Summary
Acute pulmonary embolism (PE) is a heterogenous condition, with varying early and long term clinical outcomes. The mortality rate in PE patients is higher than in patients with acute myocardial infarction, exceeding 10% at 30 days and 16% at 3 months [
1]. Within 30 days, the most common cause of death is right ventricular failure, and most deaths beyond 30 days often are due to underlying chronic conditions, including cancer, congestive heart failure, or chronic lung disease.
With therapeutic levels of anticoagulation, most patients will likely have an uneventful clinical course. Some patients, however, suffer rapid clinical deterioration, including death from right ventricular failure or the need for cardiopulmonary resuscitation, mechanical ventilation, administration of pressors for systolic arterial hypotension, rescue thrombolysis, or surgical embolectomy.
Contemporary PE risk stratification tools are (1.) the clinical evaluation, (2.) cardiac biomarkers, (3.) twelve-lead electrocardiography, (4.) echocardiography, and (5.) chest computed tomography.
Key words: pulmonary embolism; prognosis
Zusammenfassung
Die akute Lungenembolie (LE) ist eine heterogene Erkrankung mit einem variablen kurzund langfristigen klinischen Verlauf. Die Mortalitätsrate bei LE liegt mit über 10% nach 30 Tagen und 16% nach 3 Monaten höher als beim akuten Myokardinfarkt [
1]. Die häufigste Todesursache innerhalb von 30 Tagen ist eine Rechtsherzinsuffizienz. Die meisten Todesfälle nach einem Ablauf von 30 Tagen sind häufig auf zugrunde liegende chronische Krankheiten wie Krebs, dekompensierte Herzinsuffizienz oder chronische Lungenerkrankungen zurückzuführen.
Durch die Gabe von Antikoagulantien in therapeutischer Dosierung kann bei den meisten Patienten ein komplikationsloser klinischer Verlauf erreicht werden. Bei manchen Patienten tritt jedoch eine rasche klinische Verschlechterung ein, die mit dem Tod durch Rechtsherzversagen einhergeht oder eine kardiopulmonale Wiederbelebung, künstliche Beatmung, Katecholamin-Gabe, Fibrinolyse oder Embolektomie erforderlich machen kann.
Folgende Kriterien werden heute für die Risikostratifizierung bei LE verwendet: (1.) klinische Bewertung, (2.) kardiale Biomarker, (3.) 12-Kanal-EKG, (4.) Echokardiographie und (5.) Thorax-CT.
Key words: Lungenembolie; Prognose
Clinical evaluation
Profound dyspnea, cyanosis, and syncope usually indicate haemodynamically significant PE. The clinical examination often shows signs of acute right ventricular dysfunction, including tachycardia, a low arterial blood pressure, distended neck veins, an accentuated pulmonic component of the second heart sound, or a tricuspid regurgitation murmur. Conversely, pleuritic chest pain usually indicates anatomically smaller PE, often accompanied by subsegmental pulmonary infarction.
Comorbidities increase the risk of adverse clinical events, even in the presence of anatomically small PE. In the International Cooperative Pulmonary Embolism (ICOPER) registry [
1], advanced age, congestive heart failure, cancer, or chronic lung disease were identified as independent predictors of 3-month mortality, with an approximately two-fold increase in the risk of death.
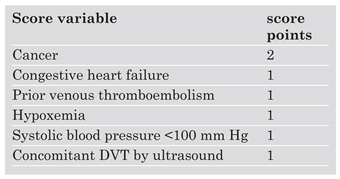
The Geneva Prognostic Index (
Table 1) is a clinical risk assessment tool based mainly on the findings from the past medical history and the clinical examination [
2]. This index was used to identify low-risk patients who were managed successfully on a completely outpatient basis. Risk stratification was performed using a clinical score with a maximum of 8 points. The score had to be less than or equal to 2 to qualify for outpatient treatment. During 3-month follow-up, none of the 43 patients died, and there was no major bleeding. One of the 43 outpatients had an objectively confirmed DVT during follow-up. In conclusion, this study showed that carefully selected PE patients at low risk for right heart failure and recurrent VTE can be treated safely on an entirely outpatient basis.
