Impact of Diabetes on Acute Coronary Syndrome Management
Summary
Zusammenfassung
Introduction
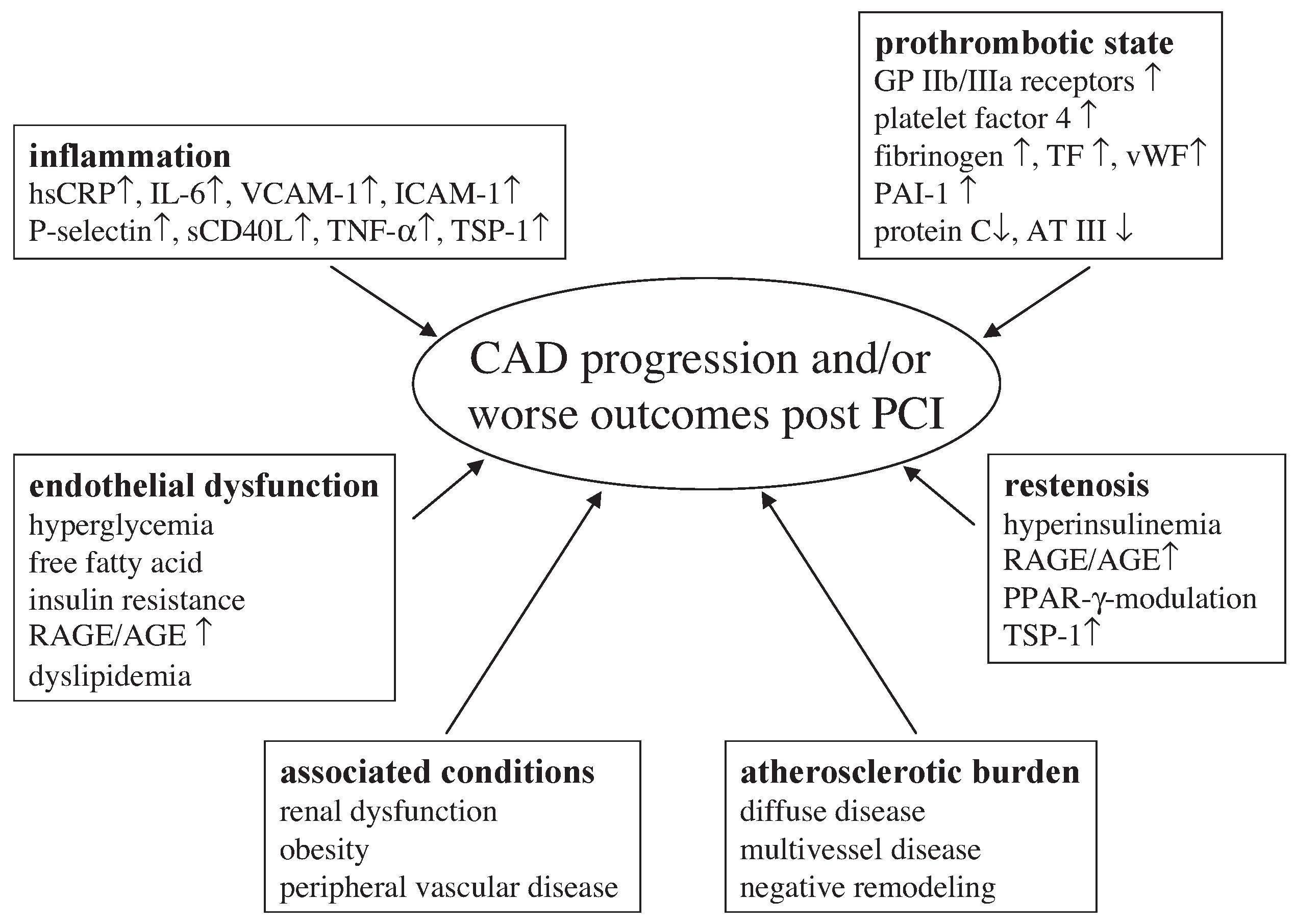
Outcome of diabetic patients with acute coronary syndromes
Percutaneous coronary intervention
Restenosis and drug eluting stents
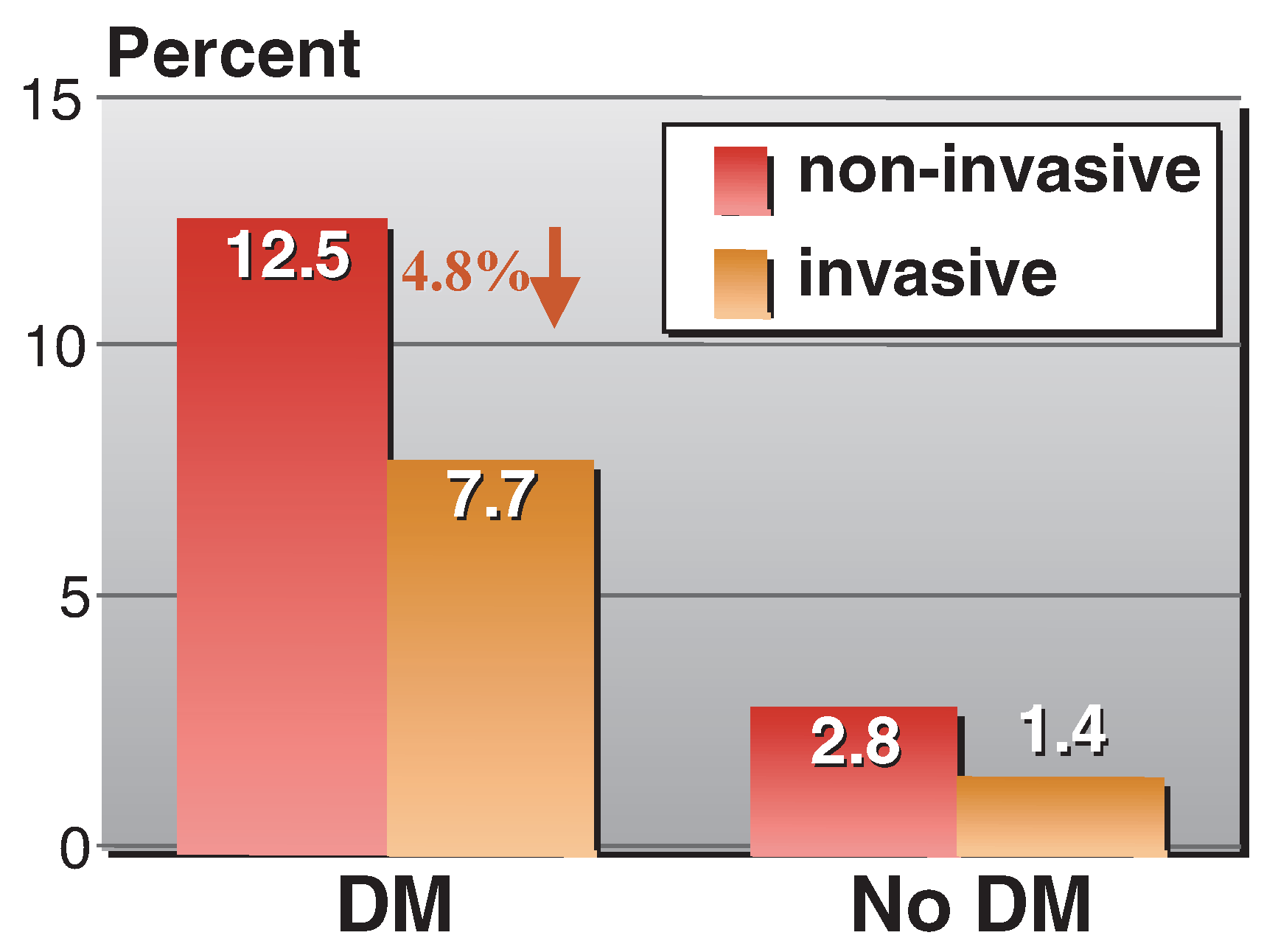
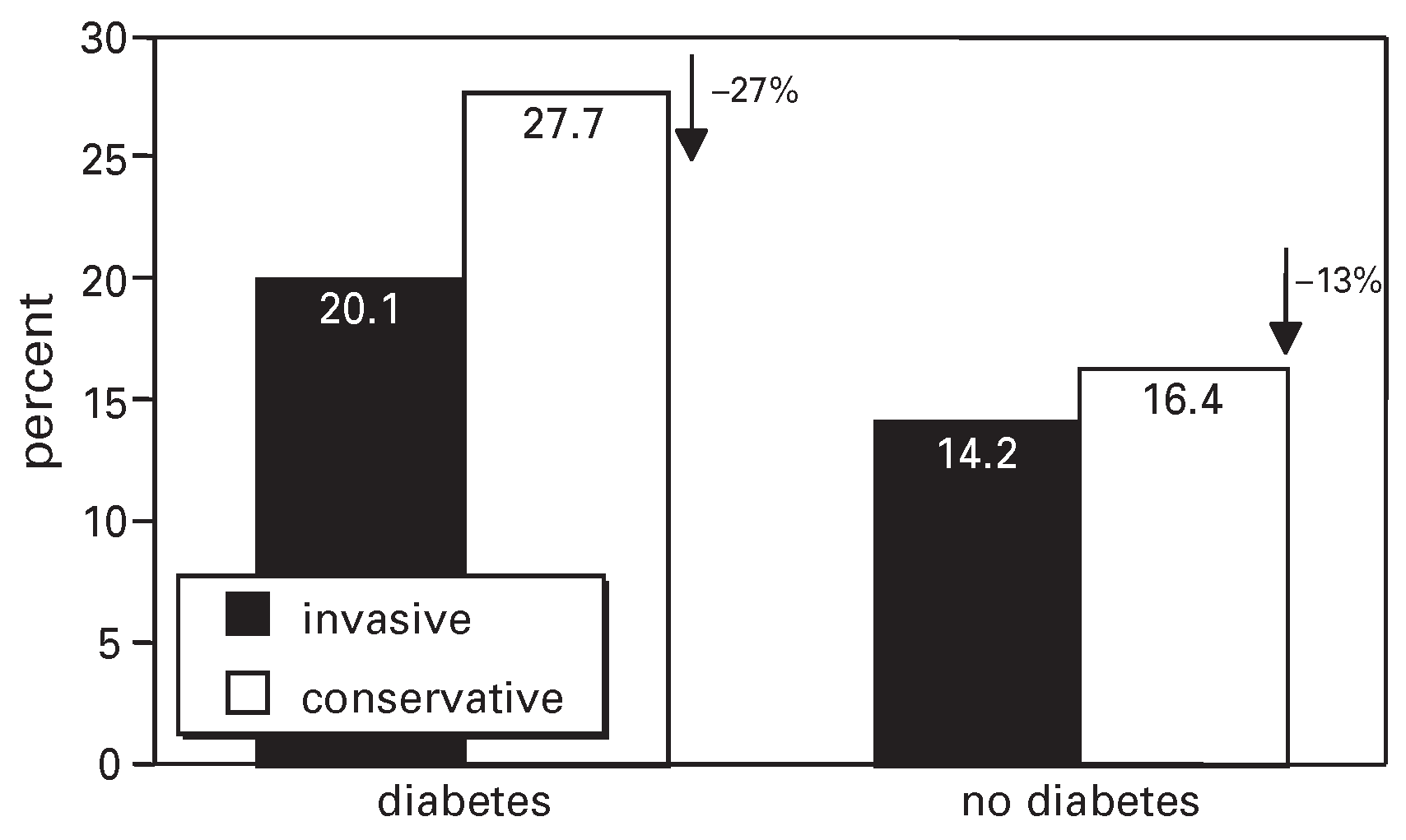
Early invasive versus conservative strategy
Adjunctive pharmacologic treatment
Clopidogrel
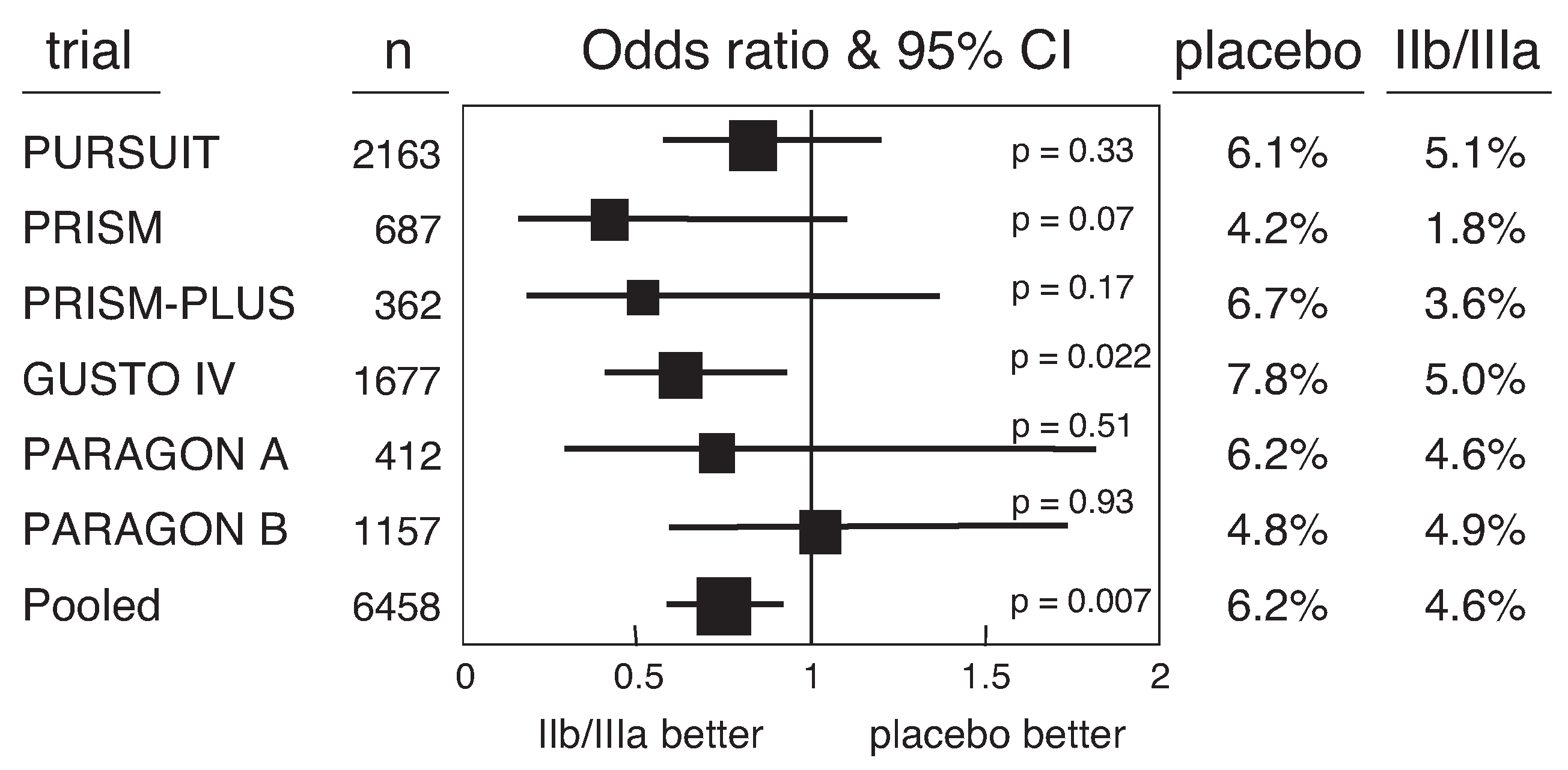
Glycoprotein IIb/IIIa receptor antagonists
Conclusions
References
- McKinlay, J.; Marceau, L. US public health and the 21st century: Diabetes mellitus. Lancet 2000, 356, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Topol, E.J. Percutaneous