Mechanical Versus Manual Chest Compression During CPR in a Cardiac Catherization Setting
Abstract
Introduction
Methods
Study setting
Data acquisition
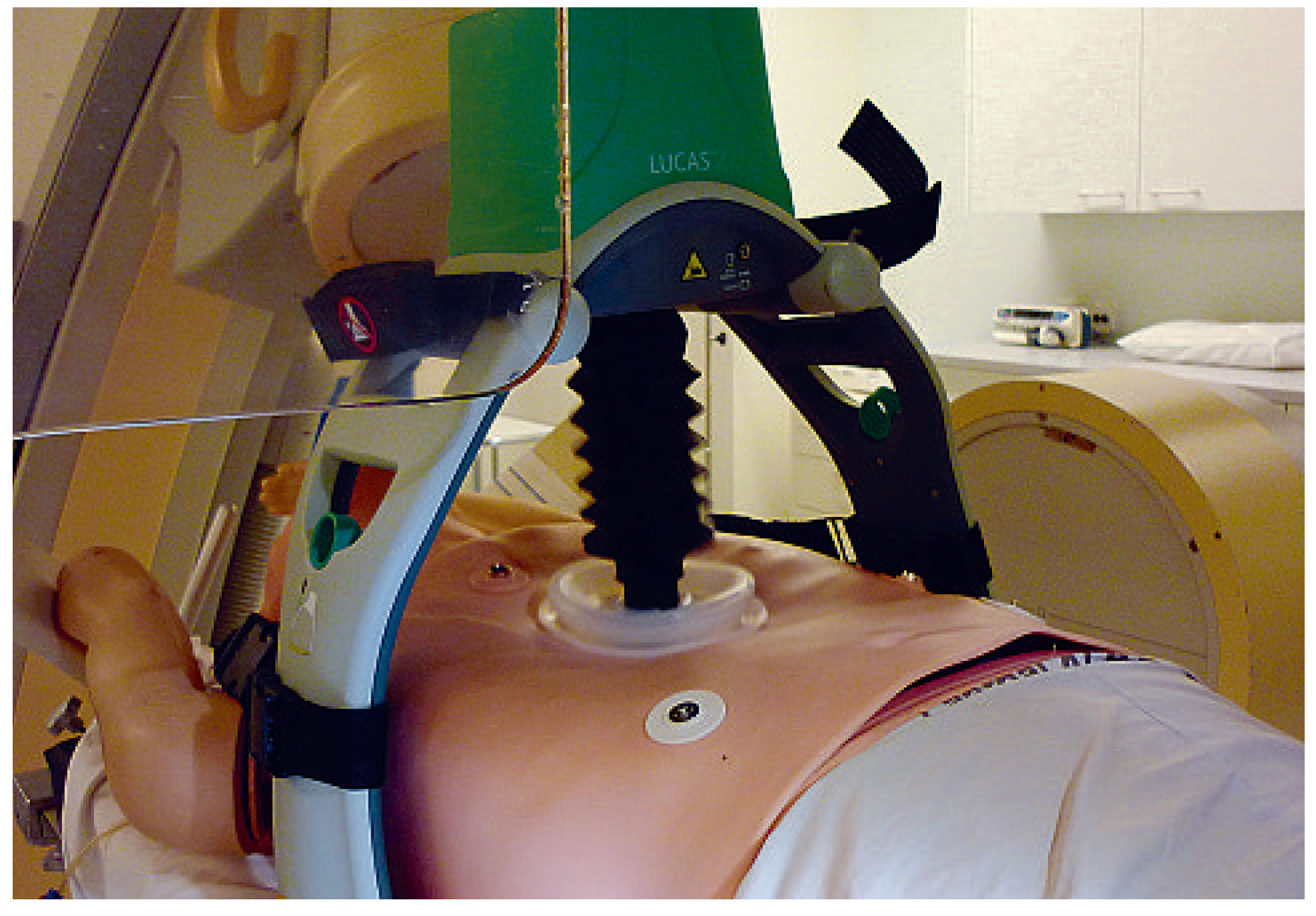
Device characteristics
Statistics
Results
Manual chest compression
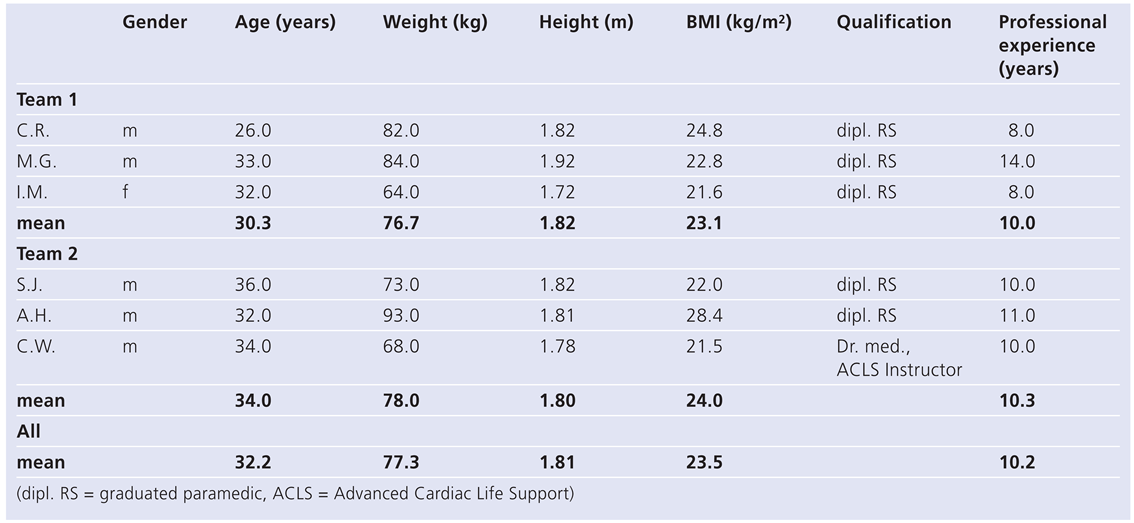
Resuscitator characteristics
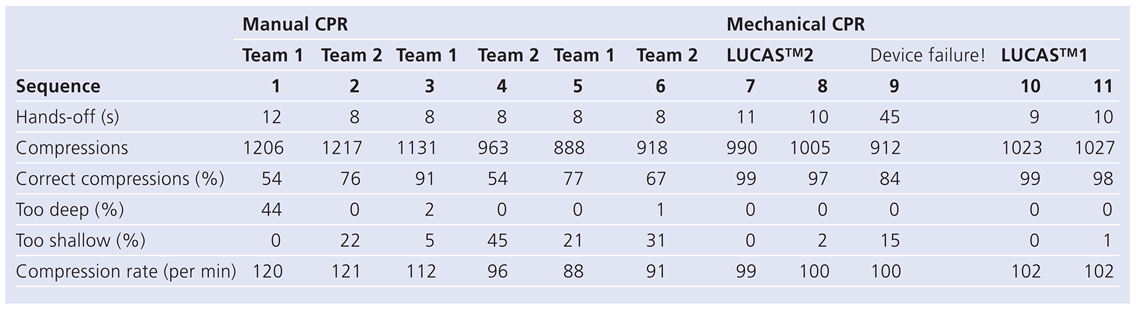
Manual CPR performance
Mechanical chest compression
Mechanical CPR performance
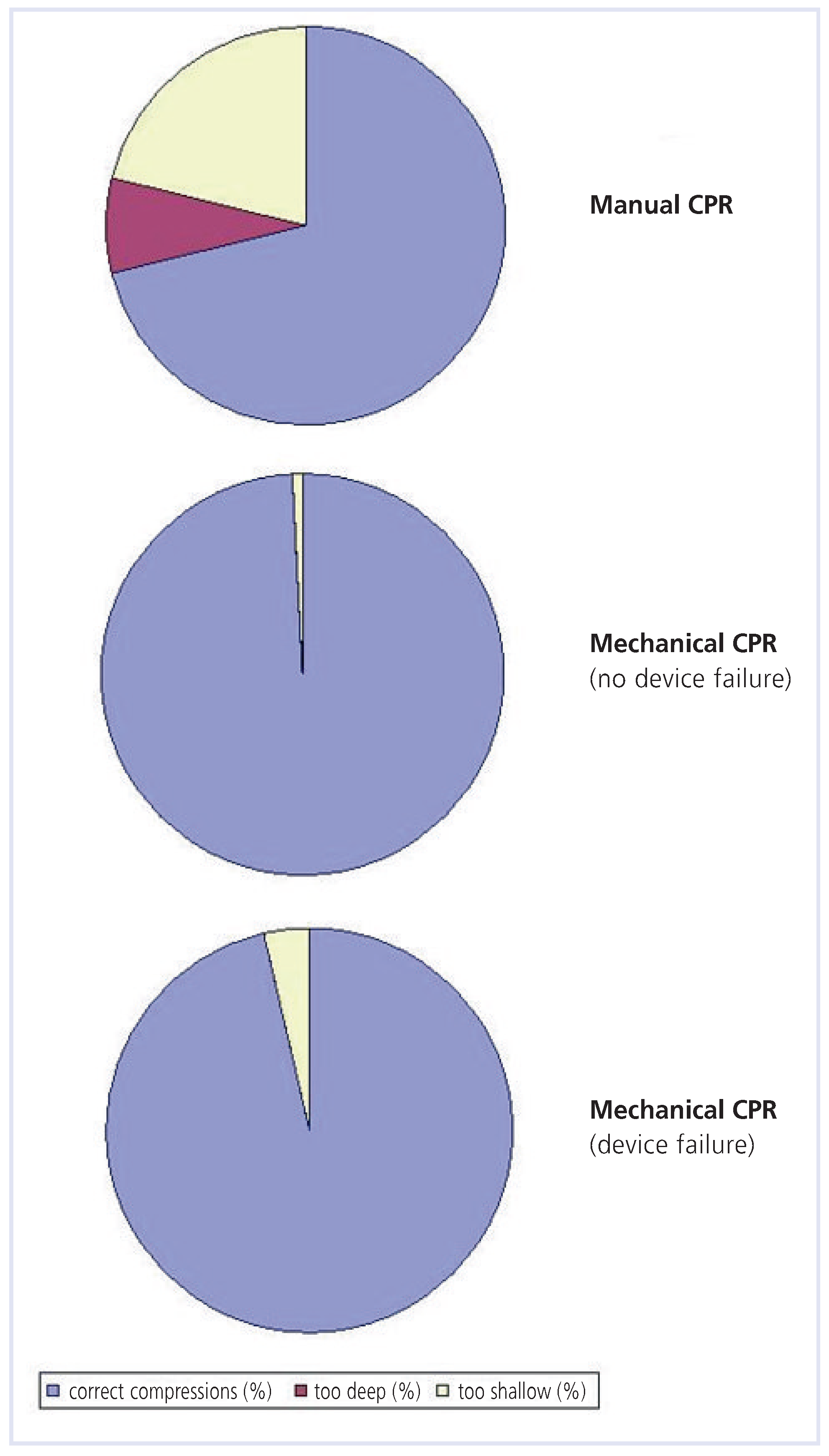
Comparison: mechanical versus manual chest compression
Performance with no device failure
Device failure
Discussion
Conclusion
Acknowledgments
Conflicts of Interest
References
- Ramaraj, R.; Ewy, G.A. Rationale for Continuous Chest Compression Cardiopulmonary Resuscitation. Heart. 2009. [Google Scholar] [CrossRef] [PubMed]
- Grogaard, H.K.; Wik, L.; Eriksen, M.; Brekke, M.; Sunde, K. Continuous mechanical chest compressions during cardiac arrest to facilitate restoration of coronary circulation with percutaneous coronary intervention. J Am Coll Cardiol. 2007, 50, 1093–1094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsen, A.I.; Hjornevik, A.S.; Ellingsen, C.L.; Nilsen, D.W. Cardiac arrest with continuous mechanical chest compression during percutaneous coronary intervention. A report on the use of the LUCAS device. Resuscitation. 