Electrocardiography
The twelve-lead electrocardiogram (ECG) helps exclude acute ST elevation myocardial infarction. McGinn described in 1935 the well known
SI QIII TIII type as a common finding among patients with acute PE. Unfortunately, about 25% of the patients with acute PE have a normal ECG without signs of right ventricular strain (
Table 2). The ECG also helps identify PE patients at increased risk of adverse clinical outcomes. T wave inversion in the anterior precordial leads V2 and V3 and the pseudoinfarction pattern, Qr in V1, usually indicate right ventricular dilation and dysfunction [
3]. Patients with right ventricular strain often present with elevated cardiac biomarker levels, including cardiac troponins and natriuretic peptides, suggesting that ECG signs correlate with the extent of right ventricular dilation and dysfunction. Furthermore, both T wave inversions and the pseudoinfarction pattern in the precordial leads were found to predict adverse clinical outcomes, including death, cardiopulmonary resuscitation, mechanical ventilation, and the administration of pressors or thrombolysis.
Echocardiography
Transthoracic echocardiography cannot be recommended to diagnose PE in haemodynamically stable patients because it is normal in about 50% of the patients with suspected acute PE. Echocardiography aims to confirm or exclude right ventricular dysfunction. The transoesophageal approach may be used to diagnose emboli in the main pulmonary artery, the right and left main pulmonary artery, but not in lobar and segmental branches [
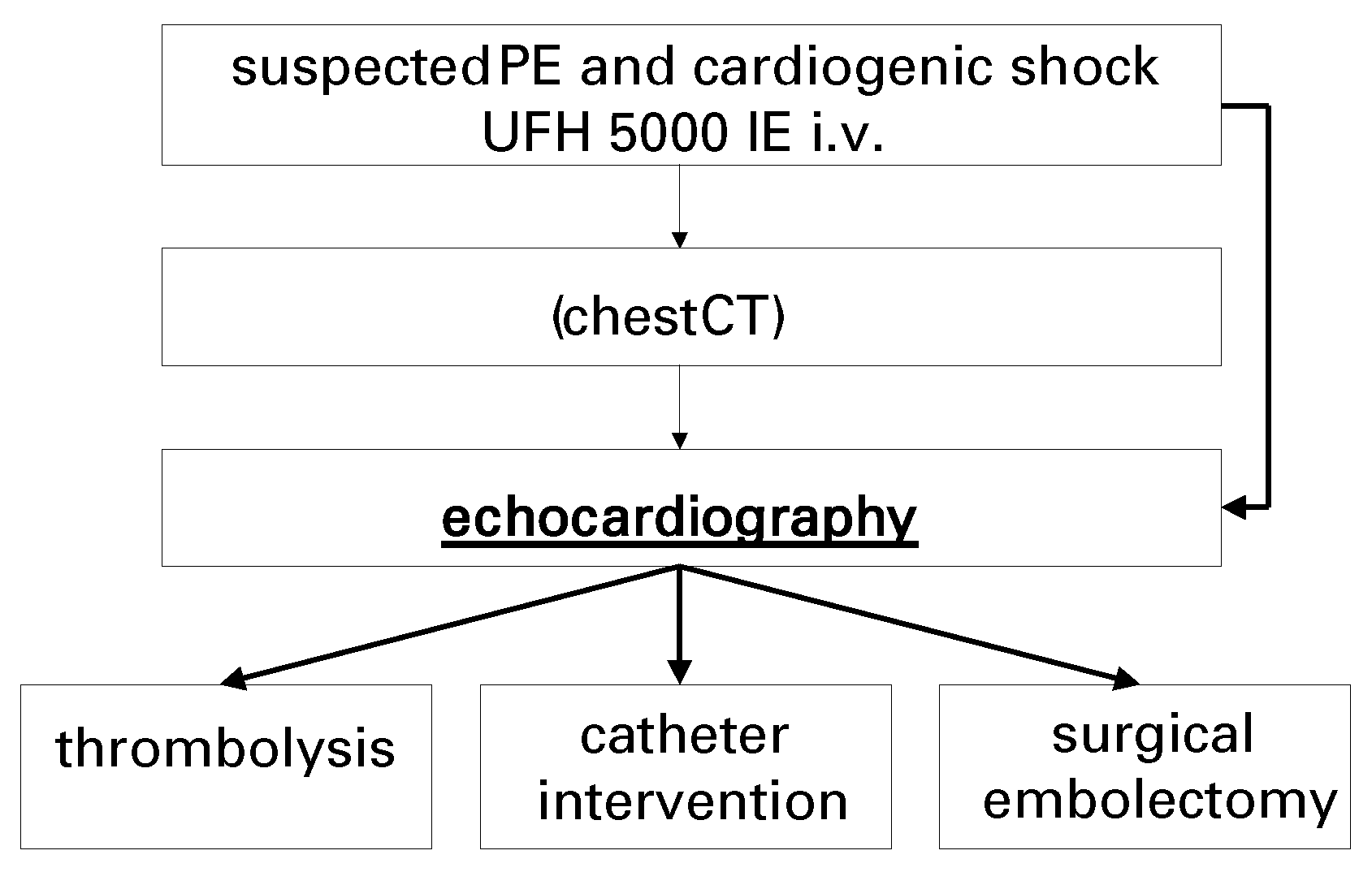
4]. Bedside echocardiography is helpful in patients with suspect-ed acute PE and haemodynamic instability because potentially life-saving treatment, including thrombolysis or surgical embolectomy, can be initiated without time delay (
Figure 1). Echocardiography also helps diagnose conditions that mimic acute PE but which are treated very differently, such as acute myocardial infarction, aortic dissection, or pericardial tamponade.
