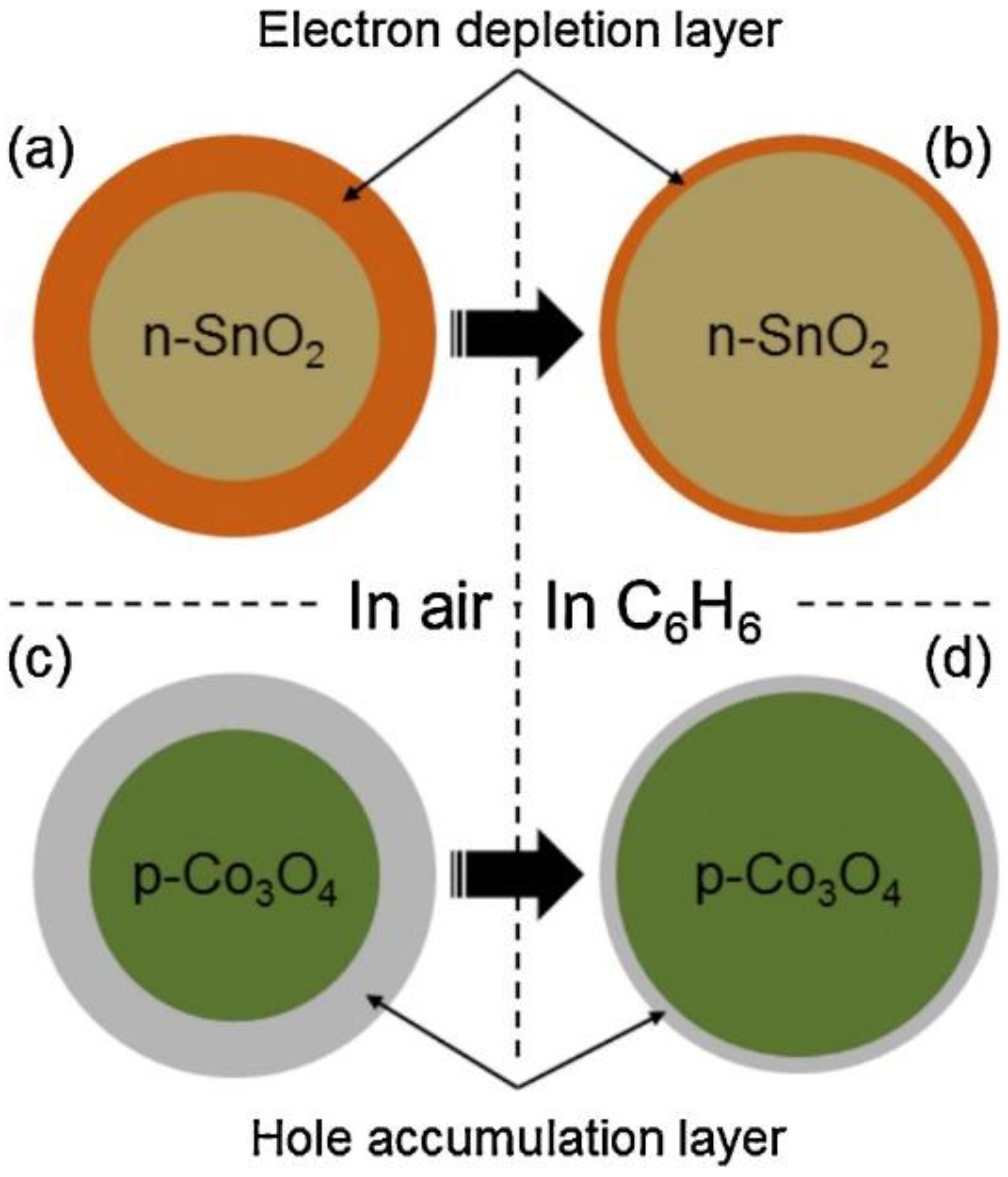
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Optimization and gas sensing mechanism of n-SnO2-p-Co3O4 composite nanofibers. Sens. Actuators B Chem. 2017, 248, 500–511. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Combination of Pd loading and electron beam irradiation for superior hydrogen sensing of electrospun ZnO nanofibers. Sens. Actuators B Chem. 2019, 284, 628–637. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Singhal, A.V.; Charaya, H.; Lahiri, I. Noble metal decorated graphene-based gas sensors and their fabrication: A review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 499–526. [Google Scholar] [CrossRef]
- Wang, C.N.; Li, Y.L.; Gong, F.L.; Zhang, Y.H.; Fang, S.M.; Zhang, H.L. Advances in doped ZnO nanostructures for gas sensor. Chem. Rec. 2020, 20, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Espid, E.; Taghipour, F. UV-LED photo-activated chemical gas sensors: A review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 416–432. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.-E. Gas sensing with heterostructures based on two-dimensional nanostructured materials: A review. J. Mater. Chem. C 2019, 7, 13367–13383. [Google Scholar] [CrossRef]
- Li, T.; Zeng, W.; Wang, Z. Quasi-one-dimensional metal-oxide-based heterostructural gas-sensing materials: A review. Sens. Actuators B Chem. 2015, 221, 1570–1585. [Google Scholar] [CrossRef]
- Nikolic, M.V. An overview of oxide materials for gas sensors. In Proceedings of the 2020 23rd International Symposium on Design and Diagnostics of Electronic Circuits & Systems (DDECS), Novi Sad, Serbia, 22–24 April 2020; pp. 1–4. [Google Scholar]
- Singh, S.; Singh, A.; Yadav, B.C.; Dwivedi, P.K. Fabrication of nanobeads structured perovskite type neodymium iron oxide film: Its structural, optical, electrical and LPG sensing investigations. Sens. Actuators B Chem. 2013, 177, 730–739. [Google Scholar] [CrossRef]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. A novel gas sensor based on Ag/Fe2O3 core-shell nanocomposites. Ceram. Int. 2016, 42, 18974–18982. [Google Scholar] [CrossRef]
- Kim, J.Y.; Voznyy, O.; Zhitomirsky, D.; Sargent, E.H.J.A.M. 25th anniversary article: Colloidal quantum dot materials and devices: A quarter-century of advances. Adv. Mater. 2013, 25, 4986–5010. [Google Scholar] [CrossRef]
- Bagher, A.M. Quantum dots applications. Sens. Transducers 2016, 198, 37. [Google Scholar]
- Mathew, M.; Preetha, K. An exploration into the quantum confinement of cts/natural dye core-shell quantum dots. Phys. B Condens. Matter 2020, 579, 411913. [Google Scholar] [CrossRef]
- Galstyan, V. Quantum dots: Perspectives in next-generation chemical gas sensors—A review. Anal. Chim. Acta 2021, 1152, 238192. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.M.; Mitra, S. Tin sulfide (SnS) nanorods: Structural, optical and lithium storage property study. RSC Adv. 2014, 4, 10358–10366. [Google Scholar] [CrossRef]
- Singh, M.K.; Mathpal, M.C.; Agarwal, A. Optical properties of SnO2 quantum dots synthesized by laser ablation in liquid. Chem. Phys. Lett. 2012, 536, 87–91. [Google Scholar] [CrossRef]
- Ren, X.; Jiang, C.H.; Li, D.D.; He, L. Fabrication of ZnO nanotubes with ultrathin wall by electrodeposition method. Mater. Lett. 2008, 62, 3114–3116. [Google Scholar] [CrossRef]
- Mamiyev, Z.Q.; Balayeva, N.O. Preparation and optical studies of PbS nanoparticles. Opt. Mater. 2015, 46, 522–525. [Google Scholar] [CrossRef]