2007, 75, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Risom, M.; Jorgensen, H.; Rasmussen, L.S.; Sorensen, A.M. Resuscitation, Prolonged Cardiac Arrest, and an Automated Chest Compression Device. J Emerg Med. 2009. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Cornelis, K.; Vermeersch, P. Successful percutaneous treatment of an intraprocedural left main stent thrombosis with the support of an automatic mechanical chest compression device. Int J Cardiol. 2008, 124, e19–21. [Google Scholar] [CrossRef] [PubMed]
- Paradis, N.A.; Martin, G.B.; Rivers, E.P.; Goetting, M.G.; Appleton, T.J.; Feingold, M.; Nowak, R.M. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990, 263, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Ewy, G.A. Cardiocerebral resuscitation: the new cardiopulmonary resuscitation. Circulation. 2005, 111, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, T.D.; Kern, K.B.; Clark, L.L.; Berg, R.A.; Berg, M.D.; Berg, D.D.; Hilwig, R.W.; Otto, C.W.; Newburn, D.; Ewy, G.A. Interruptions of chest compressions during emergency medical systems resuscitation. Circulation. 2005, 112, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Sugerman, N.T.; Edelson, D.P.; Leary, M.; Weidman, E.K.; Herzberg, D.L.; Vanden Hoek, T.L.; Becker, L.B.; Abella, B.S. Rescuer fatigue during actual in-hospital cardiopulmonary resuscitation with audiovisual feedback: a prospective multicenter study. Resuscitation. 