The University of Padua and the tradition of the study of cardiovascular system
The roots of the University of Padua trace back to the Middle Ages. In 1222, a new
Gymnasium Omnium Disciplinarum was founded in Padua, following a migration from the Bologna of Scholars and Lecturers, with interest in Theology and Jurisprudence [
2].
In 1399, the Universitas Artistarum Medicinae Physicae et Naturae was added to the Universitas Iuristarum.
The major step forward occurred in 1404 when the city of Padua fell under the rule of the rich Republic of Venice. The Senate of St. Mark Republic invested a lot on the Studium for the best education of their young patricians and for transforming Padua into an international campus of scholars. For this aim on 1517, the Senate of the Venice Republic designated three “Re-formers” to take over the government of the University, seeking the best teachers, both in Italy and Europe [
3].
On September 26, 1592, Galileo Galilei (1564–1642), lecturer in Pisa, was appointed as Professor of Mathematics at the University of Padua, at the early age of 28 years (
Figure 1). For the selection of the young Galileo, a major contribution was given by Leonardo Donà (Leonardo Donato) (1536–1612), who was one of the three Reformers of the Studium at that time and became the 90th Doge of Venice on January 10, 1606 (
Figure 2). Donà was a strong defender of political and cultural autonomy of the Republic against the influence of the Pope. In 1606, he opposed Pope Paolo V (Camillo Borghese) who claimed the delivery of two criminal friars arrested in Venice. He threatened to excommunicate Venice, raising an international controversy at risk of the outbreak of war. The crisis was diplomatically solved thanks to the intervention of King Henry IV of France. Borghese and Donato had met years before in Rome, when Camillo Borghese was cardinal and Leonardo Donà Ambassador of Venice at the Vatican. They had a verbal dispute with Borghese saying “If I were Pope I would excommunicate you” and Donà replying “If I were Doge, I would do not care of it” [
4].
It was the policy of the Reformers to call the best and young lecturers and Galileo appeared very talented and quite promising. He introduced the experimental method according to which Science is Measure, not simply observation. The discovery of the four moons of Jupiter was made on January 7–13, 1610 (“the nights that changed the history”) by using the telescope (“occhiale”) with a magnification of only 20×, and by calculating their movements. The Copernican eliocentric theory was thus proven, and established Padua as the place of the scientific revolution [
5].
With the invention of “occhiale”, Galileo was awarded by Leonardo Donà on August 25, 1609 with an increase of his salary from the original 180 to 1000 florins/year.
During the 18 years that Galileo spent in Padua (1592–1610) (“The best years of my life”) [
6] with Leonardo Donato, first Reformer and then Doge, extraordinary advances were also accomplished in Medicine. Leonardo Donato with Fra’ Paolo Sarpi and Fabrici ab Acquapendente, Professor of Anatomy and Surgery, built up a permanent anatomical theatre, which can be regarded as the first laboratory of investigation in the history of medicine [
7,
8]. In the time interval 1599–1602, William Harvey was a student in Padua and attended the lessons of Fabrici, which was doing research on venous valves, inspiring the concept of blood circulation in the mind of the young Anglican [
9]. Thanks to the teaching of Galileo, Harvey estimated that such an amount of blood passing through the heart was possible only in a closed circulatory system [
10].
In the 16th century, the golden century of Anatomy [
8], the Studium of Padua was considered the “most famous gymnasium in the world” according to a statement of the same Vesalius [
11]. The lure of Padua was related to the strategic geographic position in the North, the civil and religious freedom and tolerance, the value and fame of the teachers, the international language of Latin and the averroistic Aristotelian philosophy, with more interest in physics than in metaphysics [
12]. A chair of natural philosophy (=physics) was formally established and a predecessor of mine (Caietanus Thieneus, 1380–1465) took over the responsibility.
The lure of the Patavian Studium was granted, even by William Shakespeare. In The Taming of the Shrew, Act I, Scene I, the protagonist Lucentio, in a public place of Padua, to explain the reason why he has come to Padua, says “… for the great desire I had to see fair Padua, nursery of arts …” [
9].
Many English students, who thereafter became professionists with high reputation in their country of origin, were captured by the lure of Padua, where they had studied and graduated [
13]. Thomas Linacre, who translated the books of Galeno and founded the Royal College of Physicians in 1518, under the auspices of Henry VIII, had graduated in Padua in 1496; John Caius, who graduated in Padua in 1541 and had been a close friend of Andreas Vesalius, founded the Caius and Gonville College in Cambridge; William Harvey, who graduated in Padua in 1602, was a prominent physician at the St. Bartholemeus’ Hospital [
9].
The success of the University was due to a farsighted academic policy of the Reformers [
12]. Venetian patricians were barred from major lectureship, to avoid familism or nepotism; even native citizens of Padua could not hold ordinary chairs. Vacant chairs were given to smart candidates outside the University, to be filled by promotion from within the ranks of the existing faculty. The position of Professor was not permanent and had to be confirmed every four years, according to the results in research and teaching, with the approval of the students [
13]. Only men who demonstrated excellence in their profession were given the charge over the education of the young [
14].
The great achievements and progression of knowledge in Medicine at the University of Padua during Renaissance is well shown by the titles of the published books: first in anatomy (De Humani Corporis Fabrica by Andreas Vesalius in 1543, De Re Anatomica by Realdo Colombo in 1559, Observationes Anatomicae by Gabriele Falloppia in 1561, De Venarum Ostiolis by Fabrici ab Acquapendente in 1603), then in physiology (Exercitatio Anatomicae de Motu Cordis et Sanguinis by William Harvey in 1628) and finally in pathology (De Sedibus et Causis Morborum per Anatomen Indagatis by Giovanni Battista Morgagni in 1761).
The availability of cadavers for anatomical dissection was fundamental, for research and teaching purposes, and was obtained through the Reformers. There is a document dated December 15th, 1556, with a request from a Reformer to the Podestà (Mayor) of Padua: “Since anatomy is very useful to students of medicine and the present time is very appropriate, I beg your magnificence to give some subjects sentenced to the stake to the most excellent Fallopius who will make dissection with great expectation and satisfaction of those scholars…” [
2].
The fashion of how to hold anatomical lessons changed radically. Previous anatomists, like Mondino de Liuzzi in Bologna, used to teach “ex-cathedra” by reading the Book of the Ancients and comparing the anatomical findings with the book content, leaving the technician to dissect. The Patavian anatomists radically changed the style by doing the dissection on their own and questioning the content of the books when the findings were opposite. Thus, the Professor moved from “sapiens” to “faber”, with personal experience more important than “ipse dixit” [
8].
Thus, the course of medicine changed. Andreas Vesalius in Tabulae Sex, 1540, [
15] perfectly depicted the cardiopulmonary system, however he still believed in the existence of pores in the interventricular septum and of pulmonary veins draining air from the lung, according to the Galeno theory.