- Pan, D.; Zhao, N.; Wang, Q.; Jiang, S.; Ji, X.; An, L. Facile synthesis and characterization of luminescent TiO2 nanocrystals. Adv. Mater. 2005, 17, 1991–1995. [Google Scholar] [CrossRef]
- Gayou, V.L.; Salazar Hernández, B.; Delgado Macuil, R.; Zavala, G.; Santiago, P.; Oliva, A.I. Structural studies of ZnS nanoparticles by high resolution transmission electron microscopy. J. Nano Res. 2010, 9, 125–132. [Google Scholar] [CrossRef]
- Sohila, S.; Ramesh, R.; Ramya, S.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and characterization of SnS/ZnO nanocomposite by chemical method. J. Mater. Sci. Mater. Electron. 2013, 24, 4807–4811. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, L. Competitive growth of In2O3 nanorods with rectangular cross sections. Appl. Phys. A 2008, 92, 401–405. [Google Scholar] [CrossRef]
- Barve, A.V.; Lee, S.J.; Noh, S.K.; Krishna, S. Review of current progress in quantum dot infrared photodetectors. Laser Photonics Rev. 2010, 4, 738–750. [Google Scholar] [CrossRef]
- Jun, H.K.; Careem, M.A.; Arof, A.K. Quantum dot-sensitized solar cells—Perspective and recent developments: A review of cd chalcogenide quantum dots as sensitizers. Renew. Sustain. Energy Rev. 2013, 22, 148–167. [Google Scholar] [CrossRef]
- Kouhnavard, M.; Ikeda, S.; Ludin, N.; Khairudin, N.A.; Ghaffari, B.; Mat-Teridi, M.; Ibrahim, M.; Sepeai, S.; Sopian, K. A review of semiconductor materials as sensitizers for quantum dot-sensitized solar cells. Renew. Sustain. Energy Rev. 2014, 37, 397–407. [Google Scholar] [CrossRef]
- Moon, H.; Lee, C.; Lee, W.; Kim, J.; Chae, H.J.A.M. Stability of quantum dots, quantum dot films, and quantum dot light-emitting diodes for display applications. Adv. Mater. 2019, 31, 1804294. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Huang, Z.; Liu, J.; Hu, Z.; Zhang, J.; Zhang, G.; Yi, F.; Jiang, S.; Lian, J.; Yan, J.; et al. Fully stretchable and humidity-resistant quantum dot gas sensors. ACS Sens. 2018, 3, 51048–51055. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Lee, J.-H.J.S.; Chemical, A.B. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous sno2-based elements. Sens. Actuators B Chem. 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Liu, J.; Lv, J.; Shi, J.; Wu, L.; Su, N.; Fu, C.; Zhang, Q. Size effects of tin oxide quantum dot gas sensors: From partial depletion to volume depletion. J. Mater. Res. Technol. 2020, 9, 16399–16409. [Google Scholar] [CrossRef]
- Chen, D.; Huang, S.; Huang, R.; Zhang, Q.; Le, T.-T.; Cheng, E.; Hu, Z.; Chen, Z. Highlights on advances in SnO2 quantum dots: Insights into synthesis strategies, modifications and applications. Mater. Res. Lett. 2018, 6, 462–488. [Google Scholar] [CrossRef] [Green Version]
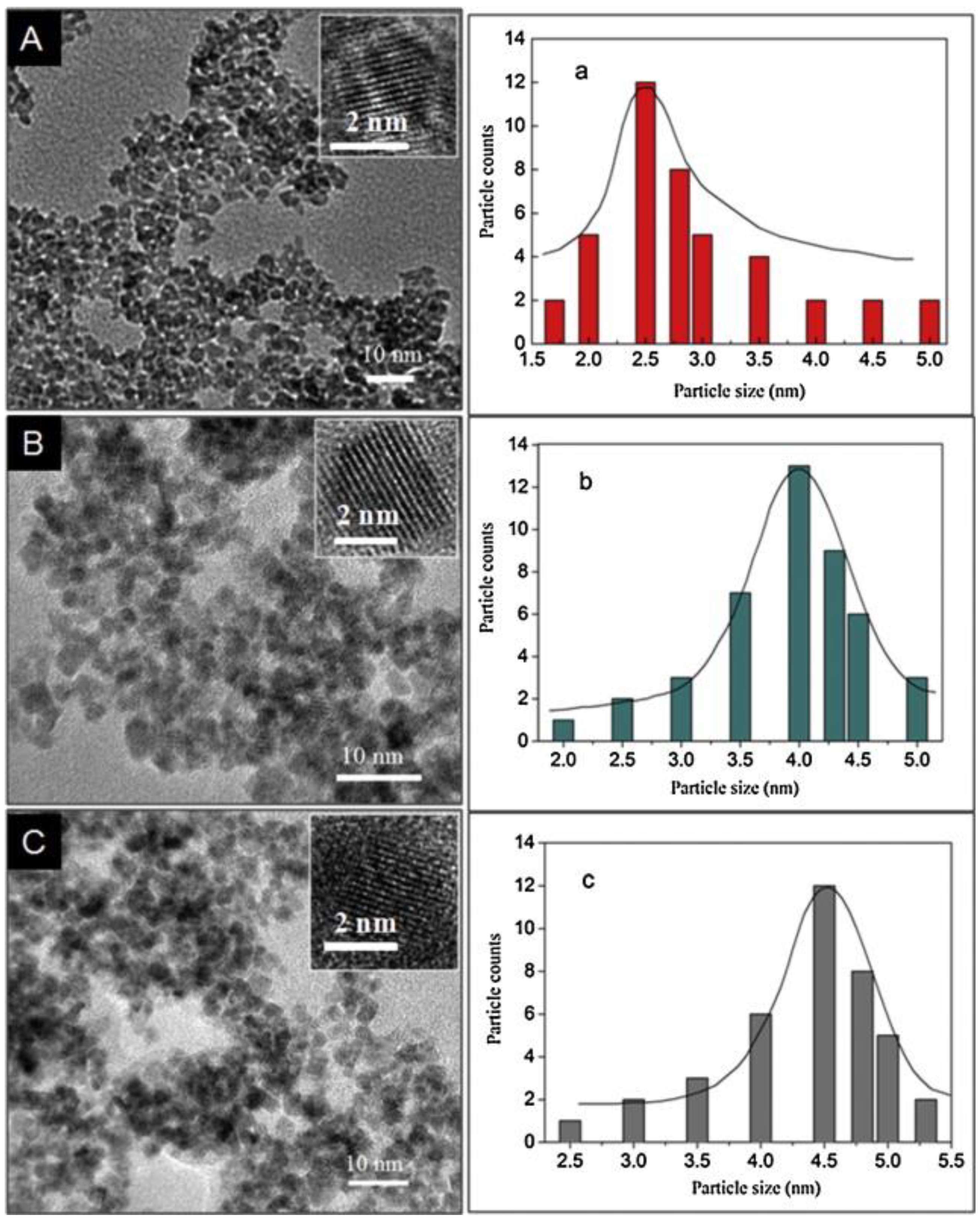
- Du, J.; Zhao, R.; Xie, Y.; Li, J. Size-controlled synthesis of SnO2 quantum dots and their gas-sensing performance. Appl. Surf. Sci. 2015, 346, 256–262. [Google Scholar] [CrossRef]
- He, Y.; Tang, P.; Li, J.; Zhang, J.; Fan, F.; Li, D. Ultrafast response and recovery ethanol sensor based on SnO2 quantum dots. Mater. Lett. 2016, 165, 50–54. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, M.; Kwan Lam, T.; Zhang, C.; Du, H.; Li, B.; Yao, Y. Fast microwave-assisted synthesis of gas-sensing SnO2 quantum dots with high sensitivity. Sens. Actuators B Chem. 2016, 236, 646–653. [Google Scholar] [CrossRef]