2009, 80, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Baubin, M.; Rabl, W.; Pfeiffer, K.P.; Benzer, A.; Gilly, H. Chest injuries after active compression-decompression cardiopulmonary resuscitation (ACD-CPR) in cadavers. Resuscitation. 1999, 43, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Smekal, D.; Johansson, J.; Huzevka, T.; Rubertsson, S. No difference in autopsy detected injuries in cardiac arrest patients treated with manual chest compressions compared with mechanical compressions with the LUCAS device—a pilot study. Resuscitation. 2009, 80, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Abella, B.S.; Alvarado, J.P.; Myklebust, H.; Edelson, D.P.; Barry, A.; O’Hearn, N.; Vanden Hoek, T.L.; Becker, L.B. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005, 293, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Li, C.S.; Liu, Z.X.; Wu, C.J.; Zhang, G.C. A comparison of 2 types of chest compressions in a porcine model of cardiac arrest. Am J Emerg Med. 2009, 27, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Steen, S.; Liao, Q.; Pierre, L.; Paskevicius, A.; Sjoberg, T. Evaluation of LUCAS, a new device for automatic mechanical compression and active decompression resuscitation. Resuscitation. 2002, 55, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Rubertsson, S.; Karlsten, R. Increased cortical cerebral blood flow with LUCAS; a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation. 2005, 65, 357–363. [Google Scholar] [CrossRef] [PubMed]

