Case report
A 69-year-old man with recurrent epigastric pain was hospitalised by his family doctor to rule out progression of his coronary artery disease. The patient was a former smoker, with arterial hypertension and dyslipdemia. He had known coronary artery disease with prior aortocoronary bypass surgery in 1997.
The current problem was an intense and stabbing epigastric pain, occurring 20–30 minutes after most meals and lasting for up to two hours thereafter. Occasionally, the pain was accompanied by non-bloody diarrhea. The patient began to avoid heavy meals because of the postprandial pain. In addition, he lost six kilograms of bodyweight within four months. The physical examination revealed an abdominal arterial bruit but was otherwise normal. Peptic ulcer disease was ruled out by gastroscopy and gall bladder disease by ultrasound.
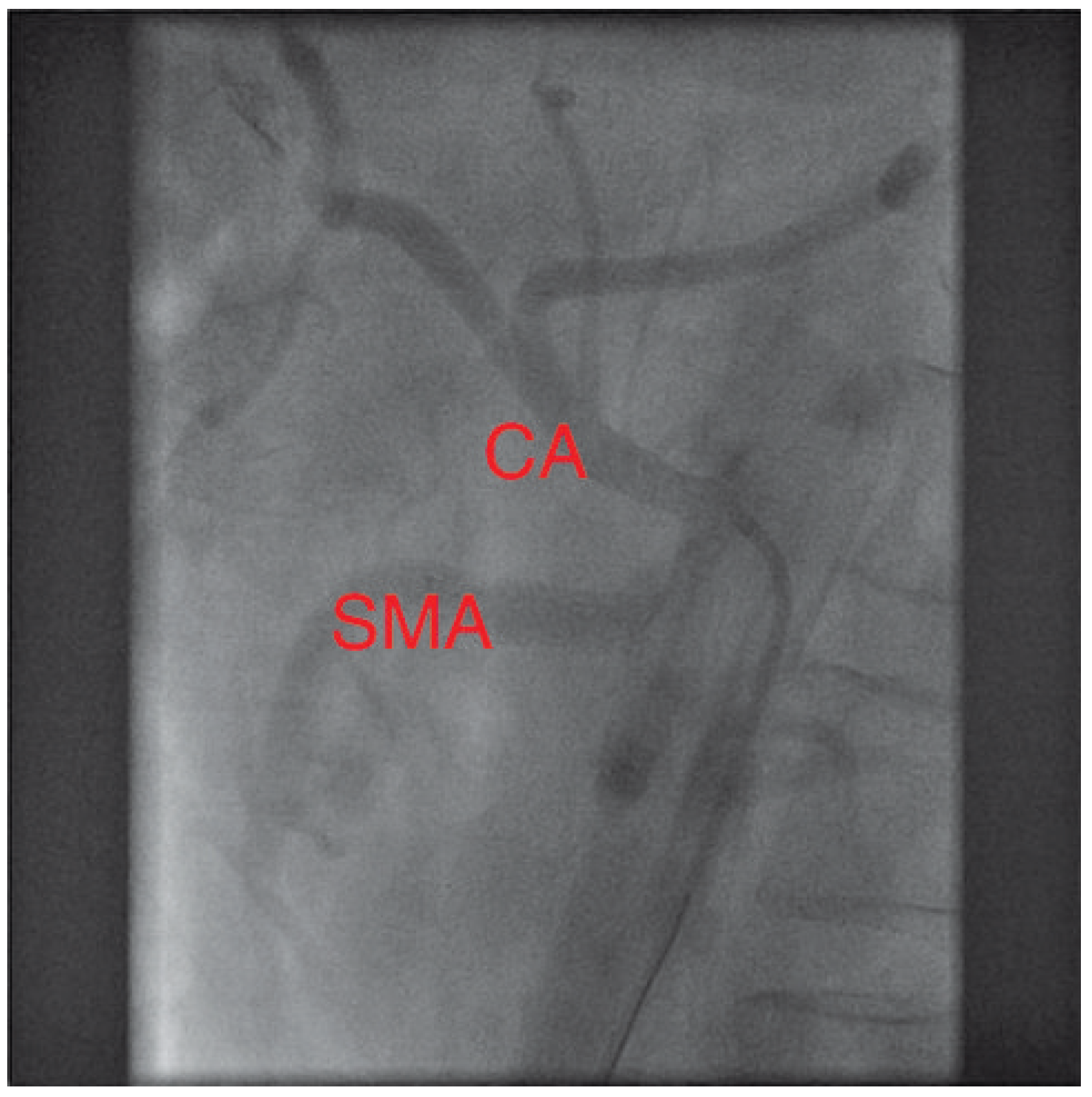
The coronary angiogram showed open coronary bypasses with no relevant progression of coronary atherosclerosis. Due to the clinical picture, suggesting mesenteric ischemia, selective angiography of the major splanchnic arteries was performed. It showed significant stenosis of the proximal superior mesenteric (SMA) and of the proximal celiac arteries (CA). The inferior mesenteric artery (IMA) and both renal arteries were normal.