- Carey, G.H.; Abdelhady, A.L.; Ning, Z.; Thon, S.M.; Bakr, O.M.; Sargent, E.H. Colloidal quantum dot solar cells. Chem. Rev. 2015, 115, 12732–12763. [Google Scholar] [CrossRef] [PubMed]
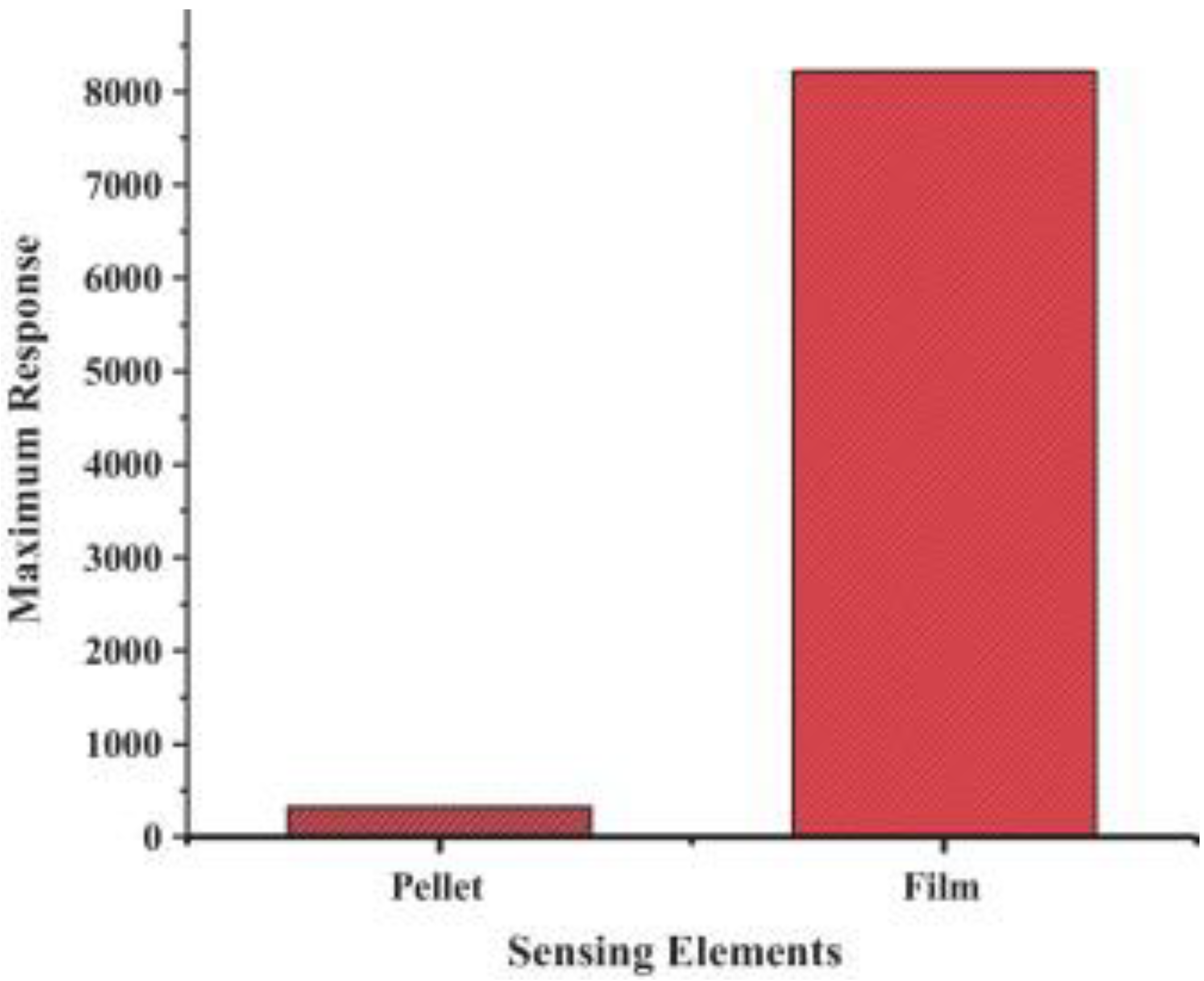
- Liu, H.; Xu, S.; Li, M.; Shao, G.; Song, H.; Zhang, W.; Wei, W.; He, M.; Gao, L.; Song, H.; et al. Chemiresistive gas sensors employing solution-processed metal oxide quantum dot films. Appl. Phys. Lett. 2014, 105, 163104. [Google Scholar] [CrossRef]
- Xu, X.; Zhuang, J.; Wang, X. Sno2 quantum dots and quantum wires: Controllable synthesis, self-assembled 2d architectures, and gas-sensing properties. J. Am. Chem. Soc. 2008, 130, 12527–12535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of zno-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Song, Z.; Kan, H.; Yu, H.; Liu, Q.; Zhang, G.; Liu, H. Sensitive H2S gas sensors employing colloidal zinc oxide quantum dots. Sens. Actuators B Chem. 2017, 249, 558–563. [Google Scholar] [CrossRef]
- Forleo, A.; Francioso, L.; Capone, S.; Siciliano, P.; Lommens, P.; Hens, Z. Synthesis and gas sensing properties of zno quantum dots. Sens. Actuators B Chem. 2010, 146, 111–115. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrogen Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Mohd Chachuli, S.A.; Hamidon, M.N.; Mamat, M.; Ertugrul, M.; Abdullah, N.H.J.S. A hydrogen gas sensor based on TiO2 nanoparticles on alumina substrate. Sensors 2018, 18, 2483. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Shen, W.; Chen, X.; Corriou, J.-P. A high-performance NH3 gas sensor based on TiO2 quantum dot clusters with ppb level detection limit at room temperature. J. Mater. Sci. Mater. Electron. 2018, 29, 18380–18387. [Google Scholar] [CrossRef]
- Li, M.; Zhou, D.; Zhao, J.; Zheng, Z.; He, J.; Hu, L.; Xia, Z.; Tang, J.; Liu, H. Resistive gas sensors based on colloidal quantum dot (cqd) solids for hydrogen sulfide detection. Sens. Actuators B Chem. 2015, 217, 198–201. [Google Scholar] [CrossRef]
- Li, M.; Kan, H.; Chen, S.; Feng, X.; Li, H.; Li, C.; Fu, C.; Quan, A.; Sun, H.; Luo, J.; et al. Colloidal quantum dot-based surface acoustic wave sensors for NO2-sensing behavior. Sens. Actuators B Chem. 2019, 287, 241–249. [Google Scholar] [CrossRef]
- Mosahebfard, A.; Jahromi, H.D.; Sheikhi, M.H. Highly sensitive, room temperature methane gas sensor based on lead sulfide colloidal nanocrystals. IEEE Sens. J. 2016, 16, 4174–4179. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Voznyy, O.; Hu, L.; Fu, Q.; Zhou, D.; Xia, Z.; Sargent, E.H.; Tang, J. Physically flexible, rapid-response gas sensor based on colloidal quantum dot solids. Adv. Mater. 2014, 26, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Mitri, F.; De Iacovo, A.; De Luca, M.; Pecora, A.; Colace, L. Lead sulphide colloidal quantum dots for room temperature NO2 gas sensors. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Mishra, R.K.; Choi, G.-J.; Choi, H.-J.; Gwag, J.-S. ZnS quantum dot based acetone sensor for monitoring health-hazardous gases in indoor/outdoor environment. Micromachines 2021, 12, 598. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Kan, H.; Li, C.; Quan, A.; Fu, C.; Luo, J.; Liu, X.; Wang, W.; Yang, Z.; et al. Surface acoustic wave no2 sensors utilizing colloidal sns quantum dot thin films. Surf. Coat. Technol. 2019, 362, 78–83. [Google Scholar] [CrossRef]