Matteo Realdo Colombo in De Re Anatomica, 1559, by making in vivo experimental investigation, discovered the pulmonary circulation “I believe the function of the pulmonary vein is to lead blood mixed with air from the lungs to the left ventricle of the heart and this is unquestionable. In fact, if you observe cadavers as well as living animals, you will always find the pulmonary vein full of blood, which is not possible if this vein was to carry only air and fumes…” [
16].
Miguel Serveto, who probably visited Padua, in Christianismi Restitutio (1553), first described the pulmonary circulation and the ventricular diastole by saying “The vital spirit is generated by the mixing inspiring air with blood, which goes from the right to the left ventricle… this blood transfer does not occur through the ventricular septum, as usually believed, but through a long conduit crossing the lungs… The blood is refined and brightened by the lungs, goes from the pulmonary artery to the pulmonary veins, is mixed with the inspired air and eliminates residual fumes… Eventually the whole mixture is sucked by the left ventricle during diastole…” [
17].
The discovery of blood circulation by William Harvey (1628) was possible thanks to the period spent in Padua where he graduated on April 25, 1602 [
10]. His friend, Robert Boyle, who was also a famous physician, told that Harvey confided in him that, while investigating the blood movement, he was inspired from the venous valves first observed by Fabrici ab Acquapendente [
9]. That was the starting point of physiology.
Then the era of morbid anatomy came. In 1712, Giovanni Battista Morgagni, called to the chair of Theoretical Medicine by the Reformers at the age of only 29, in his first lesson, in front of the college of professors, said: “We will state that it is impossible to pursue the nature and cause of any disease without dissection of respective cadavers” [
18]. He introduced the method of clinico-pathologic correlations, by doing postmortems on the same patients he had the opportunity to visit in vivo, thus collecting hundreds of cases and observations. These were eventually published in De Sedibus et Causis Morborum per Anatomen Indagatis, in 1761, [
19] a book that “established pathological anatomy as a science, thus changing the course of medical diagnosis” [
20]. He not only described several cardiovascular disorders (cardiac rupture, aortic aneurysm, endocarditis, cor bovinum, aortic incompetence, syphilis, rare pulse with seizures, sudden death), but also first used arguments of physiopathology, as clearly depicted in the anatomo-medical letter 17.12 regarding pulmonary stenosis and atrial septal defect: “…a girl, who was sick since birth, died at the age of 16. The heart was small… the right ventricle had the shape of the left one… the foramen ovale was patent… the sigmoid pulmonary valves were cartilagineous with a small orifice. I believe that the lesion was present at birth and that the livid skin was due to the blood stagnation and to the existence of foramen ovale, the valve of which was pushed from right to left …” [
19].
Cardiovascular pathology at the University of Padua 1972–2007
When I was still a student in the late 60’s and started following the call for cardiovascular (CV) pathology, the job was restricted mostly to autopsy. The discipline was the Cinderella within the large family of Anatomic Pathology and the reason was that it dealt only with cadavers.
My mentor in CV pathology was an encyclopaedic anatomist, devoted to post-mortem investigation of patients dying because of CV disease. He had superb skill to finely dissect, clearly interpreting even complex congenital heart diseases, and he was the keeper of the cardiac anatomical collection. Cardiac surgery was just starting with prohibitive operative mortality. The CV pathologist was asked to perform postmortem on surgical fatalities and was witness to the limitations in medicine. In vivo diagnosis was mostly invasive by angiography, with a poor knowledge of anatomy and pathology by the operators. Cardiopulmonary by-pass and cardiac arrest allowed surgeons to open the heart and correct the defects, lacking elementary morphological notions such as the topography of the conduction system. Most of the septal defects, which were first operated on at the Mayo Clinic with the use of a cardiopulmonary by-pass machine, weaned off the pump with atrioventricular block [
21].
There was clearly a new demand and need for a novel development in the knowledge of anatomy, and pathology. “Operable” cardiac diseases consisted of gross structural defects and clearly the mission of the pathologist was to support the clinicians and surgeons by transferring the information of postmortem into knowledge of clinical and surgical anatomy to improve the diagnosis and treatment for the benefit of the patient. The anatomical theatre was still the place “mors ubi gaudet succurrere vitae” (where death enjoys to assist life).
It soon became evident that the catherization lab, the surgical theatre and the autopsy room were places to apply the same teaching and language for fruitful clinicopathologic correlations [
22], in keeping with the Morgagni lesson [
23].
The visit I made to the Mayo Clinic in 1974 was inspiring in this perspective. I saw the pathologists with surgeons and cardiologists during clinicopathologic conferences and presentations of clinical cases, with such mutual knowledge of their professional skills as to make it difficult, at first sight, to identify “who is who”. I realized that clinical training to learn the elementary notions of electro-mechanical activity of the heart and circulation was mandatory, before my definitive professional choice of pathology.
I firstly became a cardiologist, but the call to pathology was reinforced during the clinical training by an episode of which I was a witness, with a patient dying in my arms because of late diagnosis, delayed surgical decision making, deficiencies in basic knowledge, lack of cardiac imaging and surgical facilities. Thus, I took the plunge into the venture of CV pathology, eager to implement a service complementary to clinical demands. Luckily enough, I soon got an assistant position in the Faculty of Medicine at the University of Padua.
I will now deal with the changing role of CV pathology in the last 30 years, which reflects the development of cardiac surgery and interventional cardiology on the one side and of the genetics and molecular biology on the other.