In a first step, the SMA was treated by balloon angioplasty and a bare metal stent (hippocampus 7.0/20 mm) was implanted. After 2 weeks, the patient was readmitted with improved but not completely resolved abdominal symptoms. The remaining stenosis of the CA was revascularised, using another bare metal stent (hippocampus 6.0/20 mm). During a clinical follow-up of six months, the patient was asymptomatic.
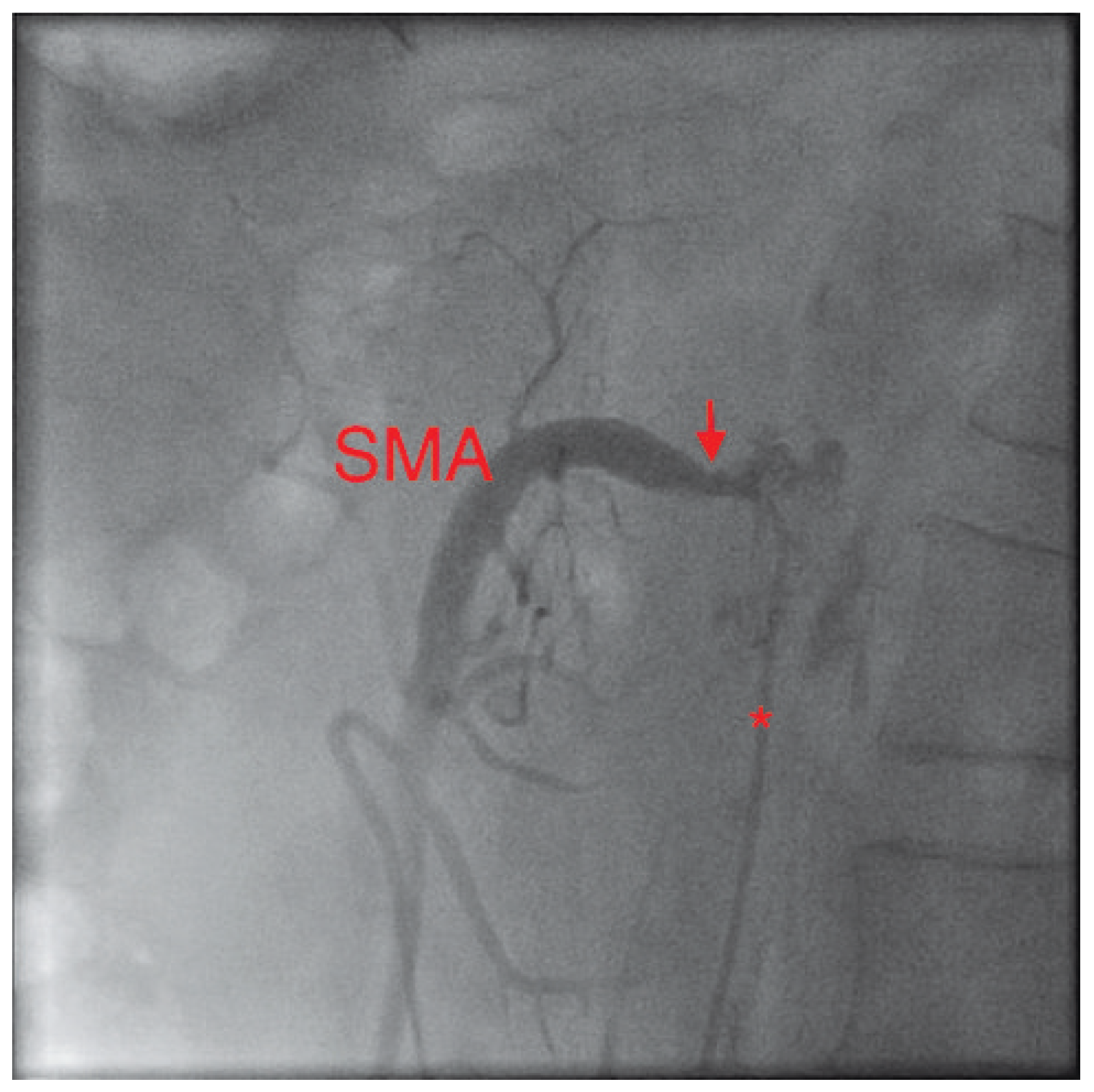
Figure 1.
In this lateral view, the catheter (*) in the abdominal aorta can be seen left of the spine. Its tip lies selectively in the superior mesenteric artery (SMA). The severely narrowed neck of the SMA can be clearly seen (arrow).
Figure 1.
In this lateral view, the catheter (*) in the abdominal aorta can be seen left of the spine. Its tip lies selectively in the superior mesenteric artery (SMA). The severely narrowed neck of the SMA can be clearly seen (arrow).