- Hu, F.F.; Tang, H.Y.; Tan, C.J.; Ye, H.Y.; Chen, X.P.; Zhang, G.Q. Nitrogen dioxide gas sensor based on monolayer sns: A first-principle study. IEEE Electron Device Lett. 2017, 38, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Gao, C.; Yang, H.; Sacco, L.; Sokolovskij, R.; Zheng, H.; Ye, H.; Vollebregt, S.; Yu, H.; Fan, X.; et al. Room temperature ppt-level no2 gas sensor based on sno x/sns nanostructures with rich oxygen vacancies. 2D Mater. 2021, 8, 045006. [Google Scholar] [CrossRef]
- Rana, C.; Bera, S.R.; Saha, S. Growth of sns nanoparticles and its ability as ethanol gas sensor. J. Mater. Sci. Mater. Electron. 2019, 30, 2016–2029. [Google Scholar] [CrossRef]
- Wang, J.; Lian, G.; Xu, Z.; Fu, C.; Lin, Z.; Li, L.; Wang, Q.; Cui, D.; Wong, C.-P. Growth of large-size sns thin crystals driven by oriented attachment and applications to gas sensors and photodetectors. ACS Appl. Mater. Interfaces 2016, 8, 9545–9551. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Li, S.; Mawella-Vithanage, L.; Ma, T.; Kilani, M.; Wang, B.; Ma, L.; Hewa-Rahinduwage, C.C.; Shafikova, A.; Nikolla, E.; et al. Atomically dispersed pb ionic sites in pbcdse quantum dot gels enhance room-temperature no2 sensing. Nat. Commun. 2021, 12, 4895. [Google Scholar] [CrossRef] [PubMed]
- Nemade, K.R.; Waghuley, S.A. Strontium oxide quantum dot decorated graphene composites for liquid petroleum gas sensing. J. Chin. Adv. Mater. Soc. 2013, 1, 219–228. [Google Scholar] [CrossRef]
- Upadhyaya, S.; Gogoi, B.; Sen Sarma, N. Poly(n-vinylpyrrolidone-co-acrylonitrile-co-methacrylic acid)–graphene quantum dot conjugate: Synthesis and characterization for sensing ammonia vapour. J. Mater. Chem. C 2021, 9, 2165–2177. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, Y.; Guan, X.; Cao, J.; Li, W.; Long, X.; Tan, X. Enhanced performances of PbS quantum-dots-modified MoS2 composite for NO2 detection at room temperature. ACS Appl. Mater. Interfaces 2019, 11, 9438–9447. [Google Scholar] [CrossRef]
- Nath, S.S.; Choudhury, M.; Chakdar, D.; Gope, G.; Nath, R.K. Acetone sensing property of zno quantum dots embedded on pvp. Sens. Actuators B Chem. 2010, 148, 353–357. [Google Scholar] [CrossRef]
- Choudhury, M.; Nath, S.S.; Nath, R.K. Zno: Pvp quantum dot ethanol sensor. J. Sens. Technol. 2011, 1, 86. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Yan, G.; Wu, C.; He, S. An ethanol vapor sensor based on a microfiber with a quantum-dot gel coating. Sensors 2019, 19, 300. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Ma, P.; Wang, A.; Yu, C.; Qian, T.; Wu, S.; Shen, J. Dopamine fluorescent sensors based on polypyrrole/graphene quantum dots core/shell hybrids. Biosens. Bioelectron. 2015, 64, 404–410. [Google Scholar] [CrossRef]
- Kumar, Y.R.; Deshmukh, K.; Sadasivuni, K.K.; Pasha, S.K.K. Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: A review. RSC Adv. 2020, 10, 23861–23898. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-s.; Yan, X. Colloidal graphene quantum dots. J. Phys. Chem. Lett. 2010, 1, 2572–2576. [Google Scholar] [CrossRef]
- Alizadeh, T.; Shokri, M.; Hanifehpour, Y.; Joo, S.W. A new hydrogen cyanide chemiresistor gas sensor based on graphene quantum dots. Int. J. Environ. Anal. Chem. 2016, 96, 763–775. [Google Scholar] [CrossRef]
- Hosseini, Z.; Ghiass, M.; Fardindoost, S.; Hatamie, S. A new approach to flexible humidity sensors using graphene quantum dots. J. Mater. Chem. C 2017, 5, 8966–8973. [Google Scholar] [CrossRef]