‘70s: the dawn of cardiac surgery and the birth of CV surgical pathology
Following improvements in anaesthesiology and myocardial-cerebral protection during cardiac arrest, which rendered operations feasible even in neonates [
24], a sound knowledge of the anatomy of complex congenital heart diseases appeared to be a prerequisite for optimising clinical diagnosis and improving surgical repair. Implementation of a cardiac registry of congenital heart disease specimens, collected since the beginning of paediatric cardiology, allowed the study of the whole anatomical spectrum of various defects. The anatomical collection represented the interface of paediatric cardiologists and cardiac surgeons with the CV pathologist, for the quality control of both clinical diagnosis and surgical treatment as well as for planning new technical solutions for diagnosis or repair. The segmental approach (atria, ventricles and great arteries) was introduced as the best way to clear up the difficulty of diagnosing complex congenital heart diseases [
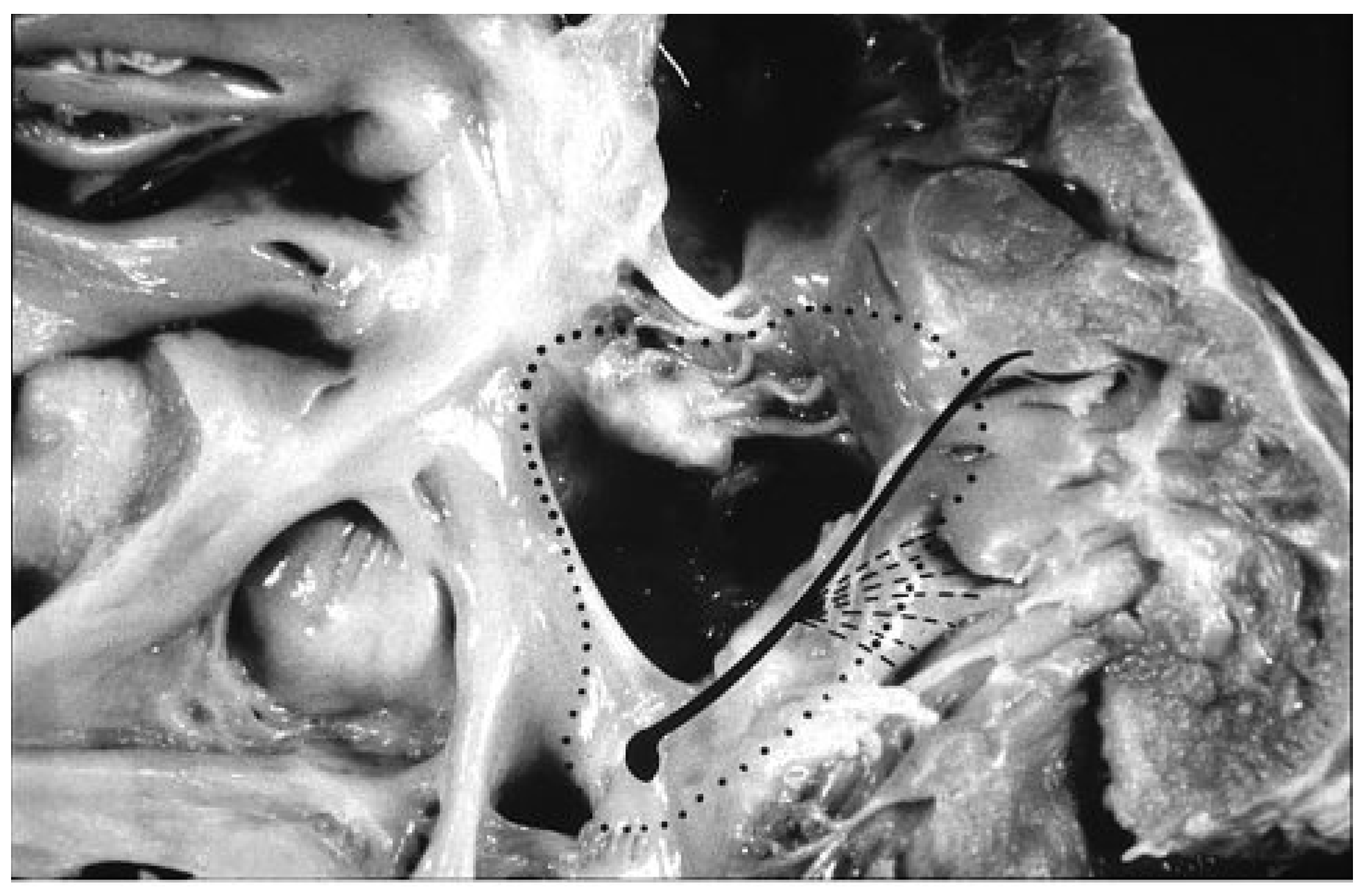
25], with the aim of reconstructing, by surgery, separated pulmonary and systemic circulations in a correct sequential manner. The identification of the precise course of the conduction system through histological serial sections virtually removed all the risks of av block during closure of septal defects (
Figure 3), by guiding the hand of the surgeon in suturing the patch [
26].
The early occurrence of pulmonary vascular disease was found to threaten the surgical repair of congenital heart disease with septal defects, and to suggest the need to plan the time of operation within the first year of life. This was supported by a histological study of lung biopsies taken in vivo at the time of total repair [
27], which demonstrated that advanced pulmonary vascular disease entailed a high risk of fatal outcome following septal closure. It is quite rewarding to realize that, thanks to the improvement of clinical diagnosis and surgical expertise, with the help of the pathologist, the mortality for repair of congenital heart disease, including quite complex forms like univentricular hearts, has decreased drastically with time.
