A Difficult Pacemaker ECG Resulting in an Unnecessary Intervention
Summary
Introduction
Case presentation
Discussion
- -
- minimise the AV/PV interval in order to ensure ventricular pacing
- -
- set atrial sensing to the lowest programmable value
- -
- shorten the PVAB to less than 100 ms
- -
- check for AFFS and, if positive, measure the V-A time interval
- -
- set the PVAB to a value at least 50 ms above the measured value
References
- Brady, W.J.; Lentz, B.; Barlotta, K.; Harrigan, R.; Chan, T. ECG Patterns confounding the ECG diagnosis of acute coronary syndrome. Emerg Med Clin N 2005, 23, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.; Kramm, B.; Schwaier, H.; Miamaitiming, A.; Geil, S.; Zellerhoff, C.; et al. Is atrial sensing of ventricular far-field signals important in single-lead VDD pacing? Pacing Clin Electrophysiol 1998, 21, 2236–2239. [Google Scholar] [CrossRef] [PubMed]
- Cools, F.J.; van Twembeke, R.R.; Backers, J.; Verpooten, G.A. Stability of far field R wave signals in different conditions. Europace 2003, 5, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Frohlig, G.; Helwani, Z.; Kusch, O.; Berg, M.; Schieffer, H. Bipolar ventricular far-field signals in the atrium. Pacing Clin Electrophysiol 1999, 22, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
 AMS with VVI back up mode is shown.
AMS with VVI back up mode is shown.
 P-waves noted in the marker channel (middle line).
P-waves noted in the marker channel (middle line).
 Intermittent Far Field sensing seen in the bottom line (signal measured between Atip
and Aring) and in the marker channel (middle line).
Intermittent Far Field sensing seen in the bottom line (signal measured between Atip
and Aring) and in the marker channel (middle line).
 Intrinsic ventricular beat.
Intrinsic ventricular beat.
 AMS with VVI back up mode is shown.
AMS with VVI back up mode is shown.
 P-waves noted in the marker channel (middle line).
P-waves noted in the marker channel (middle line).
 Intermittent Far Field sensing seen in the bottom line (signal measured between Atip
and Aring) and in the marker channel (middle line).
Intermittent Far Field sensing seen in the bottom line (signal measured between Atip
and Aring) and in the marker channel (middle line).
 Intrinsic ventricular beat.
Intrinsic ventricular beat.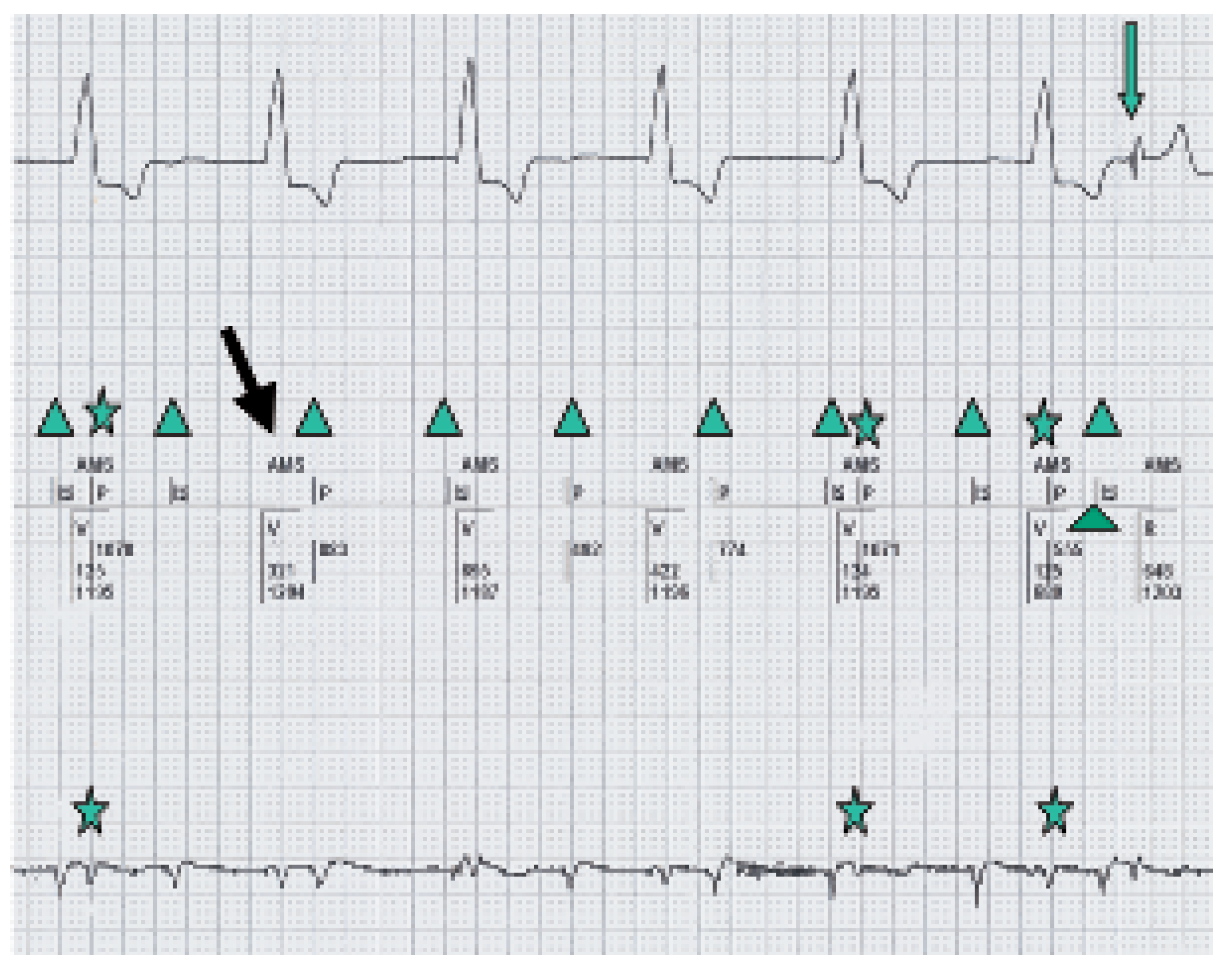
 Intrinsic ventricular complexes.