- Li, N.; Chen, X.; Chen, X.; Ding, X.; Li, X. Subsecond response of humidity sensor based on graphene oxide quantum dots. IEEE Electron Device Lett. 2015, 36, 615–617. [Google Scholar] [CrossRef]
- Ruiz, V.; Fernández, I.; Carrasco, P.; Cabañero, G.; Grande, H.J.; Herrán, J.J.S.; Chemical, A.B. Graphene quantum dots as a novel sensing material for low-cost resistive and fast-response humidity sensors. Sens. Actuators B Chem. 2015, 218, 73–77. [Google Scholar] [CrossRef]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef]
- Li, M.; Tao, C.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
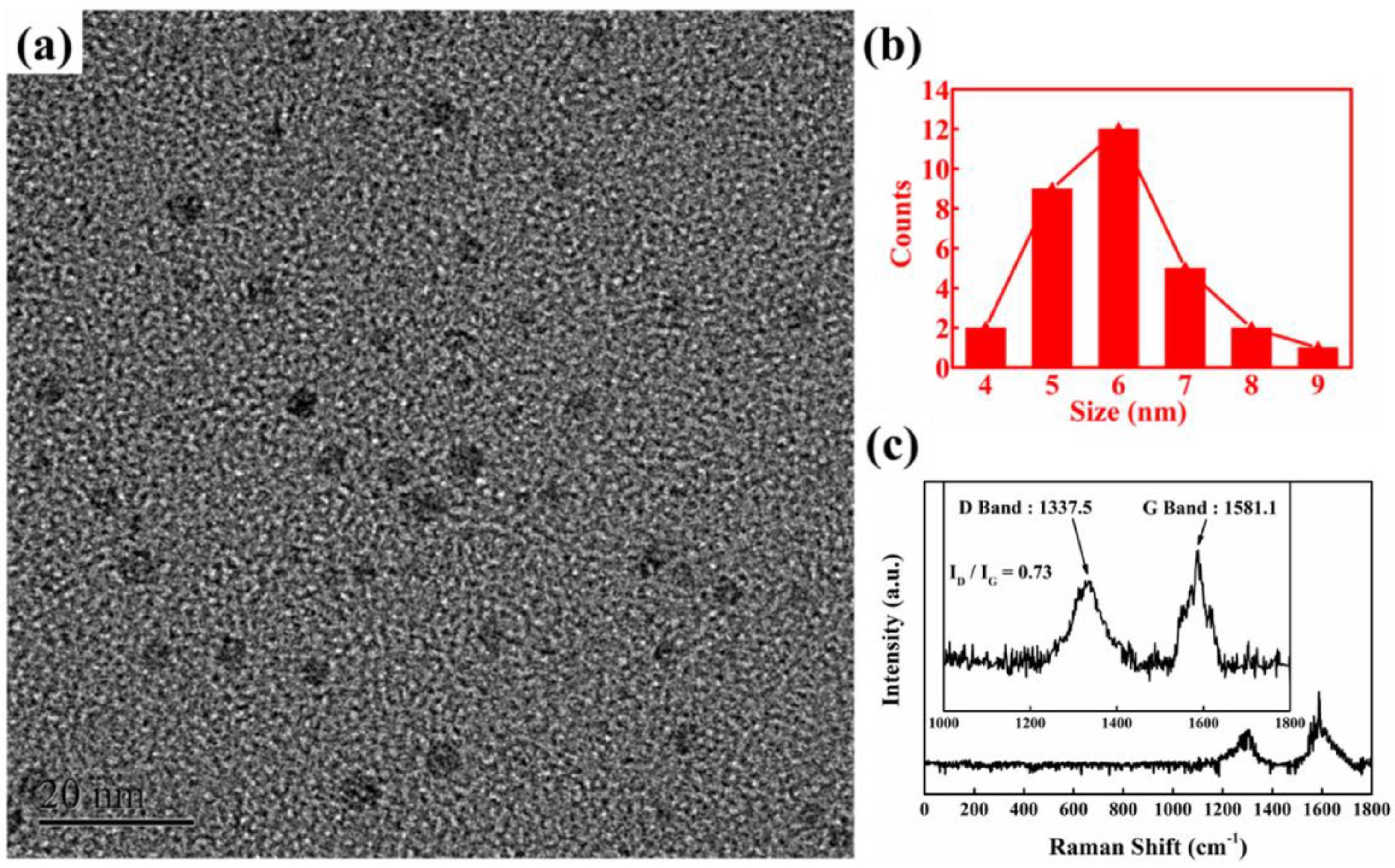
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef]
- Facure, M.H.; Schneider, R.; Mercante, L.A.; Correa, D.S. A review on graphene quantum dots and their nanocomposites: From laboratory synthesis towards agricultural and environmental applications. Environ. Sci. Nano 2020, 7, 3710–3734. [Google Scholar] [CrossRef]
- Ha Masemola, C.M.; Moloto, N.; Tetana, Z.N.; Gqoba, S.S.; Mubiayi, P.K.; Linganiso, E.C. N-doped graphene quantum dot-modified polyaniline for room-temperature sensing of alcohol vapors. Mater. Chem. Phys. 2022, 287, 126229. [Google Scholar] [CrossRef]
- Long, L.M.; Dinh, N.N.; Trung, T.Q. Synthesis and characterization of polymeric graphene quantum dots based nanocomposites for humidity sensing. J. Nanomater. 2016, 2016, 5849018. [Google Scholar] [CrossRef] [Green Version]
- Gavgani, J.N.; Hasani, A.; Nouri, M.; Mahyari, M.; Salehi, A. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens. Actuators B Chem. 2016, 229, 239–248. [Google Scholar] [CrossRef]
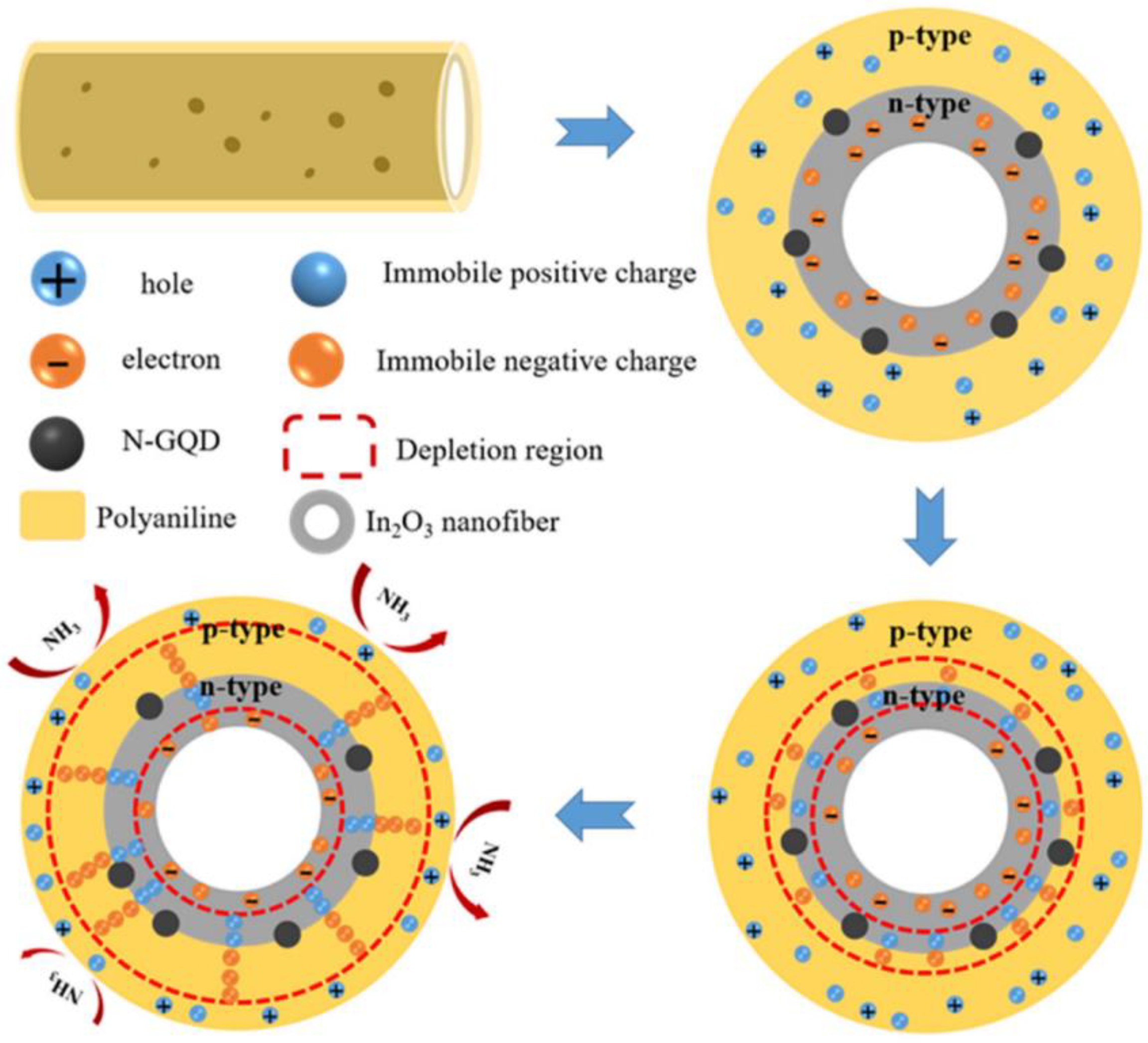
- Hong, S.-Z.; Huang, Q.-Y.; Wu, T.-M. The room temperature highly sensitive ammonia gas sensor based on polyaniline and nitrogen-doped graphene quantum dot-coated hollow indium oxide nanofiber composite. Polymers 2021, 13, 3676. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, M.; Salehi, A.; Boroumand, F.A.; Mosleh, N. Fabrication of a room temperature ammonia gas sensor based on polyaniline with n-doped graphene quantum dots. IEEE Sens. J. 2018, 18, 2245–2252. [Google Scholar] [CrossRef]
- Hakimi, M.; Salehi, A.; Boroumand, F.A. Fabrication and characterization of an ammonia gas sensor based on pedot-pss with n-doped graphene quantum dots dopant. IEEE Sens. J. 2016, 16, 6149–6154. [Google Scholar] [CrossRef]
- Montejo-Alvaro, F.; Oliva, J.; Herrera-Trejo, M.; Hdz-García, H.M.; Mtz-Enriquez, A.I. DFT study of small gas molecules adsorbed on undoped and N-, Si-, B-, and Al-doped graphene quantum dots. Theor. Chem. Accounts 2019, 138, 1–15. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Xu, L.; Zhu, S.; Yang, S.; Chen, X.; Dong, B.; Bai, X.; Lu, G.; Song, H. Graphene quantum dot-functionalized three-dimensional ordered mesoporous ZnO for acetone detection toward diagnosis of diabetes. Nanoscale 2019, 11, 11496–11504. [Google Scholar] [CrossRef]
- Lv, Y.-K.; Li, Y.-Y.; Zhou, R.-H.; Pan, Y.-P.; Yao, H.-C.; Li, Z.-J. N-doped graphene quantum dot-decorated three-dimensional ordered macroporous In2O3 for NO2 sensing at low temperatures. ACS Appl. Mater. Interfaces 2020, 12, 34245–34253. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Metal-organic frameworks-derived zinc oxide nanopolyhedra/S, N: Graphene quantum dots/polyaniline ternary nanohybrid for high-performance acetone sensing. Sens. Actuators B Chem. 2019, 288, 232–242. [Google Scholar] [CrossRef]