Any tissue resected at cardiac surgery started undergoing routine histological analysis, thus opening the era of cardiovascular surgical pathology. Valves, vessels, venous or arterial grafts, pericardium, tumors were submitted, systematically, to a precise microscopic diagnosis; an essential step in establishing the nosography and epidemiology of “operable” cardiovascular diseases. The bicuspid aortic valve was found to be not only a substrate of early dystrophic calcification and stenosis, but also a major risk factor for endocarditis, aortic incompetence and spontaneous aortic dissection. Histology of cardiac masses made it possible to distinguish between thrombotic and neoplastic tissues and to show that endocavitary atrial tumours are not only benign myxoma but also malignancies. Application of immunohistochemistry revealed to be crucial for establishing the histotype.
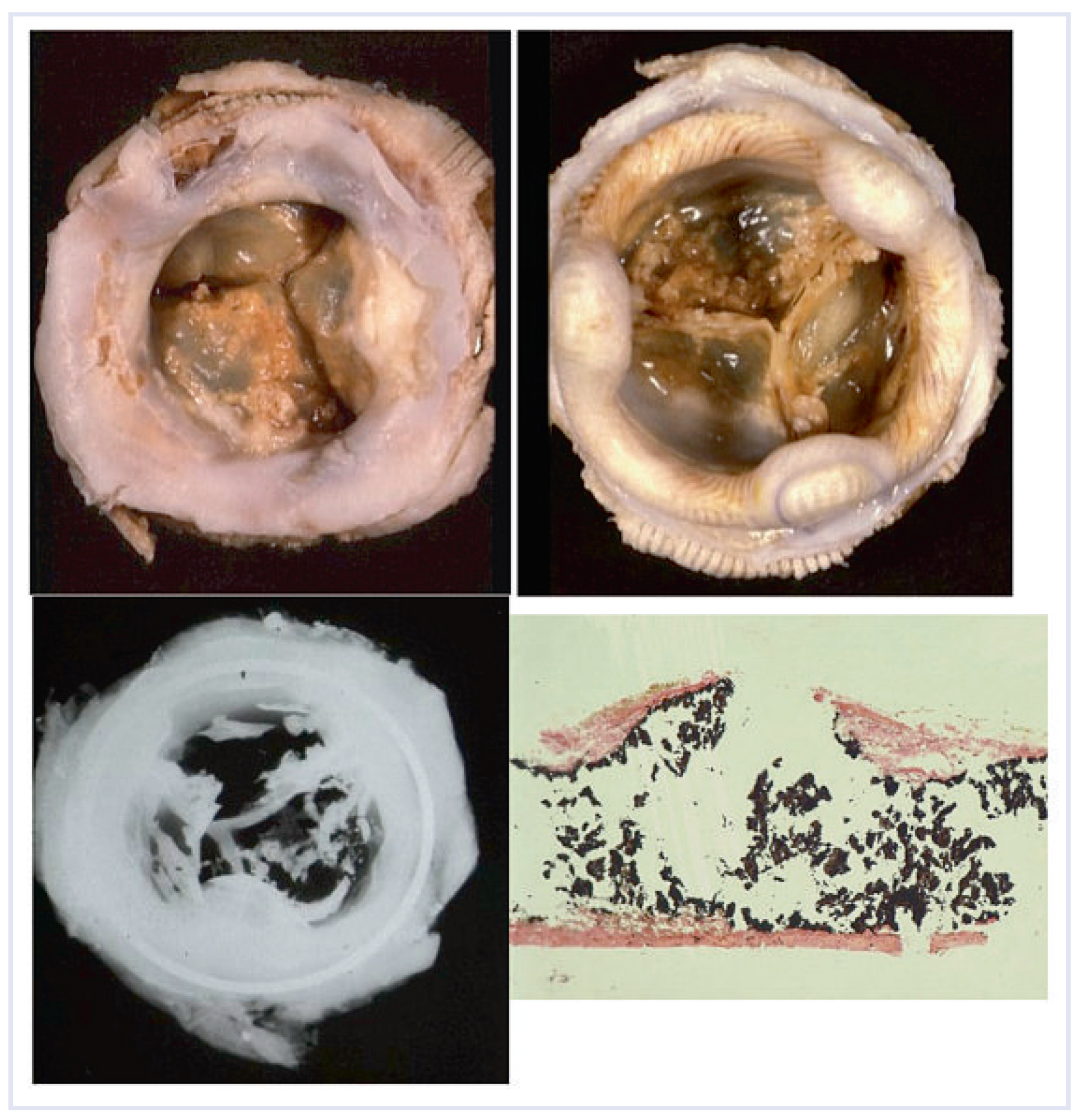
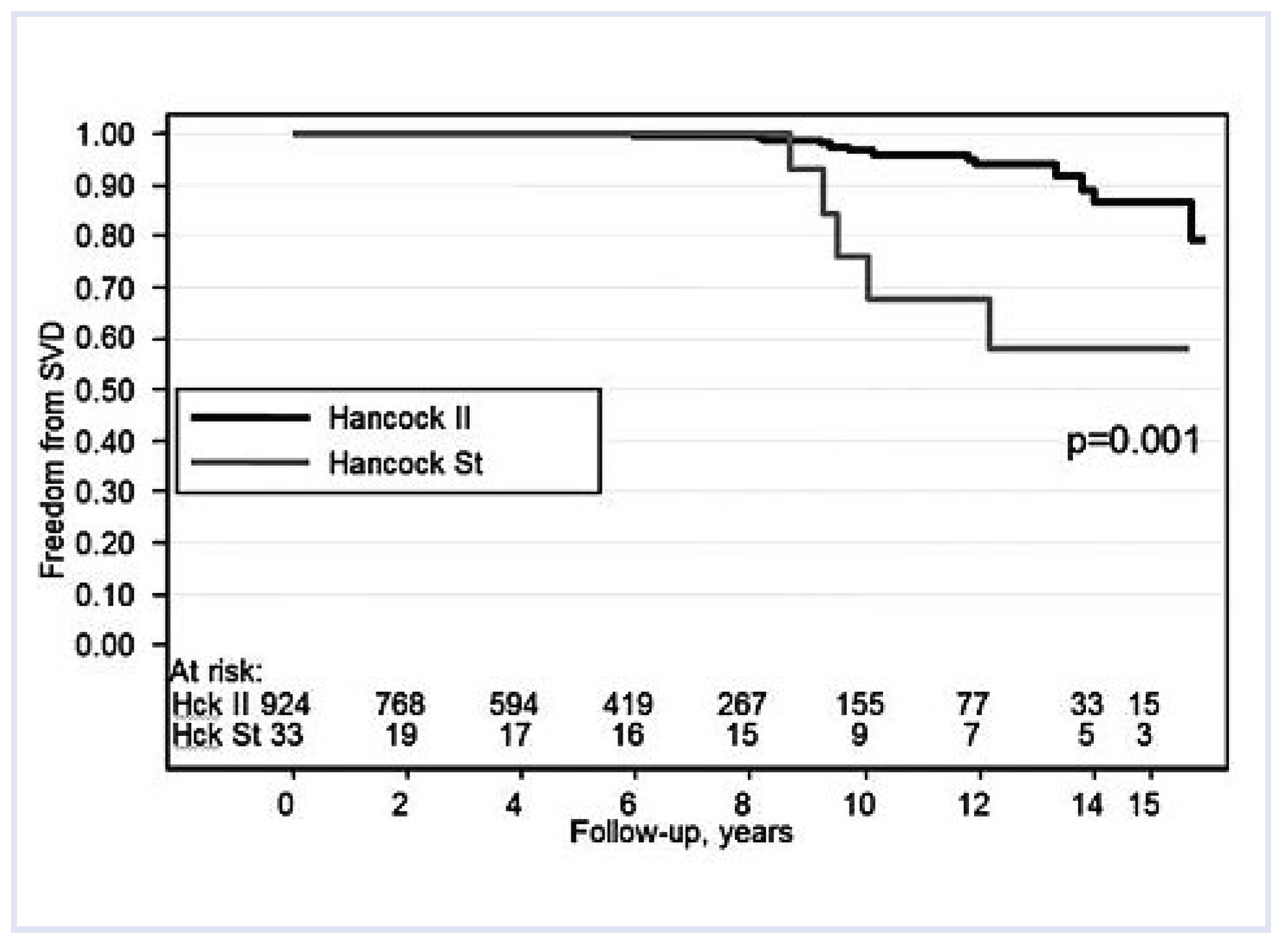
The increasing use of valvular prostheses, with an intrinsic propensity to structural failure, required a novel skill. Since the beginning, cardiac surgery at the University of Padua made the choice for biological valves, namely glutaraldehyde fixed porcine xenografts. They differ from mechanical valves, as they do not require anticoagulation and are not noisy, with fair patient compliance. The morphologic evaluation allowed physicians to discard prosthetic valves with early failure due to technical deficiencies in design, suggesting the development of more durable devices. Bioprosthetic valves were found to have limited durability, with a half-life of only 8–10 years. We accumulated great experience with a surgical pathology study of more than 400 Hancock standard porcine bioprostheses from patients who were re-operated on once or twice, due to their valvular dysfunction. Calcification proved to be the main cause of failure [
29,
30] with cusp stiffening and/or disruption (
Figure 4). This awareness emphasized the need for finding a solution to prevent or retard mineralization and to enhance long term durability. In vivo animal studies and in vitro experiments were carried out to test anti-mineralization agents with the aim to introduce new devices [
30,
31,
32], and to increase bioprosthetic longevity by 5–7 years (
Figure 5).
‘80s: the advent of interventional cardiology, endomyocardial biopsy and cardiac transplantation
In the past, there was a common quote “the pathologist knows everything, but too late”. This limitation changed in CV Pathology with the introduction of the endomyocardial biopsy. It was possible to achieve an in vivo diagnosis of heart muscle disease, thus providing vital information for clinical decision making. Endomyocardial biopsy is the gold standard today for the diagnosis of myocarditis, giving the CV pathologist the “final” word [
33].