Transthoracic echocardiography is an important tool for risk stratification because right ventricular dysfunction on the echocardiogram is a powerful and independent predictor of mortality. Even in normotensive patients with acute PE who present with a systolic arterial pressure >90 mm Hg, echocardiography is an independent predictor of early death (
Figure 2) [
5].
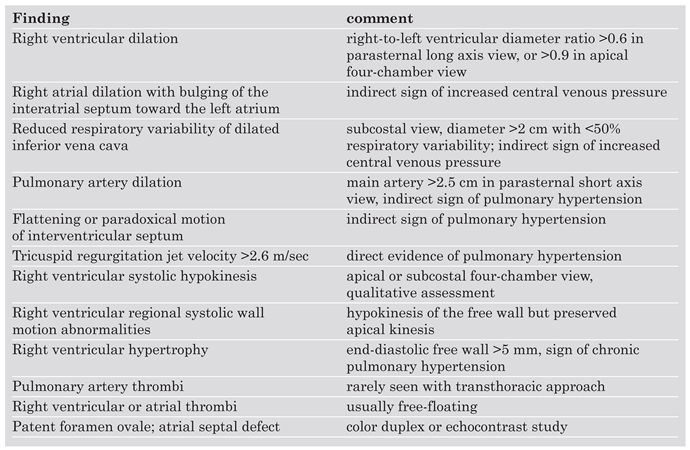
Right ventricular dysfunction is diagnosed in the presence of: (1.) right ventricular dilatation, defined as a right ventricular to left ventricular end-diastolic dimension ratio >0.6 in the parasternal long-axis view or >0.9 in the four-chamber view, (2.) right ventricular systolic free wall hypokinesis, or (3.) systolic pulmonary arterial hypertension, defined as a tricuspid regurgitant velocity >2.6 m/s (
Table 3). Indirect signs of right ventricular pressure overload are a flattened interventricular septum, paradoxical systolic motion of the interventricular septum toward the left ventricle, or a dilated inferior vena cava with reduced respiratory variability.
Echocardiography in the setting of acute PE is also useful to diagnose a patent foramen ovale or an atrial septal defect. A patent foramen ovale identifies patients at risk for paradoxical embolism and stroke. In one study of patients with acute PE [
6], the presence of a patent foramen ovale was as strong as arterial hypotension for predicting mortality. In patients with recurrent PE and paradoxical embolism, percutaneous closure of the patent foramen ovale and placement of an inferior vena cava filter prevented recurrent thromboembolic events [
7].
In patients with acute PE, free floating right heart thrombi increase the risk for adverse clinical events. In ICOPER [
1], the overall mortality rate at 14 days (21
vs 11%) and at three months (29
vs 16%) was higher in patients with than without right heart thrombi.