- Murali, G.; Reddeppa, M.; Seshendra Reddy, C.; Park, S.; Chandrakalavathi, T.; Kim, M.D.; In, I. Enhancing the charge carrier separation and transport via nitrogen-doped graphene quantum dot-TiO2 nanoplate hybrid structure for an efficient NO gas sensor. ACS Appl. Mater. Interfaces 2020, 12, 13428–13436. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.K.; Li, Y.Y.; Yao, H.C.; Li, Z.J. Nitrogen-doped graphene quantum dots-modified mesoporous SnO2 hierarchical hollow cubes for low temperature detection of nitrogen dioxide. Sens. Actuators B Chem. 2021, 339, 129882. [Google Scholar] [CrossRef]
- Hu, T.; Chu, X.; Gao, F.; Dong, Y.; Sun, W.; Bai, L. Trimethylamine sensing properties of graphene quantum Dots/α-Fe2O3 composites. J. Solid State Chem. 2016, 237, 284–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Rong, Q.; Zhao, J.; Zhang, J.; Zhu, Z.; Liu, Q. Boron-doped graphene quantum dot/Ag–LaFeO3 p–p heterojunctions for sensitive and selective benzene detection. J. Mater. Chem. A 2018, 6, 12647–12653. [Google Scholar] [CrossRef]
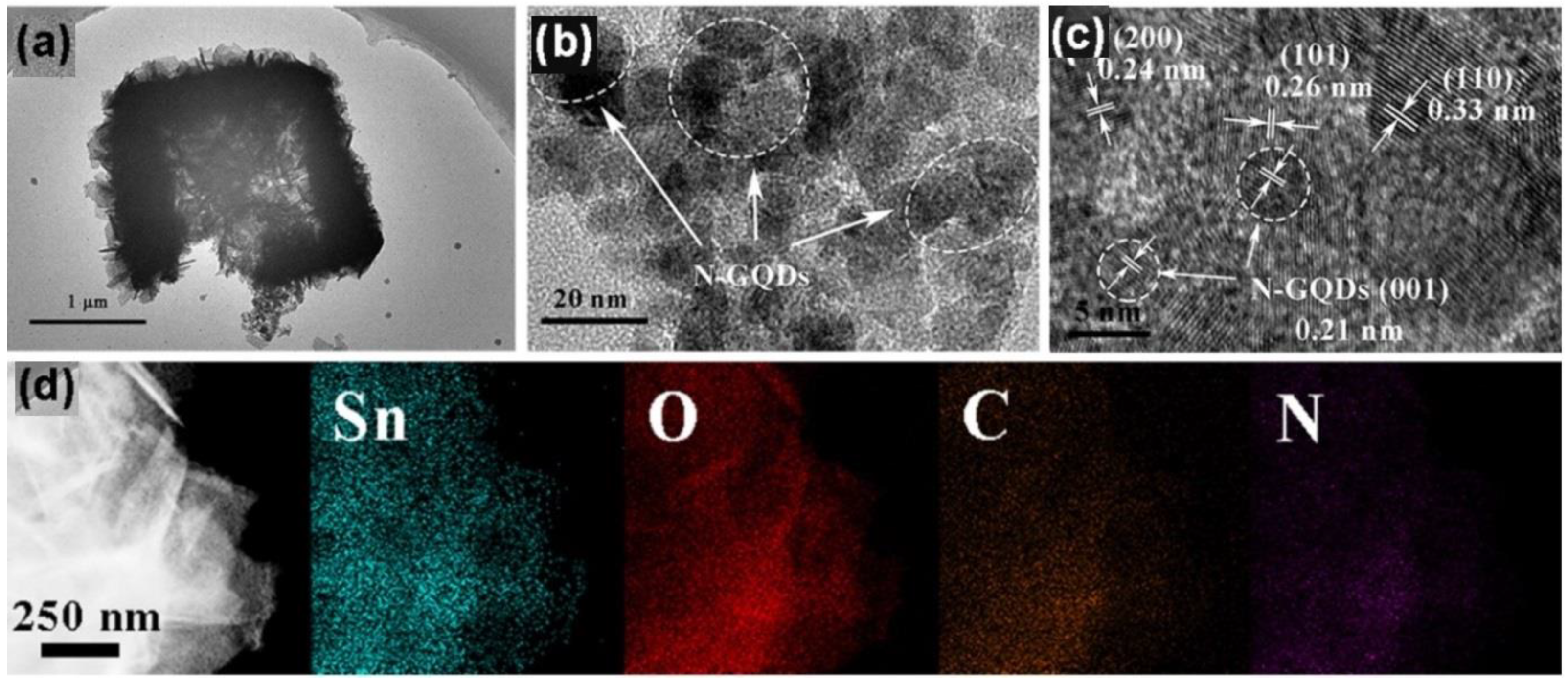
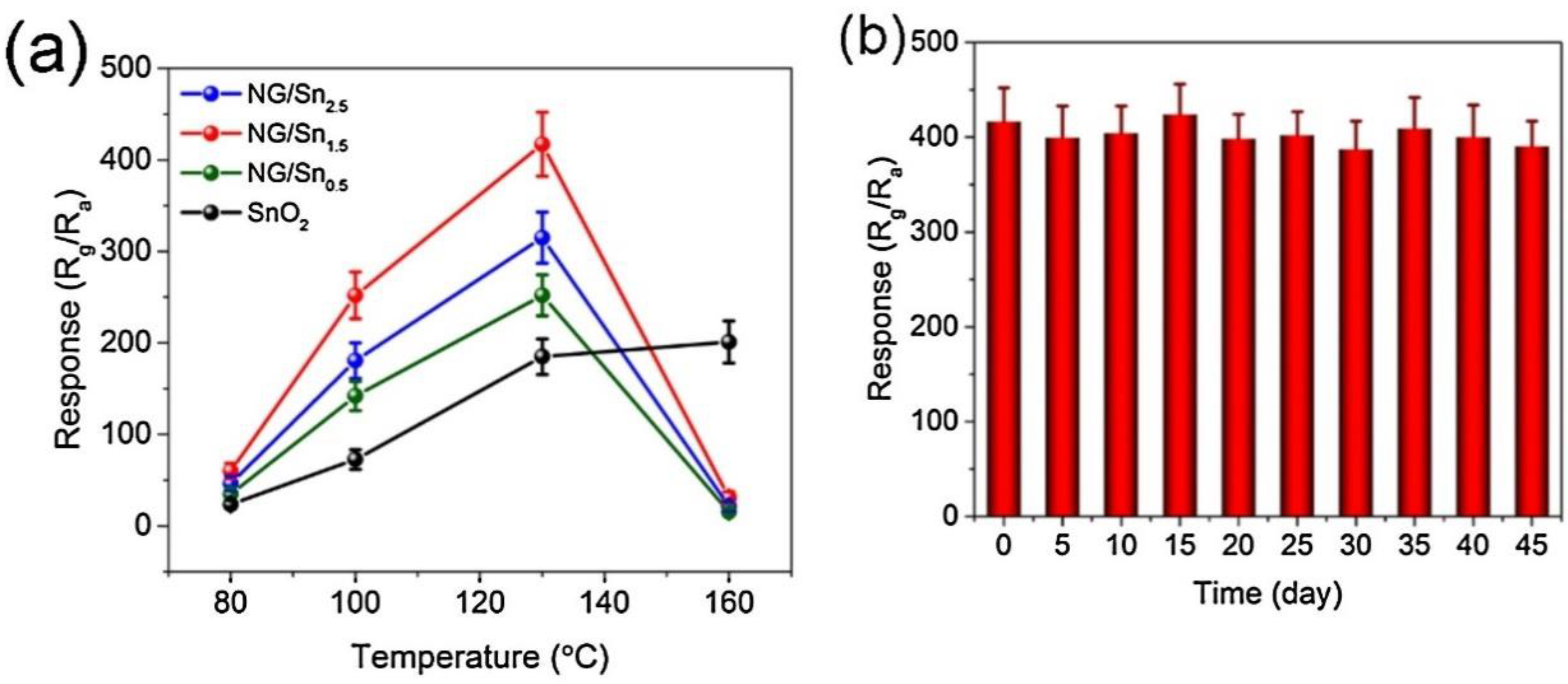
- Shao, S.; Chen, X.; Chen, Y.; Zhang, L.; Kim, H.W.; Kim, S.S. ZnO nanosheets modified with graphene quantum dots and SnO2 quantum nanoparticles for room-temperature H2S sensing. ACS Appl. Nano Mater. 2020, 3, 5220–5230. [Google Scholar] [CrossRef]
- Chen, W.; Li, F.; Ooi, P.C.; Ye, Y.; Kim, T.W.; Guo, T. Room temperature pH-dependent ammonia gas sensors using graphene quantum dots. Sens. Actuators B Chem. 2016, 222, 763–768. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Dehsari, H.S.; Hasani, A.; Mahyari, M.; Shalamzari, E.K.; Salehi, A.; Taromi, F.A. A room temperature volatile organic compound sensor with enhanced performance, fast response and recovery based on N-doped graphene quantum dots and poly (3, 4-ethylenedioxythiophene)–poly (styrenesulfonate) nanocomposite. RSC Adv. 2015, 5, 57559–57567. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Sun, H.; Zhu, Z.; Zhang, J.; Liu, Q. B, N, S, Cl doped graphene quantum dots and their effects on gas-sensing properties of Ag-LaFeO3. Sens. Actuators B Chem. 2018, 266, 364–374. [Google Scholar] [CrossRef]
- Raeyani, D.; Shojaei, S.; Ahmadi-Kandjani, S. Optical graphene quantum dots gas sensors: Theoretical study. Superlatt. Microstruct. 2018, 114, 321–330. [Google Scholar] [CrossRef]
- Chu, X.; Dai, P.; Liang, S.; Bhattacharya, A.; Dong, Y.; Epifani, M. The acetone sensing properties of ZnFe2O4-graphene quantum dots (GQDs) nanocomposites at room temperature. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 106, 326–333. [Google Scholar] [CrossRef]
- Song, Z.; Xu, S.; Liu, J.; Hu, Z.; Gao, N.; Zhang, J.; Yi, F.; Zhang, G.; Jiang, S.; Liu, H. Enhanced catalytic activity of SnO2 quantum dot films employing atomic ligand-exchange strategy for fast response H2S gas sensors. Sens. Actuators B Chem. 2018, 271, 147–156. [Google Scholar] [CrossRef]
- Wang, X.; Gao, M. Porous Co3O4/SnO2 quantum dot (QD) heterostructures with abundant oxygen vacancies and Co2+ ions for highly efficient gas sensing and oxygen evolution reaction. Nanoscale 2018, 10, 12045–12053. [Google Scholar] [CrossRef] [PubMed]
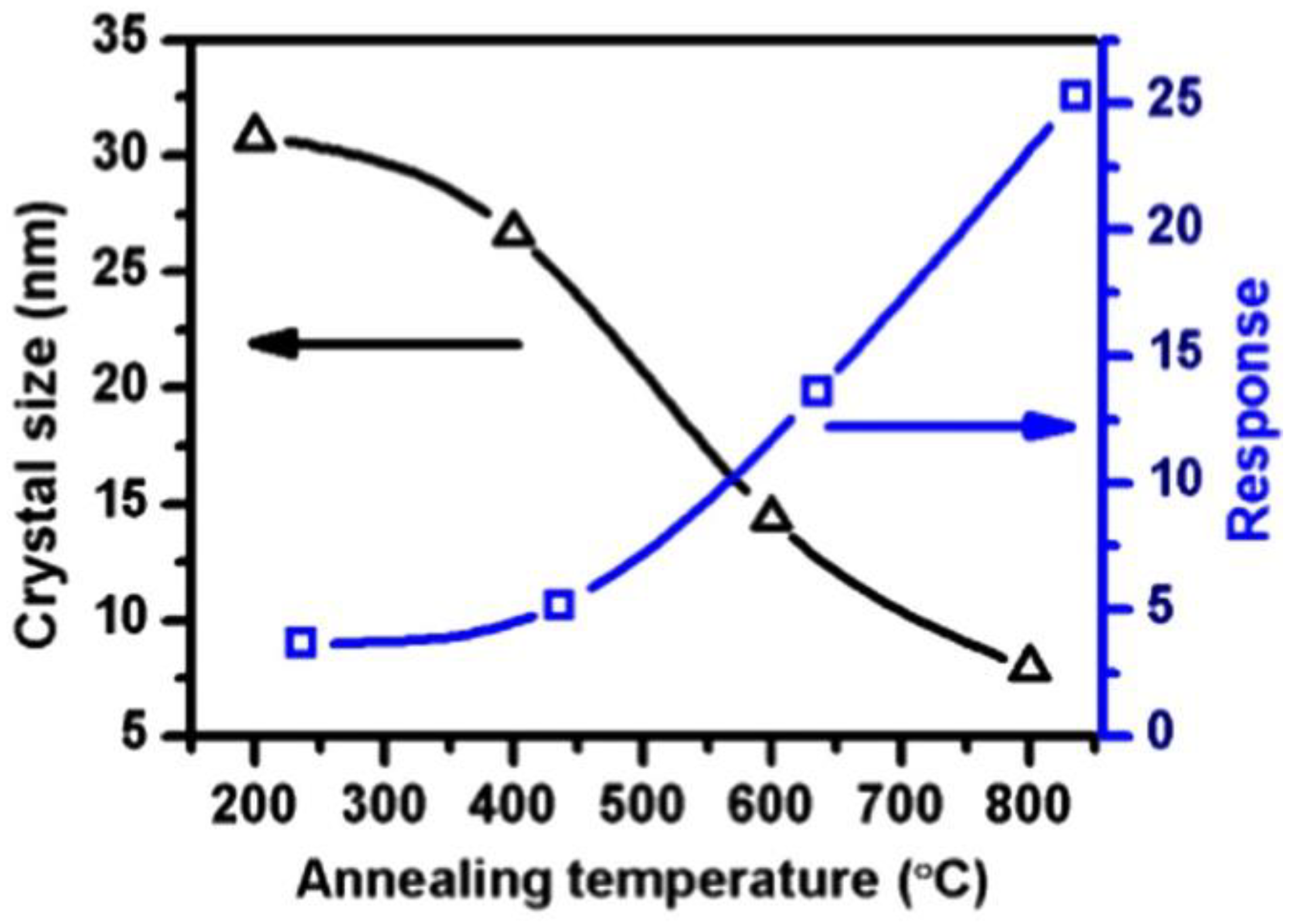
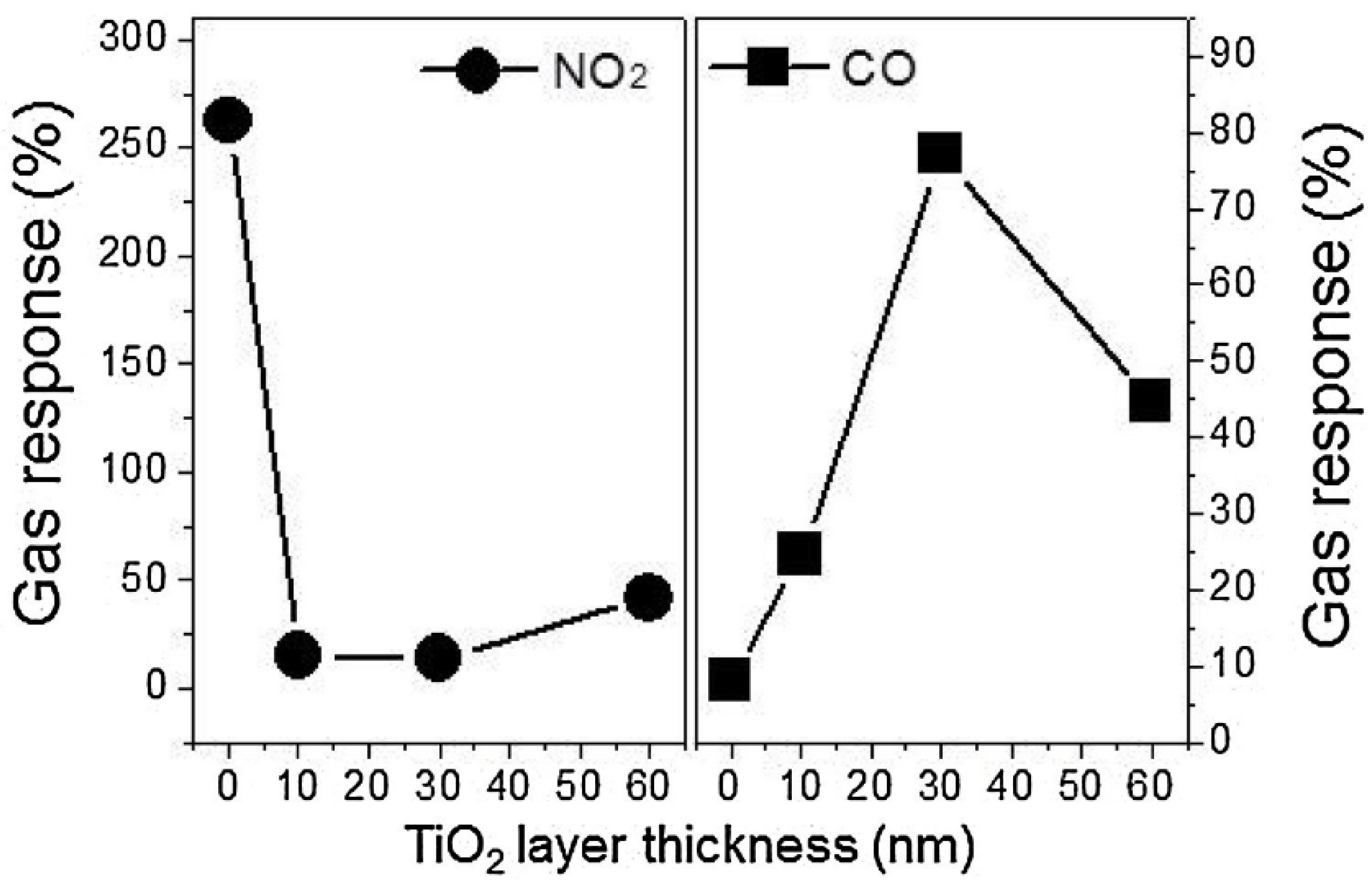