 |
 |
© 2010 by the authors. Attribution-Non-Commercial-NoDerivatives 4.0.
Share and Cite
Wyss, C.A.; Fox, J.; Franzeck, F.; Moccetti, M.; Scherrer, A.; Hellermann, J.P.; Lüscher, T.F. Mechanical Versus Manual Chest Compression During CPR in a Cardiac Catherization Setting. Cardiovasc. Med. 2010, 13, 92. https://doi.org/10.4414/cvm.2010.01480
Wyss CA, Fox J, Franzeck F, Moccetti M, Scherrer A, Hellermann JP, Lüscher TF. Mechanical Versus Manual Chest Compression During CPR in a Cardiac Catherization Setting. Cardiovascular Medicine. 2010; 13(3):92. https://doi.org/10.4414/cvm.2010.01480
Chicago/Turabian StyleWyss, Christophe A., Julia Fox, Fabian Franzeck, Marco Moccetti, Alfons Scherrer, Jens P. Hellermann, and Thomas F. Lüscher. 2010. "Mechanical Versus Manual Chest Compression During CPR in a Cardiac Catherization Setting" Cardiovascular Medicine 13, no. 3: 92. https://doi.org/10.4414/cvm.2010.01480
APA StyleWyss, C. A., Fox, J., Franzeck, F., Moccetti, M., Scherrer, A., Hellermann, J. P., & Lüscher, T. F. (2010). Mechanical Versus Manual Chest Compression During CPR in a Cardiac Catherization Setting. Cardiovascular Medicine, 13(3), 92. https://doi.org/10.4414/cvm.2010.01480