coronary intervention in diabetic patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J 2004, 25, 190–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biondi-Zoccai, G.G.; Abbate, A.; Liuzzo, G.; Biasucci, L.M. Atherothrombosis, inflammation and diabetes. J Am Coll Cardiol 2003, 41, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.J.; Norrie, J.; Caslake, M.J.; Gaw, A.; Ford, I.; Lowe, G.D.; et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 2002, 51, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.H.; Moliterno, D.J.; Granger, C.B.; Miller, D.P.; White, H.D.; Wilcox, R.G.; et al. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. GUSTO-I Investigators. Global Utilization of Streptokinase and 7Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol 1997, 30, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Cho, L.; Bhatt, D.L.; White, J.A.; Moliterno, D.J.; Harrington, R.A.; et al. Dramatic increase in 30-day mortality in diabetic patients with non-ST segment elevation acute coronary syndromes. J Am Coll Cardiol 2002, 33, 313A. [Google Scholar] [CrossRef]
- Laskey, W.K.; Selzer, F.; Vlachos, H.A.; Johnston, J.; Jacobs, A.; King, S.B., 3rd; et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002, 90, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Moliterno, D.J.; Meier, B.; Powers, E.R.; Grines, C.L.; DiBattiste, P.M.; et al. Impact of different platelet glycoprotein IIb/IIIa receptor inhibitors among diabetic patients undergoing percutaneous coronary intervention: Do Tirofiban and ReoPro Give Similar Efficacy Outcomes Trial (TARGET) 1-year follow-up. Circulation 2002, 105, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Windecker, S.; Meier, B. Drug eluting stents. In Textbook of Cardiovascular Medicine Update Series; Topol, E.J., Ed.; Lippincott Williams & Wilkins: New York, NY, USA, 2002; pp. 1–15. [Google Scholar]
- Popma, J.J. Presented at the Annual Scientific Session of the American Heart Association, Chicago; November 2002. Available online: www.tctmd.com.