- Lee, J.H.; Mirzaei, A.; Kim, J.H.; Kim, J.Y.; Nasriddinov, A.F.; Rumyantseva, M.N.; Kim, S.S. Gas-sensing behaviors of TiO2-layer-modified SnO2 quantum dots in self-heating mode and effects of the TiO2 layer. Sens. Actuators B Chem. 2020, 310, 127870. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Loo, A.H. Layered transition-metal dichalcogenides (MoS2 and WS2) for sensing and biosensing. TrAC Trends Anal. Chem. 2014, 61, 49–53. [Google Scholar] [CrossRef]
- Singh, V.K.; Yadav, S.M.; Mishra, H.; Kumar, R.; Tiwari, R.; Pandey, A.; Srivastava, A. WS2 quantum dot graphene nanocomposite film for UV photodetection. ACS Appl. Nano Mater. 2019, 2, 3934–3942. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Yang, C.-C.; Su, T.-Y.; Yang, T.-Y.; Wu, S.-C.; Hsu, Y.-C.; Chen, Y.-Z.; Lin, T.-N.; Shen, J.-L.; Lin, H.-N.; et al. Design of core–shell quantum dots–3d WS2 nanowall hybrid nanostructures with high-performance bifunctional sensing applications. ACS Nano 2020, 14, 12668–12678. [Google Scholar] [CrossRef]
- Ren, X.; Wei, Q.; Ren, P.; Wang, Y.; Peng, Y. Hydrothermal-solvothermal cutting integrated synthesis and optical properties of MoS2 quantum dots. Opt. Mater. 2018, 86, 62–65. [Google Scholar] [CrossRef]
- Jaiswal, J.; Sanger, A.; Tiwari, P.; Chandra, R. MoS2 hybrid heterostructure thin film decorated with cdte quantum dots for room temperature no2 gas sensor. Sens. Actuators B Chem. 2020, 305, 127437. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Abakumov, A.M.; Gaskov, A.M. Visible light activated room temperature gas sensors based on nanocrystalline zno sensitized with CdSe quantum dots. Sens. Actuators B Chem. 2014, 205, 305–312. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Xia, Y.; Xiang, L. Near infrared light enhanced room-temperature NO2 gas sensing by hierarchical ZnO nanorods functionalized with PbS quantum dots. Sens. Actuators B Chem. 2018, 255, 2538–2545. [Google Scholar] [CrossRef]
- Paul Choudhury, S.; Feng, Z.; Gao, C.; Ma, X.; Zhan, J.; Jia, F. BN quantum dots decorated ZnO nanoplates sensor for enhanced detection of BTEX gases. J. Alloys Compd. 2020, 815, 152376. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, W.; Chu, Z.; Nie, X.; Wang, R.; He, X. SnS2 quantum dot-based optoelectronic flexible sensors for ultrasensitive detection of no2 down to 1 ppb. ACS Appl. Mater. Interfaces 2020, 12, 25178–25188. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, X.; Wang, T.; Li, B.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; Zhou, Z.; Yang, Z. Enhancing room-temperature NO2 gas sensing performance based on a metal phthalocyanine/graphene quantum dot hybrid material. RSC Adv. 2021, 11, 5618–5628. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, M.; Wang, T.; Chen, X.; Zeng, M.; Yang, J.; Zhou, Z.; Hu, N.; Su, Y.; Yang, Z. Room temperature dmmp gas sensing based on cobalt phthalocyanine derivative/graphene quantum dot hybrid materials. RSC Adv. 2021, 11, 14805–14813. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Dong, Y.; Chi, Y.; Chen, G. Carbon quantum dot-functionalized aerogels for NO2 gas sensing. Anal. Chem. 2013, 85, 8065–8069. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Wang, X.; He, D.; Suo, H.; Zhao, C. Fabrication of ZnO/Carbon quantum dots composite sensor for detecting NO gas. Sensors 2020, 20, 4961. [Google Scholar] [CrossRef]
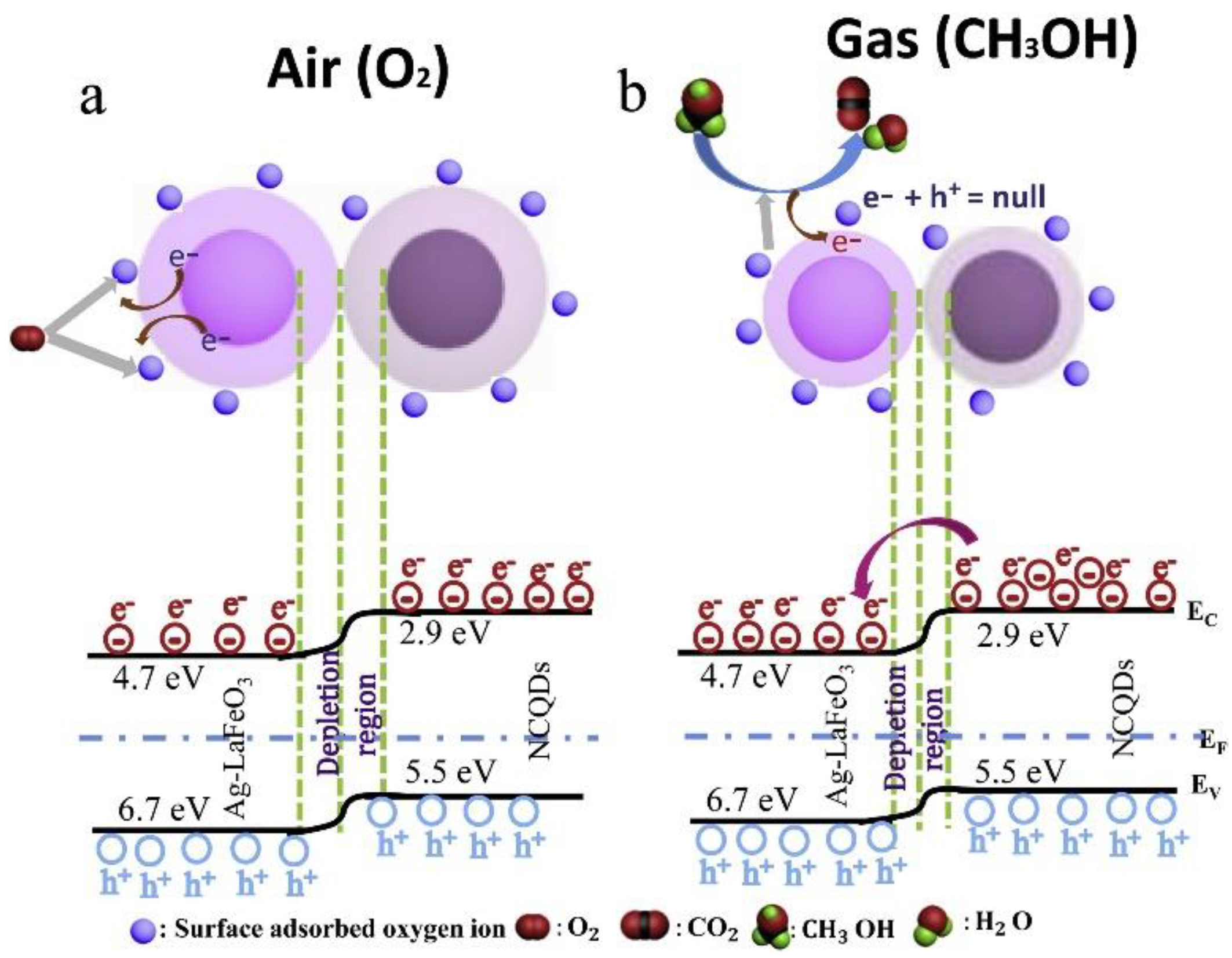