Discussion
Chronic mesenteric ischemia (CMI), also known as angina abdominalis, is a rare disease with an estimated incidence of 1/100000 [
1]. Most symptomatic patients have concomitant disease; 50% have coronary or peripheral artery disease. Due to various anastomoses and abundant collaterals in the splanchnic system (e.g., the RIOLAN arcus), stenosis of visceral arteries may remain silent. The three main splanchnic arteries consist of the celiac artery (CA), the superior mesenteric artery (SMA), and inferior mesenteric artery (IMA).
The CA supplies the foregut and divides into three branches: splenic artery, common hepatic artery and left gastric artery. The SMA originates distal to the CA, and its branches supply the biggest part of the intestinum: jejunum, ileum, and ascending as well as transverse colon. The IMA has great inter-individual variability, and brings blood to the colon, starting from the splenic flexure down to the superior portion of the rectum.
Figure 2.
Same projection: again with a catheter (*) in place. After ballooning and stent implantation the stenosis is no longer visible.
Figure 2.
Same projection: again with a catheter (*) in place. After ballooning and stent implantation the stenosis is no longer visible.
Figure 3.
Digital subtraction angiography picture of the proximal celiac artery (CA) and the distal SMA, lateral projection. The catheter (*) lies at the ostium of the severely stenosed CA (arrow) – a guidewire is in place. The patent stent (arrowhead) in the SMA is clearly visible.
Figure 3.
Digital subtraction angiography picture of the proximal celiac artery (CA) and the distal SMA, lateral projection. The catheter (*) lies at the ostium of the severely stenosed CA (arrow) – a guidewire is in place. The patent stent (arrowhead) in the SMA is clearly visible.
Figure 4.
Final result: both splanchnic arteries (SMA and CA) are now open.
Figure 4.
Final result: both splanchnic arteries (SMA and CA) are now open.
Atherosclerosis is the most common cause for CMI, accounting for up to 90% of the cases. Usually, the stenotic atheromas present as aorto-ostial lesions or in the proximal part of the involved artery. The lesions are rarely diffuse or distally located, especially in patients with diabetes or severe renal insufficiency. Rare causes of CMI include diaphragmatic compression of the CA, fibrovascular dysplasia, Takayasu’s arteritis, thromboangiitis obliterans, radiation-induced vascular injury and mesenteric venous thrombosis due to inherited thrombophilia, pancreatitis, inflammatory bowel disease, cirrhosis, portal hypertension, paraneoplastic disorders, postoperative states or trauma [
2].
The clinical picture of CMI consists of abdominal pain, nausea and/or diarrhea occurring 15–60 minutes postprandially. Reduced food intake to avoid the pain (i.e., sitophobia) may result in weight loss and malnutrition. Physical examination is often unremarkable. Sometimes, an epigastric bruit may be audible. Noninvasive imaging techniques such as duplex-sonography, CT-angiography and MR-angiography show sensitivity rates of 80–100%, while invasive arteriography remains the gold standard.
Surgical or interventional treatment of CMI is generally warranted. With medical treatment alone, 15– 50% of patient will develop devastating acute bowel ischemia with concomitant bowel infarction. Mortality is as high as 40% [
3]. Surgical revascularisation consists of transaortic endarterectomy or bypass. Due to comorbidity, surgical treatment may cause major cardiovascular complications (15–33%) and significant mortality (up to 17%). Percutaneous balloon angioplasty of the mesenteric arteries including stent implantation was first performed in the 1980s. Cohort studies report complications in up to 25% patients, with mortality rates up to 13% [
4]. Both PTA and bypass patients, appear to have similarly high primary success and patency rates of >90%.
Patients with CMI should be carefully followed using clinical visits and duplex-sonography. MRI or CT images may be needed when sonography fails to produce adequate images due to intestinal air or obesity. Reappearance of symptoms or evidence of restenosis warrant further examination and should be treated by repeated angioplasty or surgery [
4].
In conclusion, CMI is not easy to diagnose and needs to be considered in patients with chronic abdominal pain, especially in those with known atherosclerosis. Physical examination is unspecific and the diagnosis must be made from a carefully taken medical history, ruling out other, more common causes of abdominal pain, and non-invasive or invasive imaging. Stenting of mesenteric arteries seems to be a safe and effective treatment of CMI. Patients with high surgical risk or extensive atherosclerosis may especially benefit from the percutaneous approach. There are no randomised, controlled trials comparing the two strategies. The optimal approach has to be chosen individually based upon anatomy, comorbidity and patient preferences. Ideal candidates for stenting have short ostial or proximal stenosis of the SMA or CA [
5]. Mostly, bare metal stents are used because of the large diameter of intestinal arteries and the focal ostial nature of the stenosis. Instent restenosis may be treated with drugeluting stents, although sound data to support this approach are lacking.
Uncertainty exists, regarding whether two or more vessels need to be severely stenosed for patients to be symptomatic. Our patient became asymptomatic only after sequential treatment of both stenosed mesenteric arteries, questioning the classic doctrine.