The pivotal role of the CV pathologist was further enhanced by the beginning of the cardiac transplantation program, which required the use of endomyocardial biopsy for rejection monitoring. In each patient, 15–20 endomyocardial biopsies are performed during the first year following transplant [
34]. Nearly 30% of biopsy reports by a CV Pathology Service deal with transplantation. Moreover, the study of heart specimens, resected at the time of transplantation, gave an extraordinary opportunity to research with the discovery of new morbid entities like noncompacted myocardium and restrictive cardiomyopathy [
35,
36], and required a new WHO classification of cardiomyopathies [
37]. Restrictive cardiomyopathy featured the paradox of a small heart requiring transplantation, because of a refractory congestive heart failure due to elective impairment of cardiac diastole [
36].
On the autopsy side, a new demand for CV pathology came from the amazing occurrence of sudden deaths, even in young people or in athletes, fainting on the playground. The absence of coronary artery disease and myocardial infarction in most of the cases appeared intriguing to the CV pathologist, who was used to considering acute pump failure or coronary thrombosis as the obvious mode of cardiac death and the left ventricle as the main culprit cardiac chamber. The physiopathology training as well as the tight collaboration with clinicians were of great help for me in the interpretation of the event as an electrical breakdown. The concept that the heart may stop suddenly due to ventricular fibrillation, also in the absence of an ischemic milieu, was truly a revolution in the pathologist’s mind. Since the conduction system is the “power plant” of the heart, we first pointed to the specialized myocardial tissues in the search for abnormalities, able to explain cardiac arrest due to either tachyarrhythmias or block. This is the case of WolffParkinson-White syndrome where a tiny fascicle of ordinary myocardium (Kent’s fascicle), lacking decremental properties, may connect the atrial with the ventricular myocardium allowing the electrical impulse to by-pass the specialized atrioventricular junction, thus accounting for the recording of ventricular preexcitation in the twelve lead ECG [
38]. The finding of Kent’s fascicle location close to the endocardium was vital histological information to support intracavitary interventional ablation.
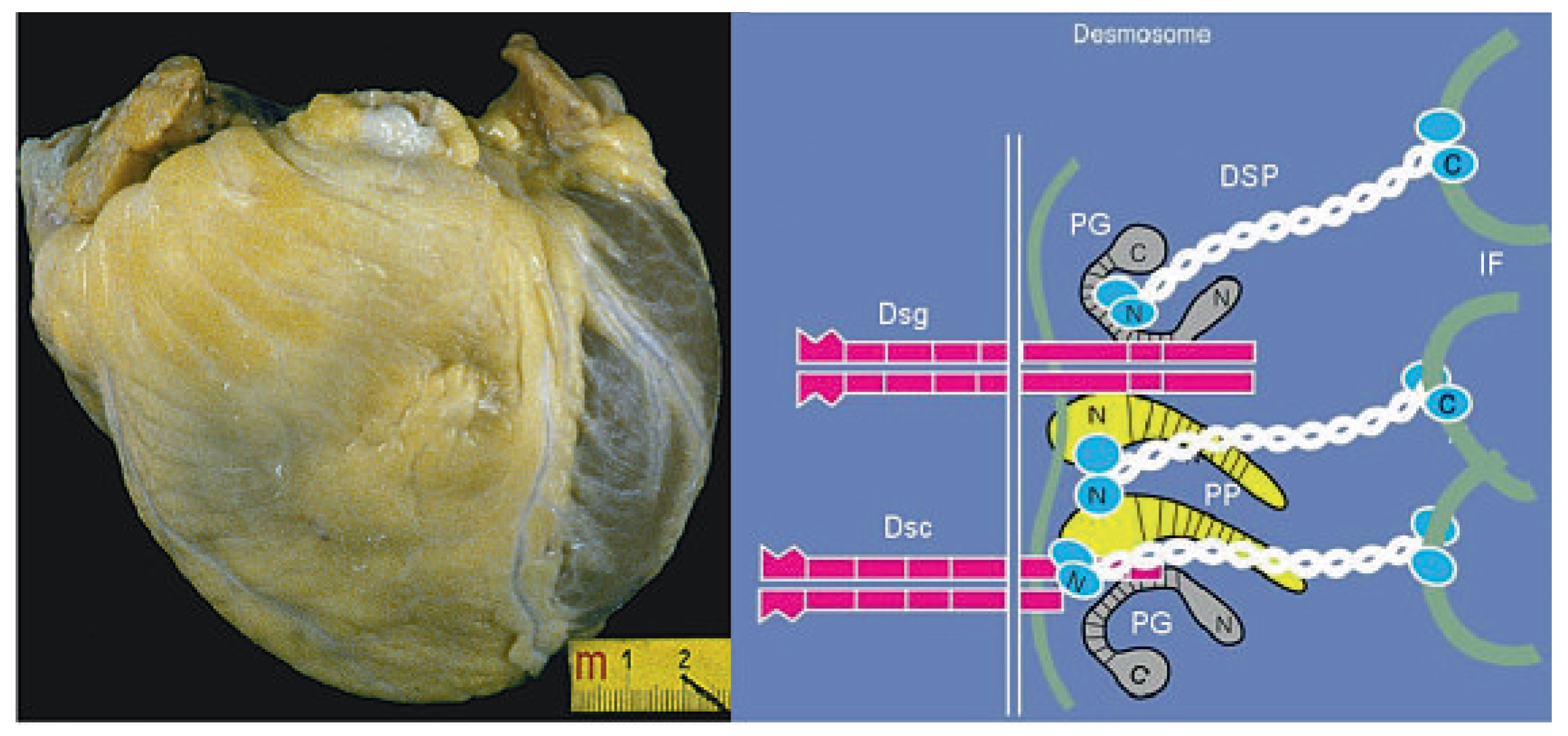
However, we soon realized that the working ventricular myocardium itself may be the substrate of lifethreatening arrhythmias, despite the integrity of the conduction system and the coronary arteries. Arrhythmogenic right ventricular cardiomyopathy, the major cause of sudden death in the young [
39], represented an alarming instance, in so far as fibro-fatty replacement of the right ventricular free wall may trigger lethal tachyarrhythmias during effort through reentrant mechanisms, even in the setting of a normal left ventricle (
Figure 6).