Echocardiographic findings at the time ofPE diagnosis also confer predictive information regarding the risk of developing chronic thromboembolic pulmonary hypertension. An estimated systolic pulmonary artery pressure of greater than 50 mm Hg at the time of PE diagnosis was associated with persistent pulmonary hypertension at one year [
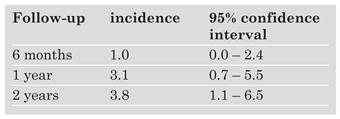
8]. In a longitudinal study of patients with acute PE, the cumulative incidence of chronic thromboembolic pulmonary hypertension was 3.1% at one year (
Table 4) [
9]. In this study, prior and idiopathic PE, younger age, or a large perfusion defect increased the risk of developing chronic thromboembolic pulmonary hypertension during the ensuing two years. Echocardiographic follow-up performed 6 weeks after the diagnosis can identify patients with persistent pulmonary hypertension and may be of value in planning the long term care of these patients. Patients with persistent symptoms and chronic thromboembolic hypertension may benefit from surgical thromboendarterectomy with insertion of a vena cava filter, percutaneous interventional therapy with stent placement, or long term vasodilator treatment.
Cardiac biomarkers
Cardiac troponins I and T as well as NT-pro brain natriuretic peptide (NT-proBNP) and brain natriuretic peptide (BNP) have emerged as promising tools for risk stratification [
10].
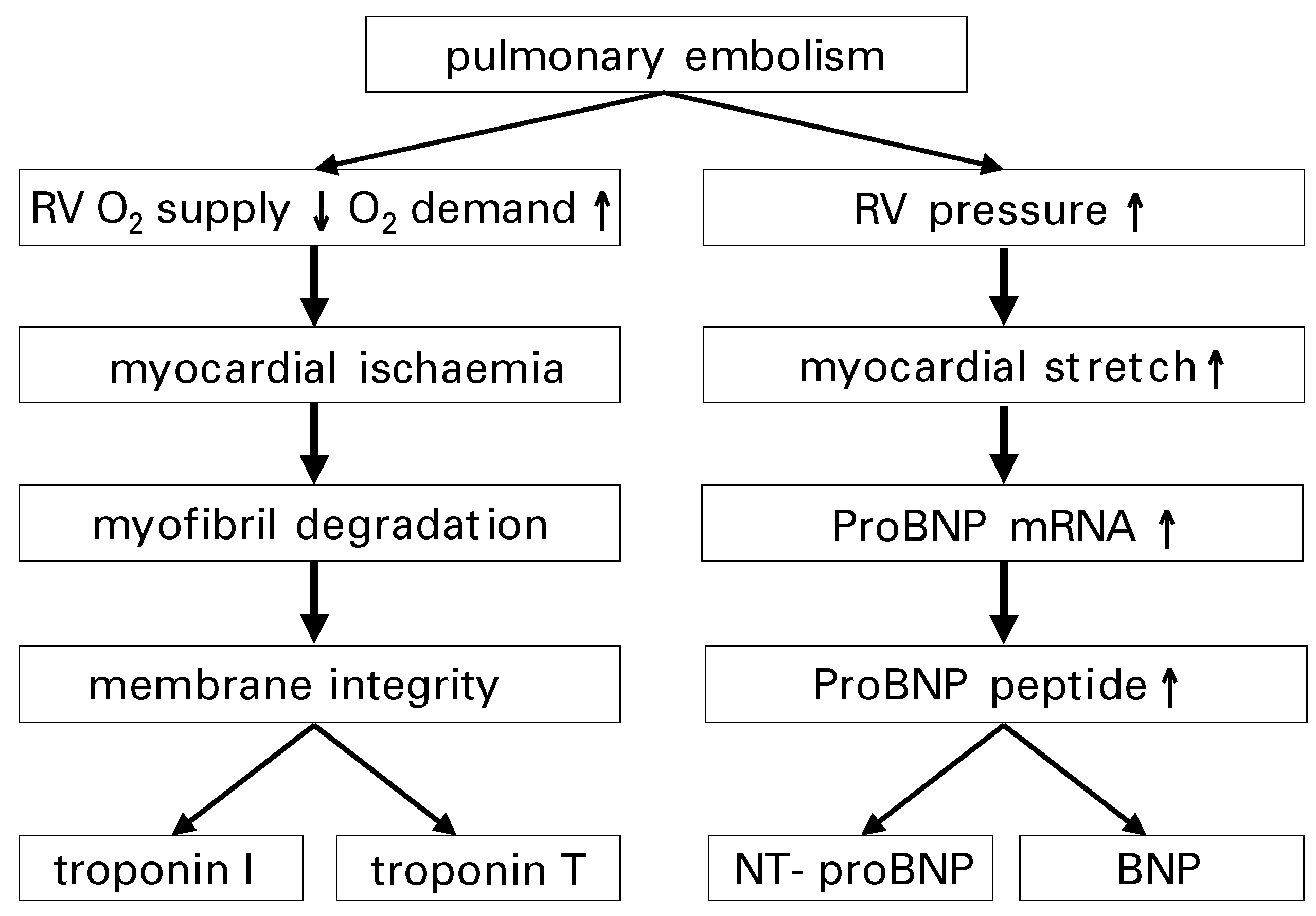