- Morice, M.C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Ban Hayashi, E.; Perin, M.; et al. A randomized comparison of a sirolimuseluting stent with a standard stent for coronary revascularization. N Engl J Med 2002, 346, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W. Taxus IV Results. In Proceedings of the Transcatheter Cardiovascular Therapeutics, Washington, DC, USA, 15–19 September 2003. [Google Scholar]
- Ragmin, F. Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Lancet 1999, 354, 708–715. [Google Scholar]
- Wallentin, L.; Lagerqvist, B.; Husted, S.; Kontny, F.; Stahle, E.; Swahn, E. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: The FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet 2000, 356, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Weintraub, W.S.; Demopoulos, L.A.; Vicari, R.; Frey, M.J.; Lakkis, N.; et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001, 344, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Topol, E.J. Anticoagulant therapy in unstable angina. In Textbook of Cardiovascular Medicine Update Series; Topol, E.J., Ed.; Lippincott Williams & Wilkins: New York, NY, USA, 2000; pp. 1–11. [Google Scholar]
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCICURE study. Lancet 2001, 358, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R.; Berger, P.B.; Mann, J.T.; 3rd Fry, E.T.; DeLago, A.; Wilmer, C.; et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA 2002, 288, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.F.; Hasselblad, V.; Harrington, R.A.; White, H.D.; Tcheng, J.E.; Kandzari, D.E.; et al. Meta-analysis of survival with platelet glycoprotein IIb/IIIa antagonists for percutaneous coronary interventions. Am J Cardiol 2003, 92, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Califf, R.M.; Moliterno, D.J.; Ellis, S.G.; Ducas, J.; Kramer, J.H.; et al. Complementary clinical benefits of coronaryartery stenting and blockade of platelet glycoprotein IIb/IIIa receptors. Evaluation of Platelet IIb/IIIa Inhibition in Stenting Investigators. N Engl J Med 1999, 341, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Marso, S.P.; Lincoff, A.M.; Wolski, K.E.; Ellis, S.G.; Topol, E.J. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000, 35, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Chew, D.; Mukherjee, D.; Bhatt, D.; White, J.; Moliterno, D.; et al. Platelet glycoprotein IIb/IIIa inhibition in acute coronary syndromes. Gradient of benefit related to the revascularization strategy. Eur Heart J 2002, 23, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Chew, D.P.; Mukherjee, D.; Bhatt, D.L.; White, J.A.; Heeschen, C.; et al. Platelet glycoprotein IIb/IIIa inhibitors reduce mortality in diabetic patients with non-ST-segmentelevation acute coronary syndromes. Circulation 2001, 104, 2767–2771. [Google Scholar] [CrossRef] [PubMed]

© 2004 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Roffi, M. Impact of Diabetes on Acute Coronary Syndrome Management. Cardiovasc. Med. 2004, 7, 325. https://doi.org/10.4414/cvm.2004.01045
Roffi M. Impact of Diabetes on Acute Coronary Syndrome Management. Cardiovascular Medicine. 2004; 7(9):325. https://doi.org/10.4414/cvm.2004.01045
Chicago/Turabian StyleRoffi, Marco. 2004. "Impact of Diabetes on Acute Coronary Syndrome Management" Cardiovascular Medicine 7, no. 9: 325. https://doi.org/10.4414/cvm.2004.01045
APA StyleRoffi, M. (2004). Impact of Diabetes on Acute Coronary Syndrome Management. Cardiovascular Medicine, 7(9), 325. https://doi.org/10.4414/cvm.2004.01045