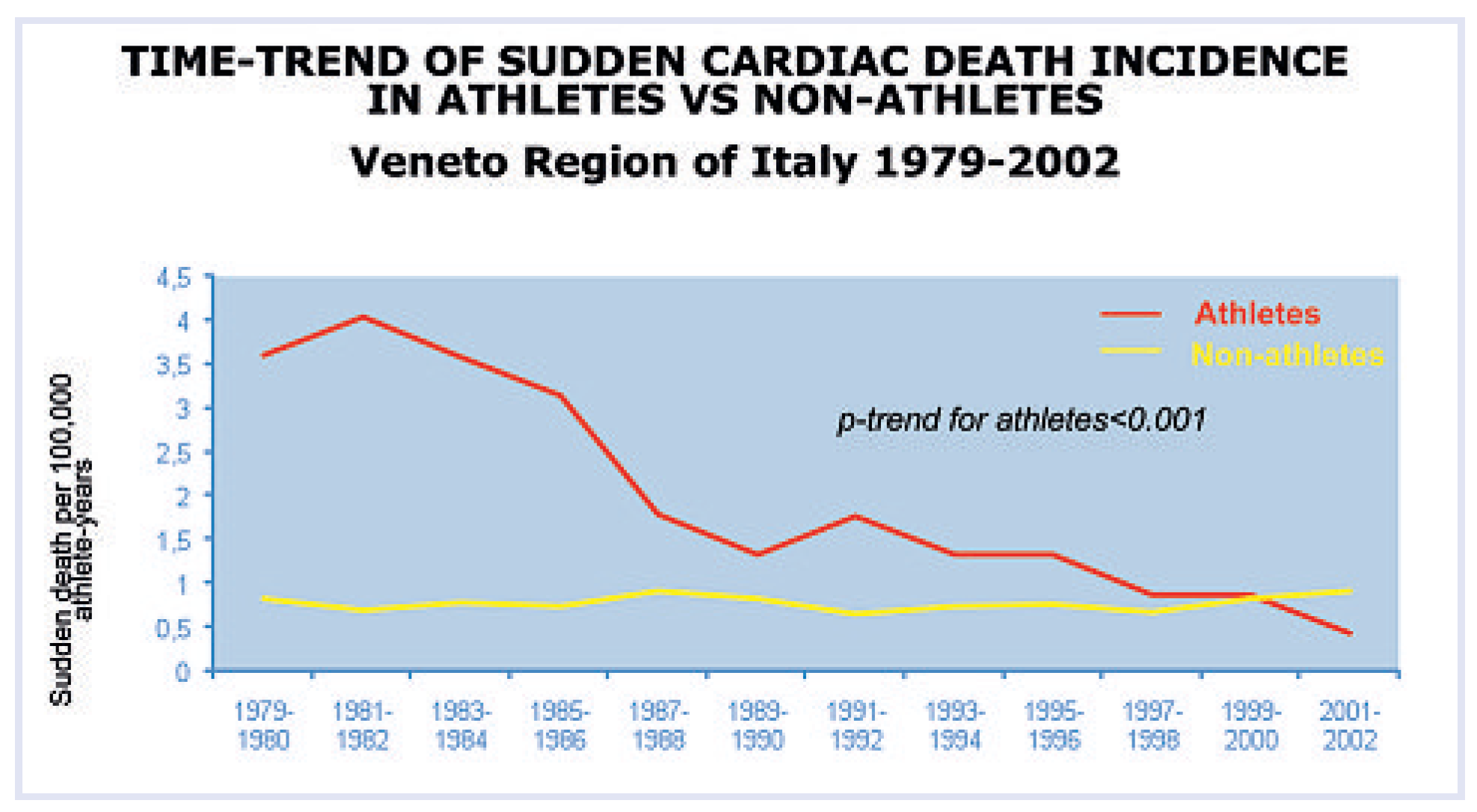
By establishing close collaboration with Pathology Services as well as with forensic pathologists of the Veneto Region, Italy, we set up a network that was able to collect all the cases of sudden death in the young. This was a unique opportunity to study the phenomenon on a large scale and to discover a wide spectrum of diseases as a cause of life-threatening cardiac electrical instability. It was now possible, at the time of screening for sport eligibility, to identify and disqualify patients at risk. A clear-cut decline of sudden death in the athletes was observed after implementation of the preparticipation screening from the time interval 1979–2002 [
40], mostly due to identification of patients bearing cardiomyopathies (
Figure 7).
‘90s: from gross to molecular pathology
The traditional tools of the pathologist are the naked eyes at autopsy for gross dissection and the light microscope for histological examination. Magnification power increased a lot with electron microscopy, allowing morphological investigation at the subcellular level. However, there are offending organisms or structural defects that escape ultrastructural imaging, emphasizing the limits of traditional morphology. The discovery of new molecular biology tools like in situ hybridisation and the polymerase chain reaction (PCR), as well as gene cloning and sequencing, opened extraordinary avenues for a new insight deep into cell and nuclear pathophysiology as well as genes and coded proteins. The collaboration with clinicians was extended to basic scientists and this policy has been rewarding, in so far as in 15 years it has been possible to move from the first postmortem observation of arrhythmogenic right ventricular cardiomyopathy to the discovery of the culprit genes encoding proteins at the level of cell junctions (desmosome) (