Cardiac troponins are sensitive and specific markers of myocardial injury. Elevations of troponin levels in PE patients are brief and subtle compared to patients with acute coronary syndrome. In acute PE, troponin levels correlate with the extent of right ventricular dysfunction. At the time of diagnosis, a few PE patients have negative troponin test results but may show an increase several hours later. Myocardial ischaemia due to alterations in oxygen supply and demand of the failing right ventricle play a major role in the pathogenesis of troponin level elevation (
Figure 3). Increased troponin levels in patients with acute PE were reported in the absence of angiographic coronary artery disease. In some patients with massive PE, a transient elevation of creatine kinase (CK) or of the CK-MB isoenzyme fraction as a result of right ventricular infarction was observed.
The natriuretic peptides are useful diagnostic and prognostic markers for patients with congestive heart failure. The stimulus for BNP synthesis and secretion is cardiomyocyte stretch (
Figure 3). Prohormone in normal ventricular myocytes is not stored to a significant amount. Thus, it takes several hours for the plasma natriuretic peptides levels to increase after the onset of acute cardiomyocyte stretch. This includes myocardial BNP messenger ribonucleic acid (mRNA) synthesis, prohormone synthesis and plasma release. Elevations in BNP and NT-proBNP are associated with right ventricular dysfunction in acute PE.
Troponins and natriuretic peptides are similarly accurate in identifying low-risk PE patients. The negative predictive value (NPV) for in-hospital death exceeds 97% for the biomarker assays. The cut-off levels for cardiac troponins usually are the lower detection limits reported by the manufacturer. The cut-off level for the BNP triage assay for predicting an uneventful clinical course in PE patients is lower (<50 pg/ml) than the “congestive heart failure” cut-off level of 90 pg/ml.