- Rong, Q.; Zhang, Y.; Li, K.; Wang, H.; Hu, J.; Zhu, Z.; Zhang, J.; Liu, Q. Ag-LaFeO3/NCQDs p-n heterojunctions for superior methanol gas sensing performance. Mater. Res. Bull. 2019, 115, 55–64. [Google Scholar] [CrossRef]
- Leghrib, R.; Dufour, T.; Demoisson, F.; Claessens, N.; Reniers, F.; Llobet, E. Gas sensing properties of multiwall carbon nanotubes decorated with rhodium nanoparticles. Sens. Actuators B Chem. 2011, 160, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, S.; Qiu, L.; Rasaki, S.A.; Qu, F.; Thomas, T.; Liu, Y.; Yang, M. Ru-decorated WO3 nanosheets for efficient xylene gas sensing application. J. Alloys Compd. 2020, 826, 154196. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Jafri, R.I.; Arockiadoss, T.; Ramaprabhu, S. Nanostructured pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor. Nanoscale 2009, 1, 382–386. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Mi, Q. A high-performance room temperature benzene gas sensor based on CoTiO3 covered TiO2 nanospheres decorated with pd nanoparticles. Sens. Actuators B Chem. 2022, 350, 130830. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Sung, S.-H.; Lee, G.; Kim, J.; Seong, J.; Shim, Y.-S.; Jun, S.C.; Jeon, S. High-performance gas sensor array for indoor air quality monitoring: The role of au nanoparticles on WO3, SnO2, and NiO-based gas sensors. J. Mater. Chem. A 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- Qin, Q.; Li, Y.; Bu, W.; Meng, L.; Chuai, X.; Zhou, Z.; Hu, C. Self-template-derived ZnCo2O4 porous microspheres decorated by ag nanoparticles and their selective detection of formaldehyde. Inorg. Chem. Front. 2021, 8, 811–820. [Google Scholar] [CrossRef]
- Liu, H.; Shen, W.; Chen, X. A room temperature operated ammonia gas sensor based on ag-decorated TiO2 quantum dot clusters. RSC Adv. 2019, 9, 24519–24526. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Sarkar, S.K. Room temperature zno and au-zno quantum dots thin film gas sensor fabrication for detecting of volatile organic compound gases. IEEE Sens. J. 2020, 20, 12602–12609. [Google Scholar] [CrossRef]
- Rathi, K.; Pal, K. Ruthenium-decorated tungsten disulfide quantum dots for a CO2 gas sensor. Nanotechnology 2020, 31, 135502. [Google Scholar] [CrossRef]
- Burman, D.; Santra, S.; Pramanik, P.; Guha, P.K. Pt decorated MoS2 nanoflakes for ultrasensitive resistive humidity sensor. Nanotechnology 2018, 29, 115504. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, W.; Jin, G.; Zhai, Z.; Lv, J.; Hong, W.; Chen, Y. Preparation of tin oxide quantum dots in aqueous solution and applications in semiconductor gas sensors. Nanomaterials 2019, 9, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzaei, A.; Kordrostami, Z.; Shahbaz, M.; Kim, J.-Y.; Kim, H.W.; Kim, S.S. Resistive-Based Gas Sensors Using Quantum Dots: A Review. Sensors 2022, 22, 4369. https://doi.org/10.3390/s22124369
Mirzaei A, Kordrostami Z, Shahbaz M, Kim J-Y, Kim HW, Kim SS. Resistive-Based Gas Sensors Using Quantum Dots: A Review. Sensors. 2022; 22(12):4369. https://doi.org/10.3390/s22124369
Chicago/Turabian StyleMirzaei, Ali, Zoheir Kordrostami, Mehrdad Shahbaz, Jin-Young Kim, Hyoun Woo Kim, and Sang Sub Kim. 2022. "Resistive-Based Gas Sensors Using Quantum Dots: A Review" Sensors 22, no. 12: 4369. https://doi.org/10.3390/s22124369
APA StyleMirzaei, A., Kordrostami, Z., Shahbaz, M., Kim, J.-Y., Kim, H. W., & Kim, S. S. (2022). Resistive-Based Gas Sensors Using Quantum Dots: A Review. Sensors, 22(12), 4369. https://doi.org/10.3390/s22124369