Figure 6) [
41].
Apoptosis was found to be a mode of cell death in cardiomyopathies. By applying the TUNEL method to endomyocardial biopsies of patients with arrhythmogenic right ventricular cardiomyopathy, myocyte apoptosis was observed at a high rate, such as to account for progressive fibro-fatty replacement [
42].
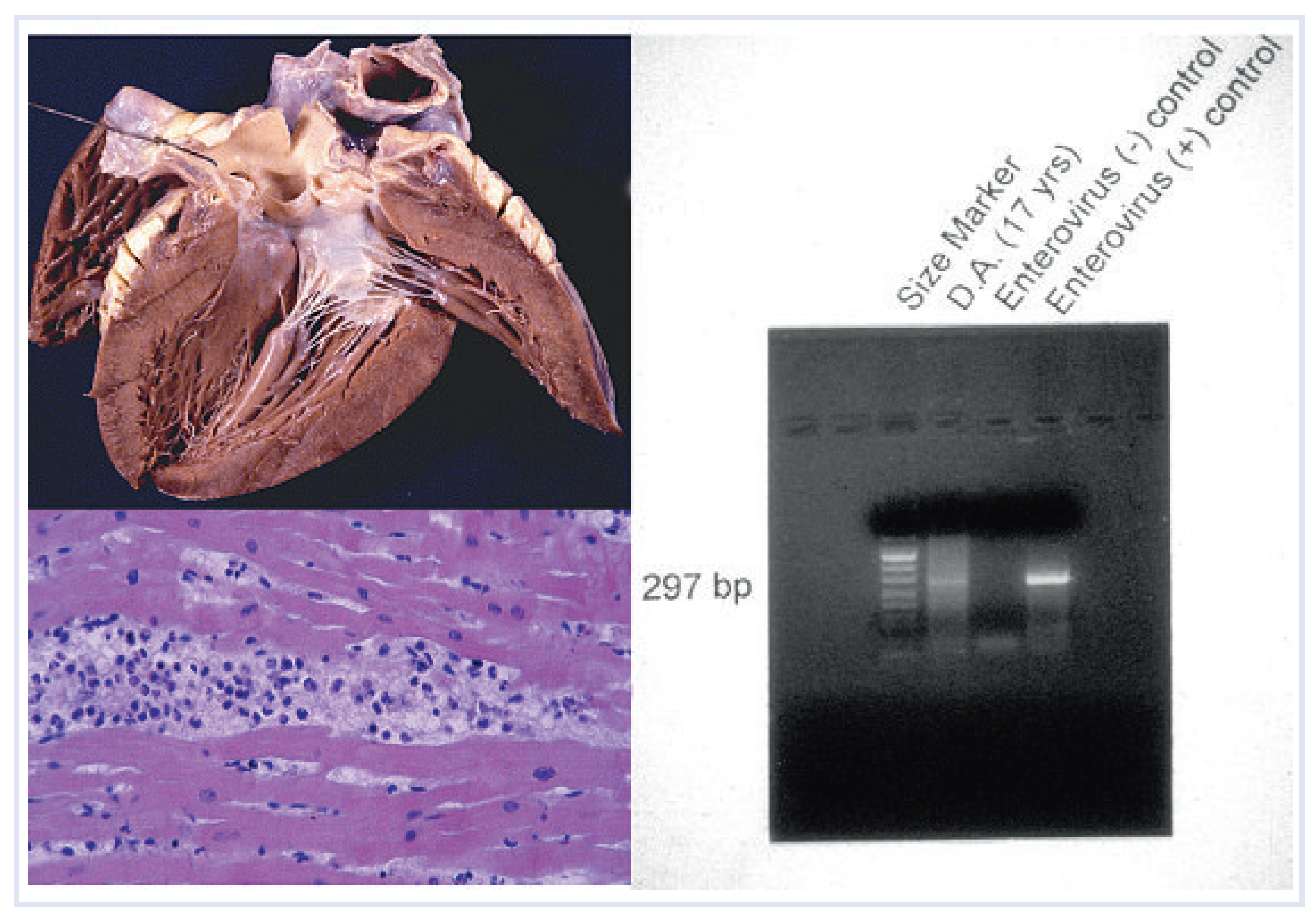
The use of PCR or reverse transcriptase PCR, able to amplify DNA or RNA by up to one billion copies, was a powerful weapon in detecting viral genomes, in up to 50% of endomyocardial biopsies from patients with myocarditis and even at autopsy (
Figure 8). DNA viruses, like adenovirus, were found to be potent cardiotropic infective agents as much as RNA enterovirus, accounting for various clinical presentations of myocarditis, from cardiogenic shock to sudden death. PCR is now an essential tool in endomyocardial biopsy evaluation and a goldstandard for diagnosis [
43]. In terms of clinical decision making, the finding of a viral etiology entails strategic therapeutic implications, since antiviral therapy may be used in PCR positive cases [
44].
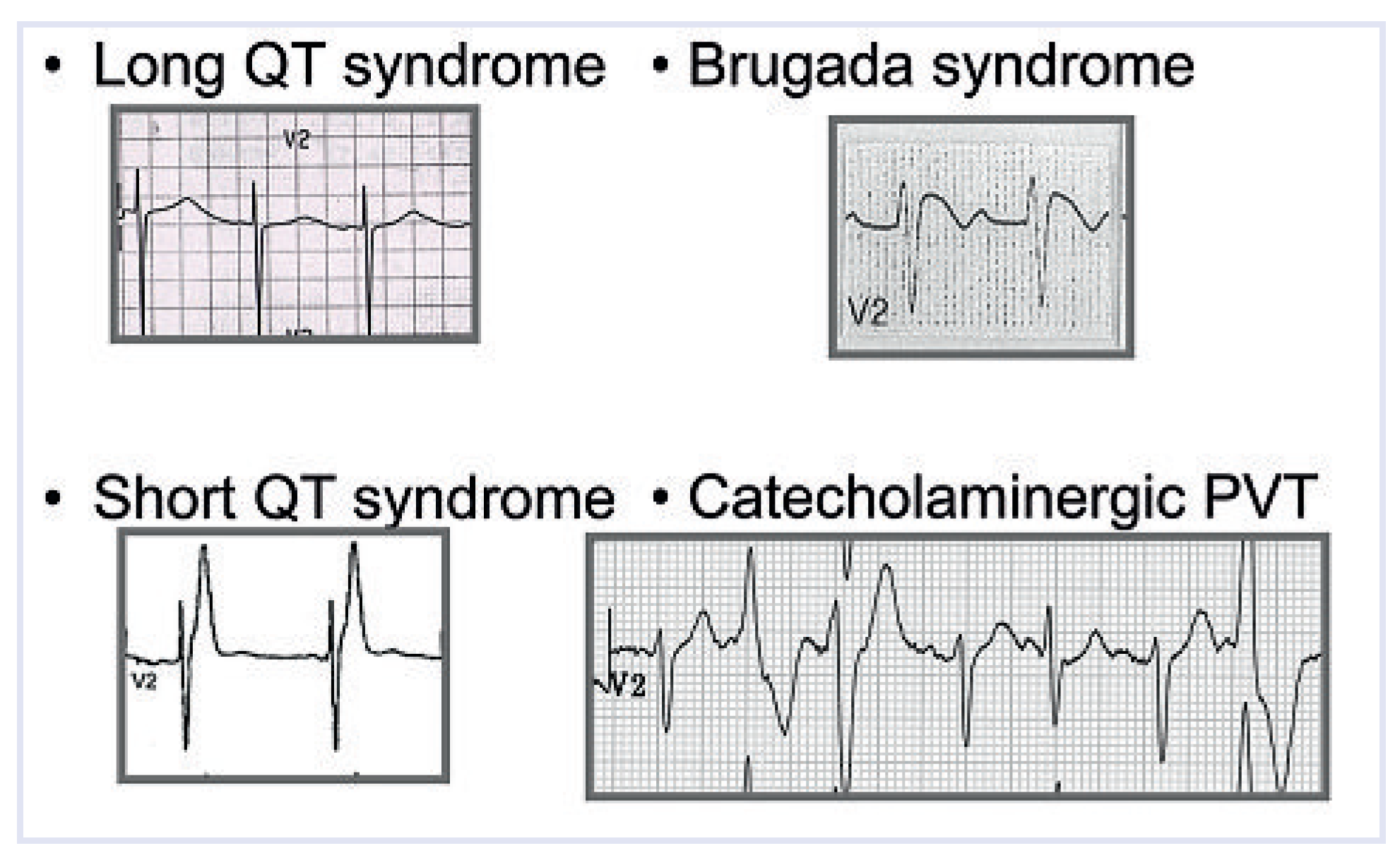
10–20% of young people who die suddenly, present with a normal heart (“mors sine materia”) [
45]. For the pathologist, it is frustrating and difficult to accept that hearts from patients who die suddenly may be normal, even after extensive morphological examination including the study of the conduction system. Molecular genetics have demonstrated that many inherited ventricular arrhythmias are ascribable to genes encoding defective proteins of cell/sarcoplasmic reticulum membranes, where ion pumps (Na, K, Ca) are located (
Figure 9). Clearly these defects are beyond the magnification power of any microscope.