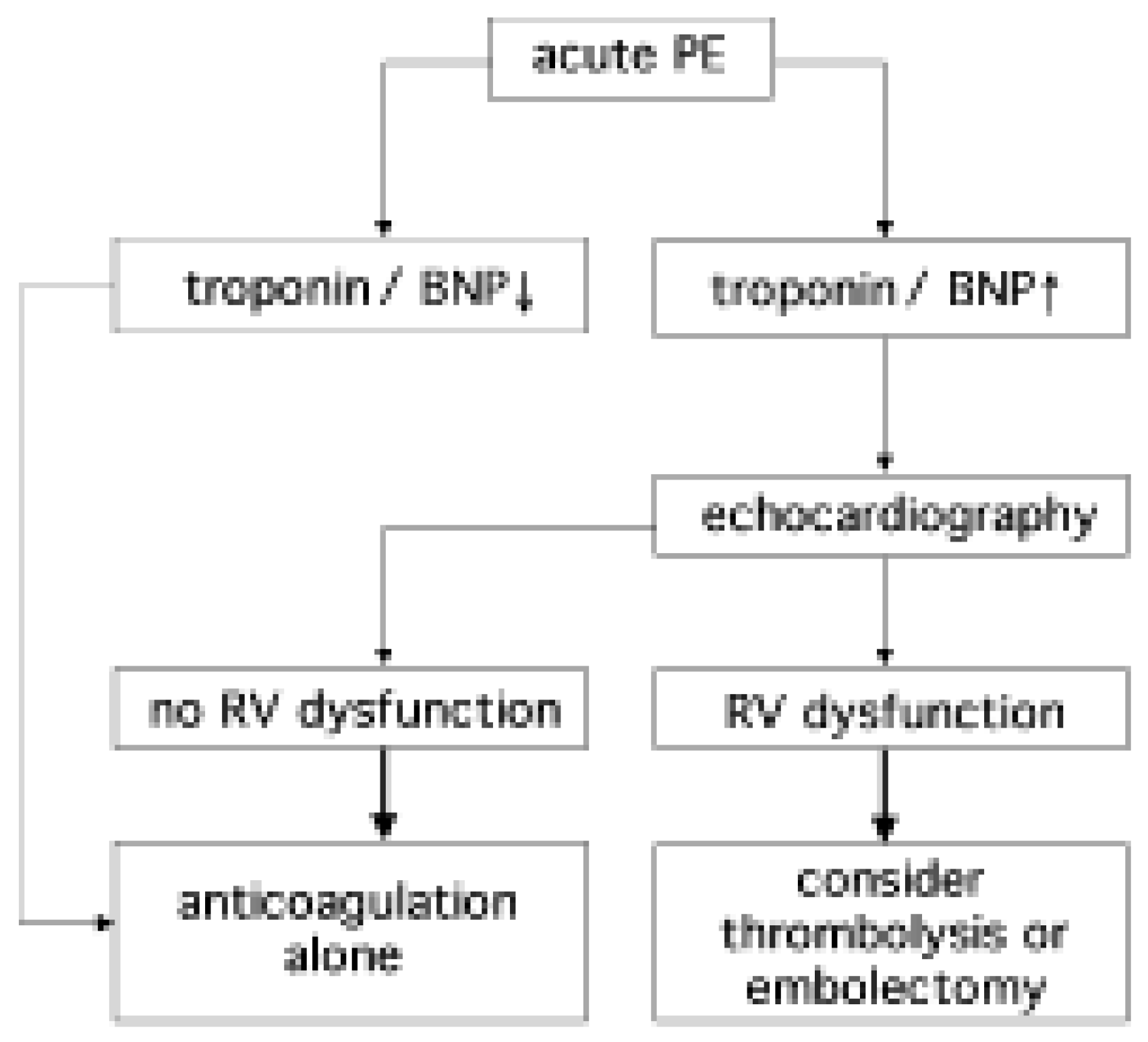
In PE patients with increased cardiac biomarker levels, further risk stratification with echocardiography is warranted due to limited specificity of the assays for predicting right ventricular dysfunction (
Figure 4). In patients with biomarker levels below the assay-specific cut-off, echocardiography will likely not add prognostic information.
Contrast-enhanced multidetector row computed tomography
Contrast enhanced chest computed tomography (CT) is increasingly used as the first-line PE imaging test and is available around the clock at most institutions. With newer generation scanners, standardised cardiac views are easily obtained in almost all patients who undergo contrast-enhanced chest CT. Most contemporary CT scanners allow online twodimensional reconstruction of standardised cardiac views, with direct measurement of ventricular dimensions (
Figure 5). In the reconstructed four-chamber view, right (RVD) and left ventricular dimensions (LVD) are then measured by identifying the maximal distance between the ventricular endocardium and the interventricular septum, perpendicular to the long axis of the heart. Right ventricular enlargement is defined as RVD/LVD >0.9.
In 63 patients with acute PE, the presence of right ventricular enlargement on the reconstructed CT four-chamber view correlated with the presence of right ventricular dysfunction on echocardiogram [
11].