Intrinsic ventricular complexes.
 P-waves.
P-waves.
 Paced ventricular complexes in AMS with VVI back up mode of 50/min.
Paced ventricular complexes in AMS with VVI back up mode of 50/min.
 Switch from VVI to VDD mode with 1:1 conduction.
Switch from VVI to VDD mode with 1:1 conduction.
 Intrinsic ventricular complexes.
Intrinsic ventricular complexes.
 P-waves.
P-waves.
 Paced ventricular complexes in AMS with VVI back up mode of 50/min.
Paced ventricular complexes in AMS with VVI back up mode of 50/min.
 Switch from VVI to VDD mode with 1:1 conduction.
Switch from VVI to VDD mode with 1:1 conduction.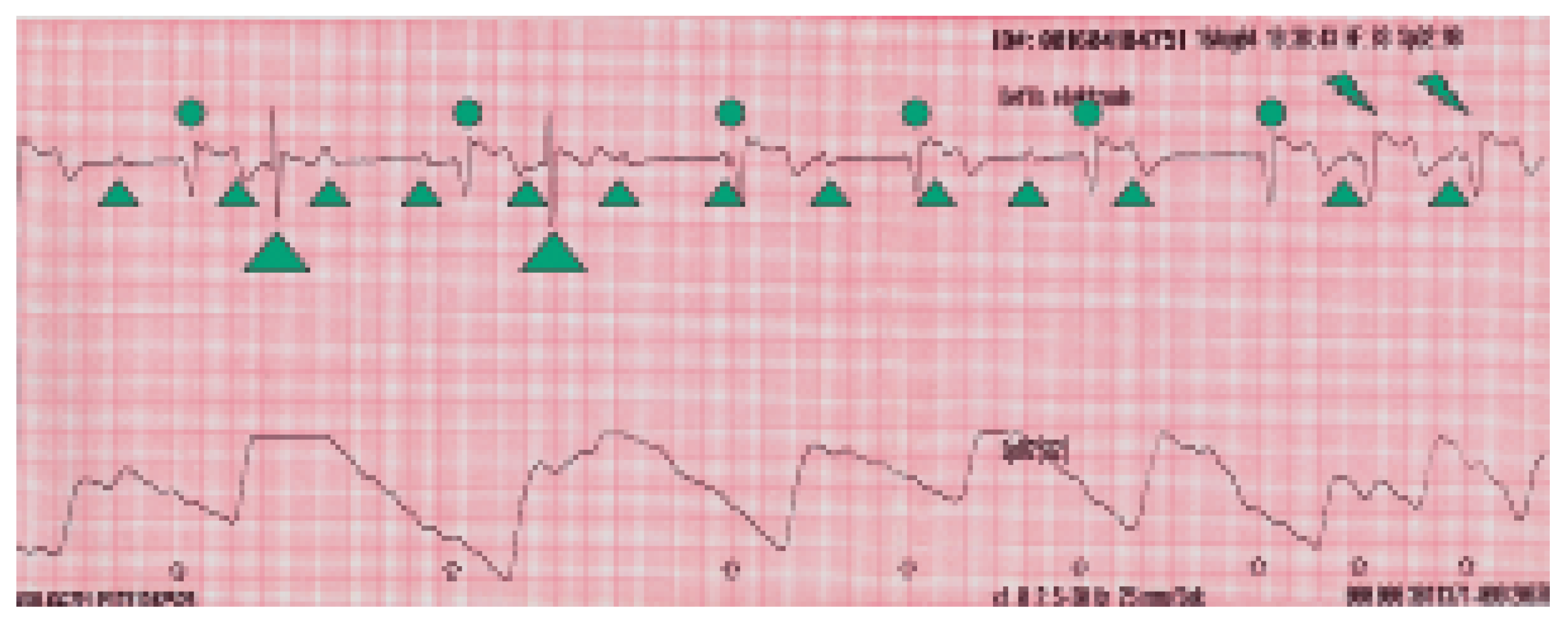
© 2006 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Gnehm, P.; Schaer, B. A Difficult Pacemaker ECG Resulting in an Unnecessary Intervention. Cardiovasc. Med. 2006, 9, 227. https://doi.org/10.4414/cvm.2006.01177
Gnehm P, Schaer B. A Difficult Pacemaker ECG Resulting in an Unnecessary Intervention. Cardiovascular Medicine. 2006; 9(6):227. https://doi.org/10.4414/cvm.2006.01177
Chicago/Turabian StyleGnehm, Peter, and Beat Schaer. 2006. "A Difficult Pacemaker ECG Resulting in an Unnecessary Intervention" Cardiovascular Medicine 9, no. 6: 227. https://doi.org/10.4414/cvm.2006.01177
APA StyleGnehm, P., & Schaer, B. (2006). A Difficult Pacemaker ECG Resulting in an Unnecessary Intervention. Cardiovascular Medicine, 9(6), 227. https://doi.org/10.4414/cvm.2006.01177