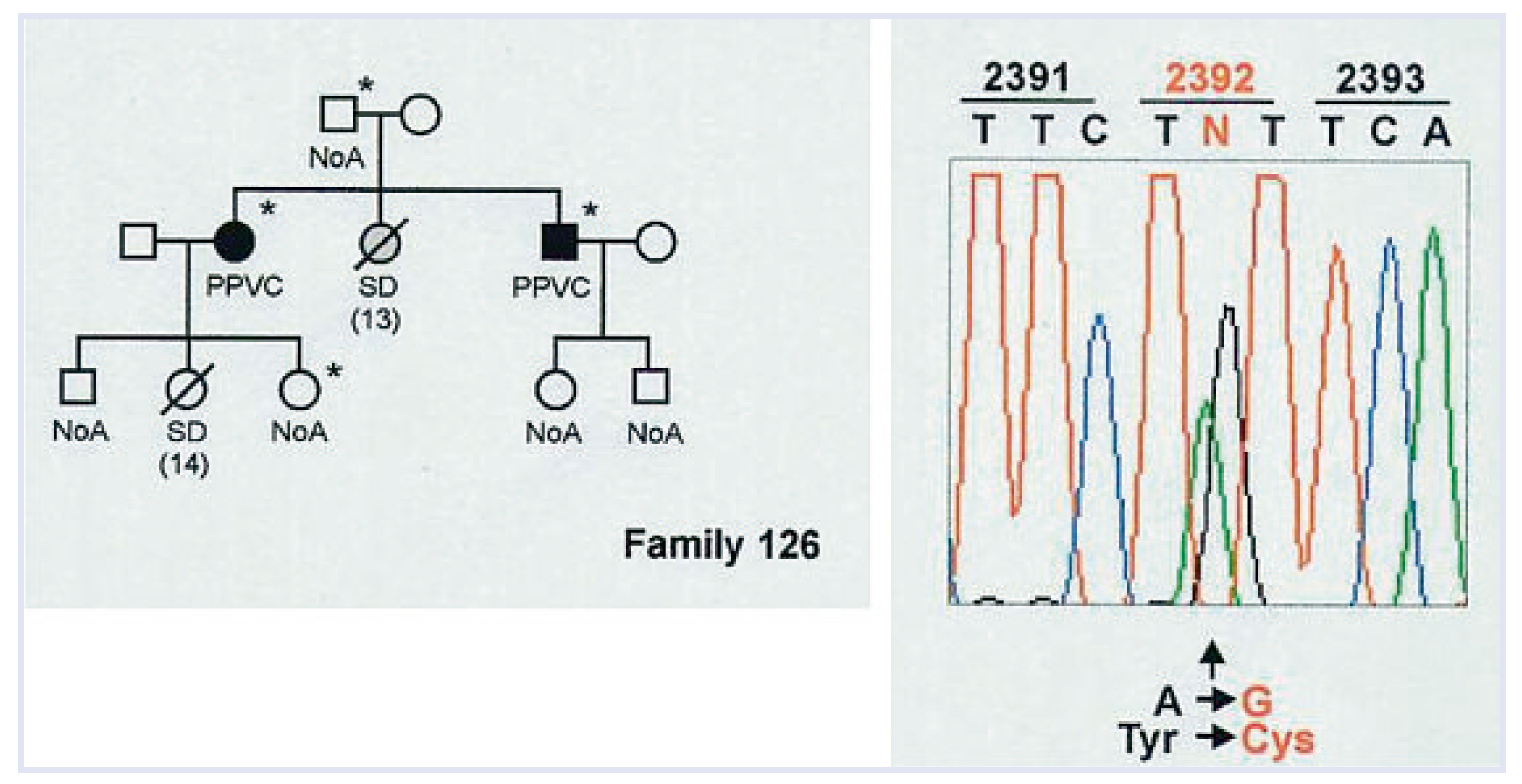
Some of them were proven by molecular genetics to be affected by missense gene mutations, like those encoding the cardiac Ryanodine receptor, responsible for Ca++ release from the smooth endoplasmic reticulum for excitation-contraction coupling [
46]. Just a mutation of a single base may account for an altered coded protein with only one different aminoacid (missense mutation), enough to impair the receptor function and Ca++ release, thus jeopardizing the electrical stability during effort (
Figure 10) [
47]. These molecular techniques can also be applied at post-mortem, making it possible to achieve molecular diagnosis in the so-called “mors sine materia” (“molecular autopsy”) [
48]. Guidelines for autopsy investigation of sudden cardiac death, which have been recently published by the Association for European Cardiovascular Pathology, include molecular investigation [
49].
One may wonder whether this infinitesimal gene sequence abnormality may be enough to deserve the name of congenital heart disease, namely a cardiac structural defect present at birth. Most probably, with the advent of molecular genetics, the definition of congenital disease will change to include not only gross structural defects but even gene mutations present at birth. A molecular classification of cardiomyopathies was advanced, according to the site of hereditary molecular structural defects (cytoskeleton, sarcomere, ion channels, desmosome) [
50].
Cardiovascular pathology in the future: what is still needed?
Death is an unavoidable destiny of human life and there will be always a need for autopsy, whether clinical or forensic. Since CV diseases account for nearly half of the causes of death, an increasing demand for pathologists with expertise in cardiac anatomy and pathology is expected.
The anatomy theatre will continue to be the main laboratory of CV pathology and dissection skill will remain a prerequisite in professional training [
51]. The autopsy rate in our University still continues to exceed 30% of the deaths occurring at the hospital. However, it is becoming more and more evident that the ability to interpret the morphologic alterations, occurring in the natural history of the human body, should be based on a solid clinical background [
52]. The information that comes from a thorough autopsy examination is of vital importance for the clinical management of future patients. A precise diagnosis based on a complete autopsy is essential in cases of sudden death due to heredofamilial disorders, where the autopsy may represent the opportunity not only to establish the cause of death but also to guide clinicians to family investigation, including genetic screening. Perhaps, CV pathologists should consider starting their career by training in a clinical milieu, as I did, to become confident with the diagnosis and treatment of heart diseases, and then move to pathology [
53].
The pathologist will never be alone and powerless, as he was in the past, witnessing the defeat of Medicine in the silence of the mortuary. The interdisciplinary approach to diagnosis, therapy, and prevention of CV diseases will place the CV pathologist in a team, as a bridge between the cardiologist and the cardiac surgeon on one hand and the genetist and molecular biologist on the other [
52,
53]. Today, a wide spectrum of diagnostic tools are at the disposal of the pathologist and some of them do not require the traditional microscope. They make the jobs more useful and the CV pathologists’ career can be intellectually and professionally appealing to the young generation of doctors, with the perspective to become protagonists in the fight against heart disease. The position of the CV pathologist, between the bench and bedside, allows him to play a strategic role in the study of CV disease [
54].
Historically, human pathology has paid more attention to morphological substrates and pathogenesis than to the etiology of disease. We now know which morbid entities affect heart and vessels, we know the signs and symptoms of the diseases as well as their natural history, we know which medical or surgical therapy may best be applied, even extreme examples like cardiac transplantation. It is time for CV pathology to move on. The extraordinary technological breakthroughs in molecular biology, including proteomics and microarrays, provide exciting opportunities for investigating the etiopathogenesis in addition to the substrate of cardiovascular diseases [
55].