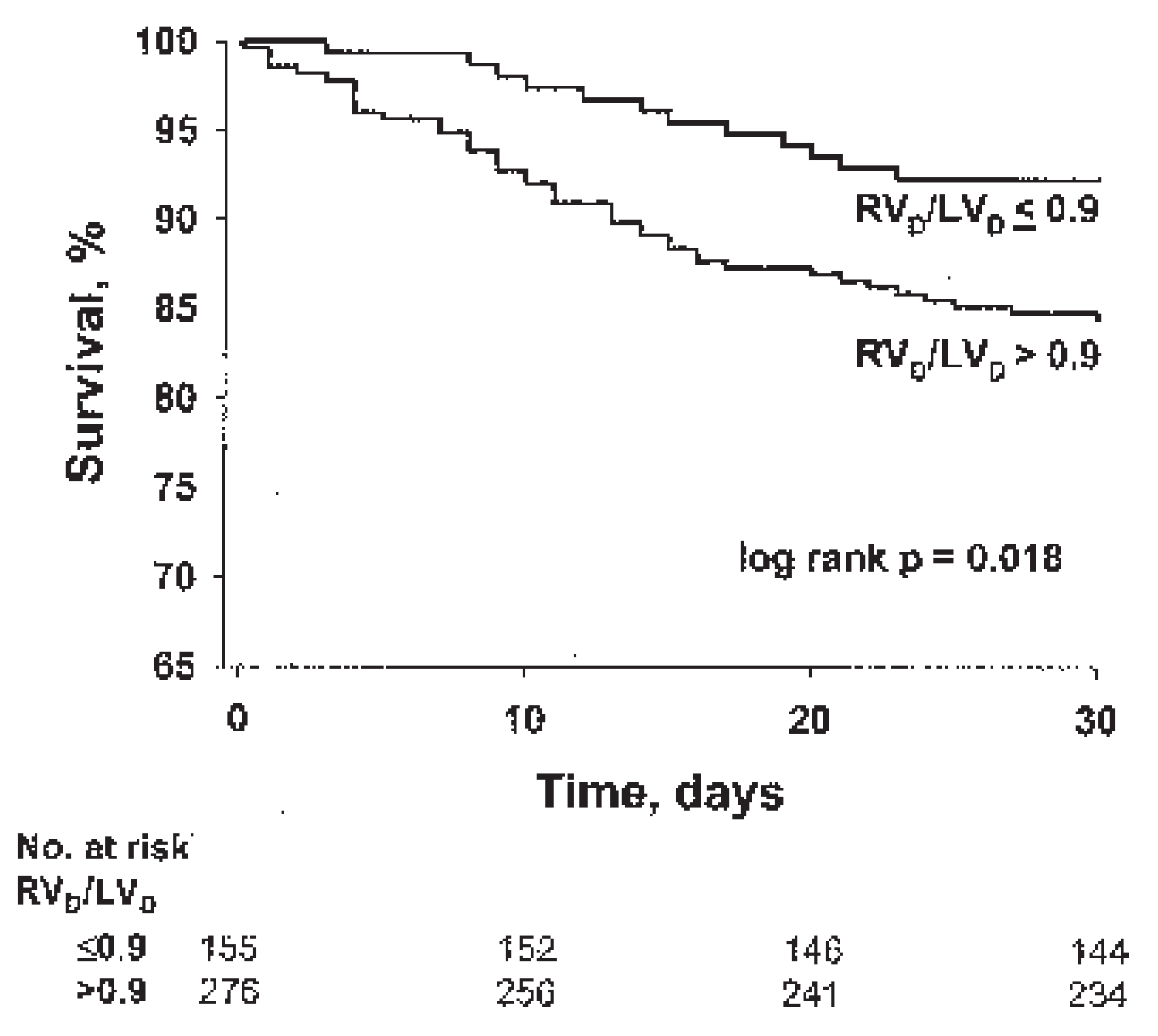
Right ventricular enlargement on chest CT helps identify patients at risk of death from right ventricular failure. In a study of 431 consecutive patients with acute PE, right ventricular enlargement on the reconstructed CT four-chamber view was an independent predictor of 30-day mortality after adjustment for important patient characteristics (
Figure 6) [
12]. Prospective management studies are needed to investigate whether cardiac measurements on reconstructed CT 4-chamber views are useful to guide treatment decisions in patients with acute PE.
Future research perspectives
According to the European Task Force Guidelines on Pulmonary Embolism [
13], risk stratification in PE patients is important for selecting the appropriate therapy. However, the role of reperfusion therapy, including thrombolysis, catheter interventions, or surgical embolectomy is not well defined, particularly for patients with severe PE despite preserved blood pressure.
Future research activities are warranted for identifying PE patients who may benefit from early aggressive intervention. Patients with acute PE who present with elevated cardiac biomarker levels and right ventricular dysfunction may have a survival benefit with early initiation of reperfusion therapy or embolectomy. However, this hypothesis has to be confirmed or rejected by conducting large randomised controlled trials.